Potential Linkage between Heavy Metal Pollution Risk Assessment and Dissolved Organic Matter Spectra in the WWTPs-River Integrated Area-Case Study from Ashi River
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Preparation
2.3. Risk Assessment
2.4. UV–Vis Spectroscopy Analysis
2.5. Fluorescence Spectroscopy Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Heterogeneity of Elements in the WWTPs-River Integrated Area
| Location | Source | Cd | Pb | Cr | Cu | Ni | Zn | As | Hg | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Ashi River, Harbin | This Study | 0.19–2.23 | 17.45–86.85 | 32.00–254.49 | 33.38–384.71 | 42.05–224.87 | 131.01–894.57 | 1.87–19.85 | 0.38–8.98 | 4.98–62.56 |
| Vidy Bay, Lausanne, Switzerland | [72] | 0.74–10.13 | 43–498 | - | 75–362 | - | 170–1564 | 8–22 | 0.24–8.98 | 18.2–47.8 |
| Biebrza River, Poland | [77] | - | - | 8.4–28.3 | 3.1–54.7 | - | 17.4–162.7 | - | - | 1.07–1.57 |
| Cape Town, South Africa | [79] | 0.25–1.77 | - | - | - | - | 178.16–949.87 | 5.70–34.66 | 0.01–0.3 | - |
| Lake Mariout, Egypt | [73] | 0.9–1.0 | 91.6–93.0 | 79.8–89.9 | 129.4–148.5 | 46.3–59.8 | - | - | - | - |
| Golden Horn, Istanbul, Turkey | [76] | 0.1–2 | 8–50 | 26–110 | 70–135 | 10–38 | 70–260 | - | - | - |
3.2. Risk of Heavy Metal Pollution
3.2.1. Mono-Metal Pollution Risk
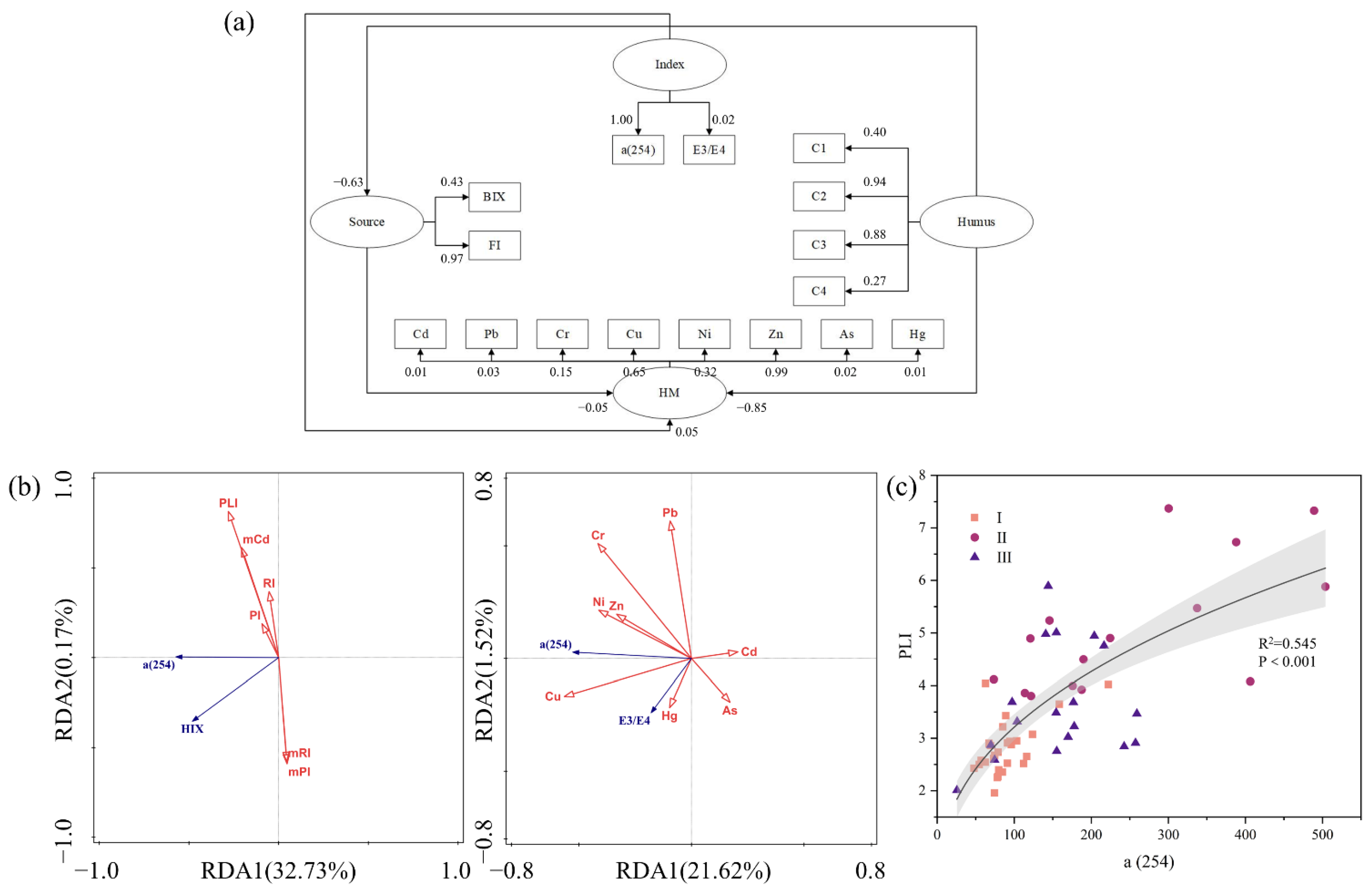
3.2.2. Integrated Pollution Risk
3.3. Component and Spectral Indices of DOM
3.4. Potential Connection between DOM and HM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, T.; Skyllberg, U.; Bjorn, E.; Green, N.W.; Tang, J.; Wang, D.; Gao, J.; Li, C. Characteristics of dissolved organic matter (DOM) and relationship with dissolved mercury in Xiaoqing River-Laizhou Bay estuary, Bohai Sea, China. Environ. Pollut. 2017, 223, 19–30. [Google Scholar] [CrossRef]
- Jahan, S.; Strezov, V. Comparison of pollution indices for the assessment of heavy metals in the sediments of seaports of NSW, Australia. Mar. Pollut. Bull. 2018, 128, 295–306. [Google Scholar] [CrossRef]
- Xiao, Y.-H.; Huang, Q.-H.; Vahatalo, A.V.; Li, F.-P.; Chen, L. Effects of Dissolved Organic Matter from a Eutrophic Lake on the Freely Dissolved Concentrations of Emerging Organic Contaminants. Environ. Toxicol. Chem. 2014, 33, 1739–1746. [Google Scholar] [CrossRef]
- Yan, M.; Korshin, G.V. Comparative Examination of Effects of Binding of Different Metals on Chromophores of Dissolved Organic Matter. Environ. Sci. Technol. 2014, 48, 3177–3185. [Google Scholar] [CrossRef]
- Ren, W.; Wu, X.; Ge, X.; Lin, G.; Zhou, M.; Long, Z.; Yu, X.; Tian, W. Characteristics of dissolved organic matter in lakes with different eutrophic levels in southeastern Hubei Province, China. J. Oceanol. Limnol. 2021, 39, 1256–1276. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Bao, H.; Gan, S.; Zhang, J. Sources, Transport, and Transformation of Dissolved Organic Matter in a Large River System: Illustrated by the Changjiang River, China. J. Geophys. Res.-Biogeosci. 2019, 124, 3881–3901. [Google Scholar] [CrossRef]
- Patidar, S.K.; Chokshi, K.; George, B.; Bhattacharya, S.; Mishra, S. Dominance of cyanobacterial and cryptophytic assemblage correlated to CDOM at heavy metal contamination sites of Gujarat, India. Environ. Monit. Assess. 2015, 187, 4118. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Wang, S.; Zhao, H.; Zhang, R. Composition, structural characteristics and indication of water quality of dissolved organic matter in Dongting Lake sediments. Ecol. Eng. 2016, 97, 370–380. [Google Scholar] [CrossRef]
- Omar, T.F.T.; Aris, A.Z.; Yusoff, F.M.; Mustafa, S. Occurrence, distribution, and sources of emerging organic contaminants in tropical coastal sediments of anthropogenically impacted Klang River estuary, Malaysia. Mar. Pollut. Bull. 2018, 131, 284–293. [Google Scholar] [CrossRef]
- He, Y.; Men, B.; Yang, X.; Li, Y.; Xu, H.; Wang, D. Relationship between heavy metals and dissolved organic matter released from sediment by bioturbation/bioirrigation. J. Environ. Sci. 2019, 75, 216–223. [Google Scholar] [CrossRef]
- Derrien, M.; Kim, M.-S.; Ock, G.; Hong, S.; Cho, J.; Shin, K.-H.; Hur, J. Estimation of different source contributions to sediment organic matter in an agricultural-forested watershed using end member mixing analyses based on stable isotope ratios and fluorescence spectroscopy. Sci. Total Environ. 2018, 618, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Shen, Z.-y.; Feng, C.-h.; Zhang, X. Investigation of the interaction between As and Sb species and dissolved organic matter in the Yangtze Estuary, China, using excitation-emission matrices with parallel factor analysis. Environ. Sci. Pollut. Res. 2015, 22, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Huguet, A.; Vacher, L.; Saubusse, S.; Etcheber, H.; Abril, G.; Relexans, S.; Ibalot, F.; Parlanti, E. New insights into the size distribution of fluorescent dissolved organic matter in estuarine waters. Org. Geochem. 2010, 41, 595–610. [Google Scholar] [CrossRef]
- Sekabira, K.; Origa, H.O.; Basamba, T.A.; Mutumba, G.; Kakudidi, E. Assessment of heavy metal pollution in the urban stream sediments and its tributaries. Int. J. Environ. Sci. Technol. 2010, 7, 435–446. [Google Scholar] [CrossRef]
- Cui, L.; Wang, X.; Li, J.; Gao, X.; Zhang, J.; Liu, Z.; Liu, Z. Ecological and health risk assessments and water quality criteria of heavy metals in the Haihe River. Environ. Pollut. 2021, 290, 117971. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Y.; Lang, M.; Guo, X.; Xia, T.; Wang, T.; Jia, H.; Zhu, L. Identification of sources, characteristics and photochemical transformations of dissolved organic matter with EEM-PARAFAC in the Wei River of China. Front. Environ. Sci. Eng. 2021, 15, 96. [Google Scholar] [CrossRef]
- Huang, M.; Li, Z.; Luo, N.; Yang, R.; Wen, J.; Huang, B.; Zeng, G. Application potential of biochar in environment: Insight from degradation of biochar-derived DOM and complexation of DOM with heavy metals. Sci. Total Environ. 2019, 646, 220–228. [Google Scholar] [CrossRef]
- Xu, H.; Yu, G.; Yang, L.; Jiang, H. Combination of two-dimensional correlation spectroscopy and parallel factor analysis to characterize the binding of heavy metals with DOM in lake sediments. J. Hazard. Mater. 2013, 263, 412–421. [Google Scholar] [CrossRef]
- Tipping, E. Cation Binding by Humic Substances; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Berto, S.; De Laurentiis, E.; Tota, T.; Chiavazza, E.; Daniele, P.G.; Minella, M.; Isaia, M.; Brigante, M.; Vione, D. Properties of the humic-like material arising from the photo-transformation of L-tyrosine. Sci. Total Environ. 2016, 545, 434–444. [Google Scholar] [CrossRef]
- Guo, X.-J.; He, X.-S.; Li, C.-W.; Li, N.-X. The binding properties of copper and lead onto compost-derived DOM using Fourier-transform infrared, UV-vis and fluorescence spectra combined with two-dimensional correlation analysis. J. Hazard. Mater. 2019, 365, 457–466. [Google Scholar] [CrossRef]
- Craven, A.M.; Aiken, G.R.; Ryan, J.N. Copper(II) Binding by Dissolved Organic Matter: Importance of the Copper-to-Dissolved Organic Matter Ratio and Implications for the Biotic Ligand Model. Environ. Sci. Technol. 2012, 46, 9948–9955. [Google Scholar] [CrossRef] [PubMed]
- Langner, P.; Mikutta, C.; Kretzschmar, R. Arsenic sequestration by organic sulphur in peat. Nat. Geosci. 2012, 5, 66–73. [Google Scholar] [CrossRef]
- Li, M.; Kong, F.; Li, Y.; Zhang, J.; Xi, M. Ecological indication based on source, content, and structure characteristics of dissolved organic matter in surface sediment from Dagu River estuary, China. Environ. Sci. Pollut. Res. 2020, 27, 45499–45512. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Wu, S.; Wang, Q.; Qian, L.; Lu, H.; Liu, J.; Lin, H.-J.; Zhang, J.; Xu, W.-B.; Yan, C. Trace metal pollution risk assessment in urban mangrove patches: Potential linkage with the spectral characteristics of chromophoric dissolved organic matter. Environ. Pollut. 2021, 272, 115996. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Du, Y.; Yu, S.; Luo, J.; Duan, H. Human activities determine quantity and composition of dissolved organic matter in lakes along the Yangtze River. Water Res. 2020, 168, 115132. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, J.; Kong, F.; Li, Y.; Li, M.; Dong, Y.; Xi, M. Identification of source apportionment and its spatial variability of dissolved organic matter in Dagu River-Jiaozhou Bay estuary based on the isotope and fluorescence spectroscopy analysis. Ecol. Indic. 2019, 102, 528–537. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, K.; Guo, Z.; Li, X.; Chen, J.; Qi, Z.; Xu, S. Spatiotemporal variations of spectral characteristics of dissolved organic matter in river flowing into a key drinking water source in China. Sci. Total Environ. 2020, 700, 134360. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Zhang, L.; Zhao, H.; Jiao, L.; Zhao, Y.; He, X. Composition and spectroscopic characteristics of dissolved organic matter extracted from the sediment of Erhai Lake in China. J. Soils Sediments 2014, 14, 1599–1611. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, C.; He, C.; Li, P.; Wang, Y.; Pang, Y.; Shi, Q.; He, D. Characterization of dissolved organic matter processing between surface sediment porewater and overlying bottom water in the Yangtze River Estuary. Water Res. 2022, 215, 118260. [Google Scholar] [CrossRef]
- Ishii, S.K.L.; Boyer, T.H. Behavior of Reoccurring PARAFAC Components in Fluorescent Dissolved Organic Matter in Natural and Engineered Systems: A Critical Review. Environ. Sci. Technol. 2012, 46, 2006–2017. [Google Scholar] [CrossRef]
- He, W.; Hur, J. Conservative behavior of fluorescence EEM-PARAFAC components in resin fractionation processes and its applicability for characterizing dissolved organic. Water Res. 2015, 83, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Gabor, R.S.; Eilers, K.; McKnight, D.M.; Fierer, N.; Anderson, S.P. From the litter layer to the saprolite: Chemical changes in water-soluble soil organic matter and their correlation to microbial community composition. Soil Biol. Biochem. 2014, 68, 166–176. [Google Scholar] [CrossRef]
- Liu, W.; Ma, T.; Du, Y.; Wu, X.; Chen, L.; Li, J. Characteristics of dissolved organic matter in surface water and sediment and its ecological indication in a typical mining-affected river-Le’an River, China. Environ. Sci. Pollut. Res. 2022, 29, 37115–37128. [Google Scholar] [CrossRef]
- Osburn, C.L.; Handsel, L.T.; Mikan, M.P.; Paerl, H.W.; Montgomery, M.T. Fluorescence Tracking of Dissolved and Particulate Organic Matter Quality in a River-Dominated Estuary. Environ. Sci. Technol. 2012, 46, 8628–8636. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, F.; Kung, H.-T.; Ghulam, A.; Trumbo, A.L.; Yang, J.; Ren, Y.; Jing, Y. Evaluation and estimation of surface water quality in an arid region based on EEM-PARAFAC and 3D fluorescence spectral index: A case study of the Ebinur Lake Watershed, China. Catena 2017, 155, 62–74. [Google Scholar] [CrossRef]
- Tian, Z.; Bi, Z.; Wang, Y.; Zhao, H. Rapid evaluation method based on DOM for water quality by microlaser fluorescence spectrometer. Appl. Phys. B-Lasers Opt. 2020, 126, 150. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, Z.-T.; Liu, Y.-C.; Wang, Y.-H.; Zhang, S.; He, C.; Shi, Q.; Zeng, H.-X.; Wang, J.-J. Spectroscopic and Molecular-Level Characteristics of Dissolved Organic Matter in a Highly Polluted Urban River in South China. ACS Earth Space Chem. 2019, 3, 2033–2044. [Google Scholar] [CrossRef]
- He, D.; He, C.; Li, P.; Zhang, X.; Shi, Q.; Sun, Y. Optical and Molecular Signatures of Dissolved Organic Matter Reflect Anthropogenic Influence in a Coastal River, Northeast China. J. Environ. Qual. 2019, 48, 603–613. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Tian, Y.; Hou, Z.; He, K.; Fu, L.; Xu, H. Impact of land use on the DOM composition in different seasons in a subtropical river flowing through a region undergoing rapid urbanization. J. Clean. Prod. 2019, 212, 1224–1231. [Google Scholar] [CrossRef]
- Wu, F.; Evans, D.; Dillon, P.; Schiff, S. Molecular size distribution characteristics of the metal–DOM complexes in stream waters by high-performance size-exclusion chromatography (HPSEC) and high-resolution inductively coupled plasma mass spectrometry (ICP-MS). J. Anal. At. Spectrom. 2004, 19, 979–983. [Google Scholar] [CrossRef]
- Zhang, J.L.; Wang, X.; Sun, W.N.; Li, Y.P.; Liu, Z.R.; Liu, Y.R.; Huang, G.H. Application of fiducial method for streamflow prediction under small sample cases in Xiangxihe watershed, China. J. Hydrol. 2020, 586, 124866. [Google Scholar] [CrossRef]
- Cao, F.; Tzortziou, M.; Hu, C.; Mannino, A.; Fichot, C.G.; Del Vecchio, R.; Najjar, R.G.; Novak, M. Remote sensing retrievals of colored dissolved organic matter and dissolved organic carbon dynamics in North American estuaries and their margins. Remote Sens. Environ. 2018, 205, 151–165. [Google Scholar] [CrossRef]
- Chaminda, G.G.T.; Nakajima, F.; Furumai, H.; Kasuga, I.; Kurisu, F. Comparison of metal (Zn and Cu) complexation characteristics of DOM in urban runoff, domestic wastewater and secondary effluent. Water Sci. Technol. 2010, 62, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, X.C.; Liu, H.; Zheng, Y.; Zhang, Y.; Xiong, J.; Pan, P.; Liu, Y. Speciation, Distribution and Risk Assessment of Metals in Sediments from a Water Body Replenished by Effluent from a Wastewater Treatment Plant. Bull. Environ. Contam. Toxicol. 2019, 102, 525–530. [Google Scholar] [CrossRef]
- Qin, C.; Liu, H.; Liu, L.; Smith, S.; Sedlak, D.L.; Gu, A.Z. Bioavailability and characterization of dissolved organic nitrogen and dissolved organic phosphorus in wastewater effluents. Sci. Total Environ. 2015, 511, 47–53. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Yang, C.; Wang, Q.; Jiang, D. Variations of DOM quantity and compositions along WWTPs-river-lake continuum: Implications for watershed for environmental management. Chemosphere 2019, 218, 468–476. [Google Scholar] [CrossRef]
- Baken, S.; Degryse, F.; Verheyen, L.; Merckx, R.; Smolders, E. Metal Complexation Properties of Freshwater Dissolved Organic Matter Are Explained by Its Aromaticity and by Anthropogenic Ligands. Environ. Sci. Technol. 2011, 45, 2584–2590. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Tong, T.; Li, B.; He, T.; Liu, Y.; Xie, S. Eutrophication influences methanotrophic activity, abundance and community structure in freshwater lakes. Sci. Total Environ. 2019, 662, 863–872. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, W.; Jiang, M.; He, J.; Zheng, Z. Black-odor water analysis and heavy metal distribution of Yitong River in Northeast China. Water Sci. Technol. 2017, 76, 2051–2064. [Google Scholar] [CrossRef]
- Guo, X.; Wang, L.; Ma, F.; You, Y.; Ju, C. Multi-level methods to quantify risk assessment, source apportionment and identifying key risk areas of soil toxic elements in Ashi River watershed, China. Sci. Total Environ. 2021, 800, 149385. [Google Scholar] [CrossRef]
- Li, N.; Tian, Y.; Zhang, J.; Zuo, W.; Zhan, W.; Zhang, J. Heavy metal contamination status and source apportionment in sediments of Songhua River Harbin region, Northeast China. Environ. Sci. Pollut. Res. 2017, 24, 3214–3225. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Jaffe, R. Photo- and bio-reactivity patterns of dissolved organic matter from biomass and soil leachates and surface waters in a subtropical wetland. Water Res. 2014, 61, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zha, T.; Guo, X.; Meng, G.; Zhou, J. Spatial distribution of metal pollution of soils of Chinese provincial capital cities. Sci. Total Environ. 2018, 643, 1502–1513. [Google Scholar] [CrossRef] [PubMed]
- Wedepohl, K.H. The Composition of the Continental Crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Muller, G. Index of Geoaccumulation in Sediments of the Rhine River. Geojournal 1969, 2, 109–118. [Google Scholar]
- Wang, N.; Wang, A.; Kong, L.; He, M. Calculation and application of Sb toxicity coefficient for potential ecological risk assessment. Sci. Total Environ. 2018, 610, 167–174. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Brady, J.P.; Ayoko, G.A.; Martens, W.N.; Goonetilleke, A. Development of a hybrid pollution index for heavy metals in marine and estuarine sediments. Environ. Monit. Assess. 2015, 187, 306. [Google Scholar] [CrossRef]
- Duodu, G.O.; Goonetilleke, A.; Ayoko, G.A. Comparison of pollution indices for the assessment of heavy metal in Brisbane River sediment. Environ. Pollut. 2016, 219, 1077–1091. [Google Scholar] [CrossRef]
- Zahra, A.; Hashmi, M.Z.; Malik, R.N.; Ahmed, Z. Enrichment and geo-accumulation of heavy metals and risk assessment of sediments of the Kurang Nallah-Feeding tributary of the Rawal Lake Reservoir, Pakistan. Sci. Total Environ. 2014, 470, 925–933. [Google Scholar] [CrossRef]
- Lambert, T.; Bouillon, S.; Darchambeau, F.; Massicotte, P.; Borges, A.V. Shift in the chemical composition of dissolved organic matter in the Congo River network. Biogeosciences 2016, 13, 5405–5420. [Google Scholar] [CrossRef]
- Lawaetz, A.J.; Stedmon, C.A. Fluorescence Intensity Calibration Using the Raman Scatter Peak of Water. Appl. Spectrosc. 2009, 63, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods 2013, 5, 6557–6566. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr.-Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Wenig, P.; Bro, R. OpenFluor—An online spectral library of auto-fluorescence by organic compounds in the environment. Anal. Methods 2014, 6, 658–661. [Google Scholar] [CrossRef]
- Cory, R.M.; Miller, M.P.; McKnight, D.M.; Guerard, J.J.; Miller, P.L. Effect of instrument-specific response on the analysis of fulvic acid fluorescence spectra. Limnol. Oceanogr.-Methods 2010, 8, 67–78. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Hansen, A.M.; Kraus, T.E.C.; Pellerin, B.A.; Fleck, J.A.; Downing, B.D.; Bergamaschi, B.A. Optical properties of dissolved organic matter (DOM): Effects of biological and photolytic degradation. Limnol. Oceanogr. 2016, 61, 1015–1032. [Google Scholar] [CrossRef]
- Zsolnay, A.; Baigar, E.; Jimenez, M.; Steinweg, B.; Saccomandi, F. Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere 1999, 38, 45–50. [Google Scholar] [CrossRef]
- Ohno, T. Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef]
- Sauvain, L.; Bueche, M.; Junier, T.; Masson, M.; Wunderlin, T.; Kohler-Milleret, R.; Diez, E.G.; Loizeau, J.-L.; Tercier-Waeber, M.-L.; Junier, P. Bacterial communities in trace metal contaminated lake sediments are dominated by endospore-forming bacteria. Aquat. Sci. 2014, 76, S33–S46. [Google Scholar] [CrossRef]
- Abu El-Magd, S.A.; Taha, T.H.; Pienaar, H.H.; Breil, P.; Amer, R.A.; Namour, P. Assessing heavy metal pollution hazard in sediments of Lake Mariout, Egypt. J. Afr. Earth Sci. 2021, 176, 104116. [Google Scholar] [CrossRef]
- Saleh, H.M.; Mahmoud, H.H.; Aglan, R.F.; Bayoumi, T.A. Biological Treatment of Wastewater Contaminated with Cu(II), Fe(II) and Mn(II) Using Ludwigia stolonifera Aquatic Plant. Environ. Eng. Manag. J. 2019, 18, 1327–1336. [Google Scholar] [CrossRef]
- Kafil, M.; Nasab, S.B.; Moazed, H.; Bhatnagar, A. Phytoremediation potential of vetiver grass irrigated with wastewater for treatment of metal contaminated soil. Int. J. Phytoremediat. 2019, 21, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Kanat, G.; Ikizoglu, B.; Erguven, G.O.; Akgun, B. Determination of Pollution and Heavy Metal Fractions in Golden Horn Sediment Sludge (Istanbul, Turkey). Pol. J. Environ. Stud. 2018, 27, 2605–2611. [Google Scholar] [CrossRef]
- Skorbilowicz, M.; Skorbilowicz, E.; Tkaczuk, J. Assessment of metals content in river bottom sediments near sewage treatment plants. Desalination Water Treat. 2020, 199, 314–322. [Google Scholar] [CrossRef]
- Tansel, B.; Rafiuddin, S. Heavy metal content in relation to particle size and organic content of surficial sediments in Miami River and transport potential. Int. J. Sediment Res. 2016, 31, 324–329. [Google Scholar] [CrossRef]
- Olujimi, O.O.; Fatoki, O.S.; Odendaal, J.; Daso, A.P.; Oputu, O. Preliminary Investigation into Occurrence and Removal of Arsenic, Cadmium, Mercury, and Zinc in Wastewater Treatment Plants in Cape Town and Stellenbosch. Pol. J. Environ. Stud. 2012, 21, 1755–1765. [Google Scholar]
- Ying, J.; Ming, F.; You-jun, W.; Hong, L.; Yi, M.; Xue-tong, W.; Wei-xin, L.; Xiao, T. Pollution characteristics and potential ecological risk of heavy metals in river sediments of Shanghai. China Environ. Sci. 2013, 33, 147–153. [Google Scholar]
- Budi, H.S.; Catalan Opulencia, M.J.; Afra, A.; Abdelbasset, W.K.; Abdullaev, D.; Majdi, A.; Taherian, M.; Ekrami, H.A.; Mohammadi, M.J. Source, toxicity and carcinogenic health risk assessment of heavy metals. Rev. Environ. Health 2022. [Google Scholar] [CrossRef]
- Liu, C.; Du, Y.; Yin, H.; Fan, C.; Chen, K.; Zhong, J.; Gu, X. Exchanges of nitrogen and phosphorus across the sediment-water interface influenced by the external suspended particulate matter and the residual matter after dredging. Environ. Pollut. 2019, 246, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Dainard, P.G.; Gueguen, C. Distribution of PARAFAC modeled CDOM components in the North Pacific Ocean, Bering, Chukchi and Beaufort Seas. Mar. Chem. 2013, 157, 216–223. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S. Tracing the production and degradation of autochthonous fractions of dissolved organic matter by fluorescence analysis. Limnol. Oceanogr. 2005, 50, 1415–1426. [Google Scholar] [CrossRef]
- Pitta, E.; Zeri, C. The impact of combining data sets of fluorescence excitation-emission matrices of dissolved organic matter from various aquatic sources on the information retrieved by PARAFAC modeling. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2021, 258, 119800. [Google Scholar] [CrossRef]
- Scully, N.M.; Maie, N.; Dailey, S.K.; Boyer, J.N.; Jones, R.D.; Jaffe, R. Early diagenesis of plant-derived dissolved organic matter along a wetland, mangrove, estuary ecotone. Limnol. Oceanogr. 2004, 49, 1667–1678. [Google Scholar] [CrossRef]
- Bowen, J.C.; Kaplan, L.A.; Cory, R.M. Photodegradation disproportionately impacts biodegradation of semi-labile DOM in streams. Limnol. Oceanogr. 2020, 65, 13–26. [Google Scholar] [CrossRef]
- Graeber, D.; Tenzin, Y.; Stutter, M.; Weigelhofer, G.; Shatwell, T.; von Tuempling, W.; Tittel, J.; Wachholz, A.; Borchardt, D. Bioavailable DOC: Reactive nutrient ratios control heterotrophic nutrient assimilation-An experimental proof of the macronutrient-access hypothesis. Biogeochemistry 2021, 155, 1–20. [Google Scholar] [CrossRef]
- Nebbioso, A.; Piccolo, A. Molecular characterization of dissolved organic matter (DOM): A critical review. Anal. Bioanal. Chem. 2013, 405, 109–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, E.; Yin, Y.; van Dijk, M.A.; Feng, L.; Shi, Z.; Liu, M.; Qin, B. Characteristics and sources of chromophoric dissolved organic matter in lakes of the Yungui Plateau, China, differing in trophic state and altitude. Limnol. Oceanogr. 2010, 55, 2645–2659. [Google Scholar] [CrossRef]
- Cory, R.M.; McNeill, K.; Cotner, J.P.; Amado, A.; Purcell, J.M.; Marshall, A.G. Singlet Oxygen in the Coupled Photochemical and Biochemical Oxidation of Dissolved Organic Matter. Environ. Sci. Technol. 2010, 44, 3683–3689. [Google Scholar] [CrossRef]
- Li, S.; Lu, L.; Wu, Y.; Zhao, Z.; Huang, C.; Huang, T.; Yang, H.; Ma, X.; Jiang, Q. Investigation on depth-dependent properties and benthic effluxes of dissolved organic matter (DOM) in pore water from plateau lake sediments. Ecol. Indic. 2021, 125, 107500. [Google Scholar] [CrossRef]
- Roth, V.-N.; Lange, M.; Simon, C.; Hertkorn, N.; Bucher, S.; Goodall, T.; Griffiths, R.I.; Mellado-Vazquez, P.G.; Mommer, L.; Oram, N.J.; et al. Persistence of dissolved organic matter explained by molecular changes during its passage through soil. Nat. Geosci. 2019, 12, 755–761. [Google Scholar] [CrossRef]
- He, J.; Wu, X.; Zhi, G.; Yang, Y.; Wu, L.; Zhang, Y.; Zheng, B.; Qadeer, A.; Zheng, J.; Deng, W.; et al. Fluorescence characteristics of DOM and its influence on water quality of rivers and lakes in the Dianchi Lake basin. Ecol. Indic. 2022, 142, 109088. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, Y.; Bai, S.; Zhou, H.; Chen, X.; Wei, Z. New insights into the variation of dissolved organic matter components in different latitudinal lakes of northeast China. Limnol. Oceanogr. 2020, 65, 471–481. [Google Scholar] [CrossRef]
- Xiao, X.; Xi, B.-D.; He, X.-S.; Zhang, H.; Li, Y.-H.; Pu, S.; Liu, S.-J.; Yu, M.-D.; Yang, C. Redox properties and dechlorination capacities of landfill-derived humic-like acids. Environ. Pollut. 2019, 253, 488–496. [Google Scholar] [CrossRef]
- Fellman, J.B.; D’Amore, D.V.; Hood, E.; Boone, R.D. Fluorescence characteristics and biodegradability of dissolved organic matter in forest and wetland soils from coastal temperate watersheds in southeast Alaska. Biogeochemistry 2008, 88, 169–184. [Google Scholar] [CrossRef]
- Choppala, G.; Kunhikrishnan, A.; Seshadri, B.; Park, J.H.; Bush, R.; Bolan, N. Comparative sorption of chromium species as influenced by pH, surface charge and organic matter content in contaminated soils. J. Geochem. Explor. 2018, 184, 255–260. [Google Scholar] [CrossRef]
- Wong, J.W.C.; Li, K.L.; Zhou, L.X.; Selvam, A. The sorption of Cd and Zn by different soils in the presence of dissolved organic matter from sludge. Geoderma 2007, 137, 310–317. [Google Scholar] [CrossRef]
- Chen, M.; Wang, D.; Ding, S.; Fan, X.; Jin, Z.; Wu, Y.; Wang, Y.; Zhang, C. Zinc pollution in zones dominated by algae and submerged macrophytes in Lake Taihu. Sci. Total Environ. 2019, 670, 361–368. [Google Scholar] [CrossRef]
- Zhu, M.; Kong, F.; Li, Y.; Li, M.; Zhang, J.; Xi, M. Effects of moisture and salinity on soil dissolved organic matter and ecological risk of coastal wetland. Environ. Res. 2020, 187, 109659. [Google Scholar] [CrossRef]
- Ren, H.; Ma, F.; Yao, X.; Shao, K.; Yang, L. Multi-spectroscopic investigation on the spatial distribution and copper binding ability of sediment dissolved organic matter in Nansi Lake, China. J. Hydrol. 2020, 591, 125289. [Google Scholar] [CrossRef]
- Aftabtalab, A.; Rinklebe, J.; Shaheen, S.M.; Niazi, N.K.; Moreno-Jimenez, E.; Schaller, J.; Knorr, K.-H. Review on the interactions of arsenic, iron (oxy)(hydr)oxides, and dissolved organic matter in soils, sediments, and groundwater in a ternary system. Chemosphere 2022, 286, 131790. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, H.; Lu, X.; Lin, H.; Christensen, G.K.; Pierce, E.M.; Gu, B. Contrasting Effects of Dissolved Organic Matter on Mercury Methylation by Geobacter sulfurreducens PCA and Desulfovibrio desulfuricans ND132. Environ. Sci. Technol. 2017, 51, 10468–10475. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Pan, J.; Devlin, A.T. Characteristics of Absorption Spectra of Chromophoric Dissolved Organic Matter in the Pearl River Estuary in Spring. Remote Sens. 2019, 11, 1533. [Google Scholar] [CrossRef]
- Altmann, J.; Massa, L.; Sperlich, A.; Gnirss, R.; Jekel, M. UV254 absorbance as real-time monitoring and control parameter for micropollutant removal in advanced wastewater treatment with powdered activated carbon. Water Res. 2016, 94, 240–245. [Google Scholar] [CrossRef] [PubMed]



| Mono-Metal Pollution Risk | Integrated Pollution Risk | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Igeo | Qualification | EF | Enrichment | mCd | PI | mPI | Qualification | RI/mRI | Risk |
| <0 | Unpolluted | 1 | No | <1.5 | <0.7 | <1 | Unpolluted | <150 | Low |
| 0–1 | Slightly | 1–3 | Minorly | 1.5–2 | 0.7–1 | 1–2 | Slightly | 150–300 | Moderate |
| 1–2 | Moderately | 3–5 | Moderately | 2–4 | 1–2 | 2–3 | Moderately | 300–600 | Considerable |
| 2–3 | Moderately-severely | 5–10 | Moderately-severely | 4–8 | 3–5 | Moderately-heavily | >600 | Very high | |
| 3–4 | Severely | 10–25 | Severely | 8–16 | 2–3 | 5–10 | Severely | ||
| 4–5 | Severely-extremely | >25 | Extremely | 16–32 | >3 | >10 | Heavily | ||
| >5 | Extremely | 32 | Extremely | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, T.; Li, Z.; Wang, L.; Li, T.; Qiu, P.; Wang, J.; Song, H. Potential Linkage between Heavy Metal Pollution Risk Assessment and Dissolved Organic Matter Spectra in the WWTPs-River Integrated Area-Case Study from Ashi River. Toxics 2023, 11, 904. https://doi.org/10.3390/toxics11110904
Dai T, Li Z, Wang L, Li T, Qiu P, Wang J, Song H. Potential Linkage between Heavy Metal Pollution Risk Assessment and Dissolved Organic Matter Spectra in the WWTPs-River Integrated Area-Case Study from Ashi River. Toxics. 2023; 11(11):904. https://doi.org/10.3390/toxics11110904
Chicago/Turabian StyleDai, Taoyan, Zhijun Li, Liquan Wang, Tienan Li, Pengpeng Qiu, Jun Wang, and Haotian Song. 2023. "Potential Linkage between Heavy Metal Pollution Risk Assessment and Dissolved Organic Matter Spectra in the WWTPs-River Integrated Area-Case Study from Ashi River" Toxics 11, no. 11: 904. https://doi.org/10.3390/toxics11110904
APA StyleDai, T., Li, Z., Wang, L., Li, T., Qiu, P., Wang, J., & Song, H. (2023). Potential Linkage between Heavy Metal Pollution Risk Assessment and Dissolved Organic Matter Spectra in the WWTPs-River Integrated Area-Case Study from Ashi River. Toxics, 11(11), 904. https://doi.org/10.3390/toxics11110904






