Carbetocin Inhibits Behavioral Sensitization to Ethanol in Male and Female Mice, Independent of Corticosterone Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Identification of the Estrous Cycle Phase
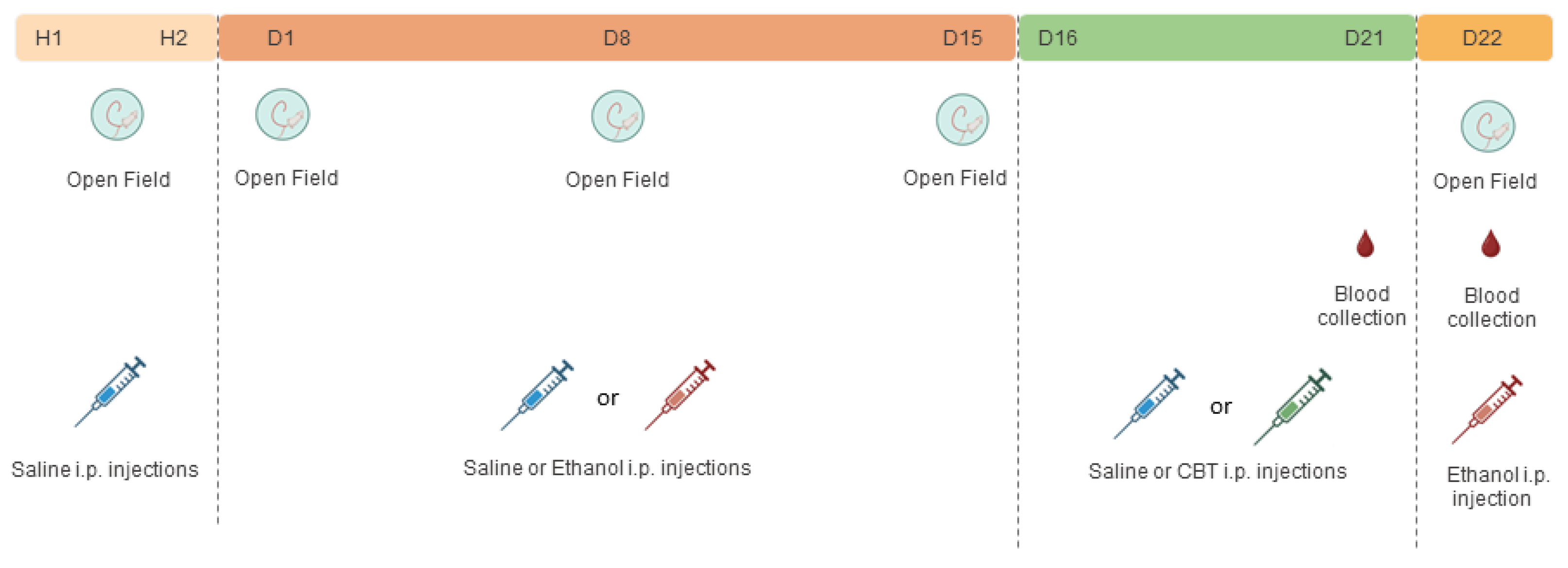
2.4. Experimental Design
2.4.1. Effects of Carbetocin on Ethanol-Induced Behavioral Sensitization
2.4.2. Effects of Carbetocin on Ethanol Consumption
2.5. Blood Collection for Biochemical Analysis
2.6. Statistical Analysis
3. Results
3.1. CBT Inhibited the Expression of Behavioral Sensitization in Male and Female Mice
3.2. CBT Influence on Behavioral Sensitization Is Not Mediated by Alterations in the Stress Hormone Corticosterone
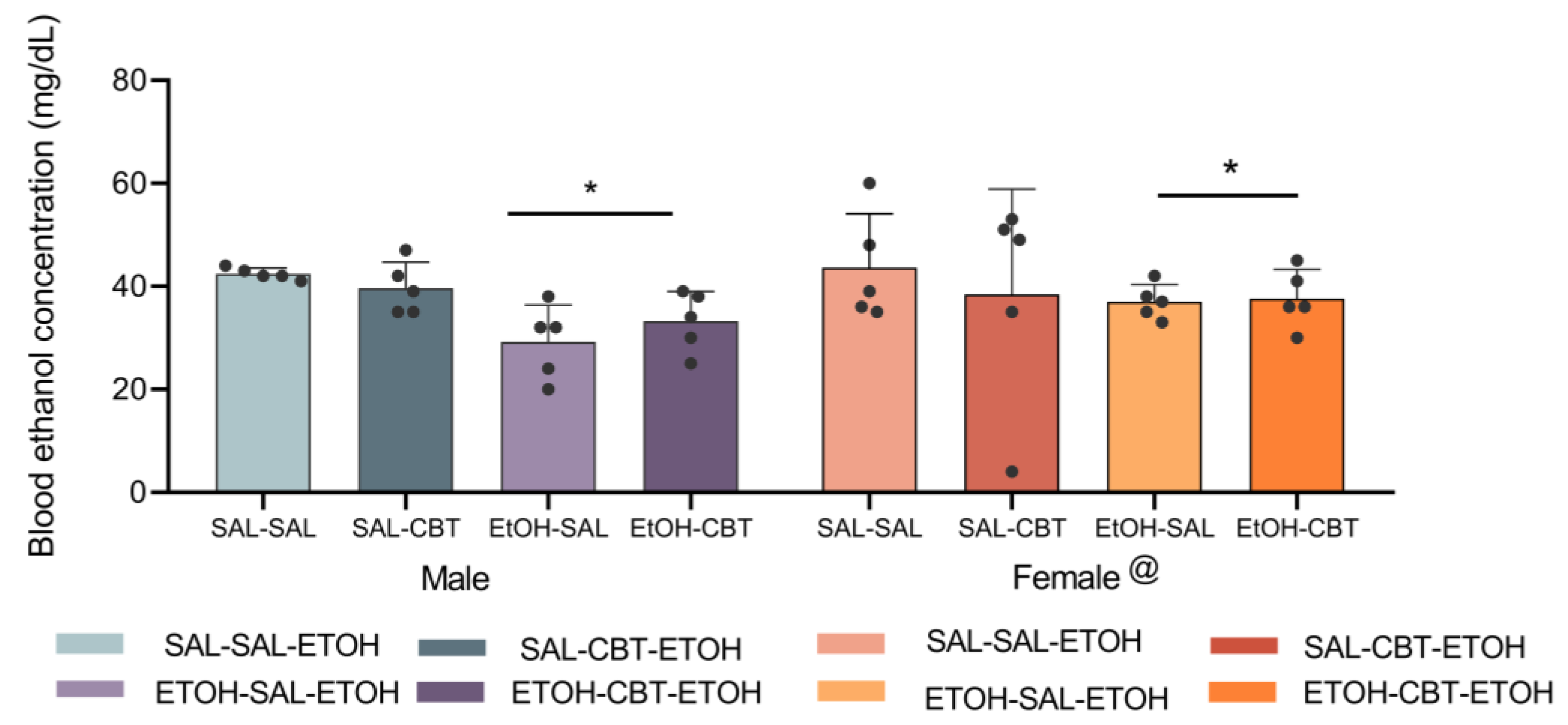
3.3. CBT Does Not Alter Ethanol Metabolism
3.4. CBT Decreases Ethanol Intake in Male Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- GBD 2016 Alcohol and Drug Use Collaborators. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry 2018, 5, 987–1012. [Google Scholar] [CrossRef] [PubMed]
- Osna, N.A.; Donohue, T.M., Jr.; Kharbanda, K.K. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol. Res. 2017, 38, 147–161. [Google Scholar] [PubMed]
- El-Mas, M.M.; Abdel-Rahman, A.A. Role of Alcohol Oxidative Metabolism in Its Cardiovascular and Autonomic Effects. Adv. Exp. Med. Biol. 2019, 1193, 1–33. [Google Scholar] [PubMed]
- Vijayraghavan, S.; Porcher, L.; Mieczkowski, P.A.; Saini, N. Acetaldehyde makes a distinct mutation signature in single-stranded DNA. Nucleic Acids Res. 2022, 50, 7451–7464. [Google Scholar] [CrossRef]
- World Health Organization. World Health Statistics 2023: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Goh, C.M.J.; Asharani, P.V.; Abdin, E.; Shahwan, S.; Zhang, Y.; Sambasivam, R.; Vaingankar, J.A.; Ma, S.; Chong, A.S.; Subramaniam, M. Gender Differences in Alcohol Use: A Nationwide Study in a Multiethnic Population. Int. J. Ment. Health Addict. 2022, 1–15. [Google Scholar] [CrossRef]
- Sudhinaraset, M.; Wigglesworth, C.; Takeuchi, D.T. Social and Cultural Contexts of Alcohol Use: Influences in a Social-Ecological Framework. Alcohol. Res. 2016, 38, 35–45. [Google Scholar]
- White, A.M. Gender Differences in the Epidemiology of Alcohol Use and Related Harms in the United States. Alcohol. Res. 2020, 40, 1. [Google Scholar] [CrossRef]
- Camarini, R. Mesenchymal stem cells as new perspective for the treatment of alcohol use disorder. Gene Ther. 2019, 27, 471–473. [Google Scholar] [CrossRef]
- Green, C.A. Gender and use of substance abuse treatment services. Alcohol. Res. Health 2006, 29, 55–62. [Google Scholar]
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef]
- Ryabinin, A.E.; Fulenwider, H.D. Alcohol and oxytocin: Scrutinizing the relationship. Neurosci. Biobehav. Rev. 2021, 127, 852–864. [Google Scholar] [CrossRef] [PubMed]
- King, C.E.; Griffin, W.C.; Luderman, L.N.; Kates, M.M.; McGinty, J.F.; Becker, H.C. Oxytocin reduces ethanol self-administration in mice. Alcohol. Clin. Exp. Res. 2017, 41, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Hansson, A.C.; Koopmann, A.; Uhrig, S.; Bühler, S.; Domi, E.; Kiessling, E.; Ciccocioppo, R.; Froemke, R.C.; Grinevich, V.; Kiefer, F.; et al. Oxytocin Reduces Alcohol Cue-Reactivity in Alcohol-Dependent Rats and Humans. Neuropsychopharmacology 2018, 43, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Hansson, A.C.; Spanagel, R. No changes in the oxytocin system in alcohol-dependent female rodents and humans: Towards a sex-specific psychopharmacology in alcoholism. Addict. Biol. 2021, 26, e12945. [Google Scholar] [CrossRef]
- Rodriguez, K.M.; Smith, B.L.; Caldwell, H.K. Voluntary alcohol consumption is increased in female, but not male, oxytocin receptor knockout mice. Brain Behav. 2020, 10, e01749. [Google Scholar] [CrossRef]
- Bardo, M.T.; Bevins, R.A. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology 2000, 153, 31–43. [Google Scholar] [CrossRef]
- Carrara-Nascimento, P.F.; Hoffmann, L.B.; Flório, J.C.; Planeta, C.S.; Camarini, R. Effects of Ethanol Exposure During Adolescence or Adulthood on Locomotor Sensitization and Dopamine Levels in the Reward System. Front. Behav. Neurosci. 2020, 14, 31. [Google Scholar] [CrossRef]
- Quigley, J.A.; Logsdon, M.K.; Turner, C.A.; Gonzalez, I.L.; Leonardo, N.B.; Becker, J.B. Sex differences in vulnerability to addiction. Neuropharmacology 2021, 187, 108491. [Google Scholar] [CrossRef]
- Masur, J.; Boerngen, R. The excitatory component of ethanol in mice: A chronic study. Pharmacol. Biochem. Behav. 1980, 13, 777–780. [Google Scholar] [CrossRef]
- Camarini, R.; Marianno, P.; Rae, M. Chapter three—Social Factors in Ethanol Sensitization. Int. Rev. Neurobiol. 2018, 140, 53–80. [Google Scholar]
- Morley-Fletcher, S.; Rea, M.; Maccari, S.; Laviola, G. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behavior and HPA axis reactivity in rats. Eur. J. Neurosci. 2003, 18, 3367–3374. [Google Scholar] [CrossRef] [PubMed]
- Santos-Rocha, J.B.; Rae, M.; Teixeira, A.M.A.; Teixeira, S.A.; Munhoz, C.D.; Muscará, M.N.; Marcourakis, T.; Szumlinski, K.K.; Camarini, R. Involvement of neuronal nitric oxide synthase in cross-sensitization between chronic unpredictable stress and ethanol in adolescent and adult mice. Alcohol 2018, 68, 71–79. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.R.B.; Rae, M.; Teixeira, S.A.; Muscará, M.N.; Szumlinski, K.K.; Camarini, R. The effect of MK-801 on stress-ethanol cross-sensitization is dissociable from its effects on nNOS activity. Alcohol 2023, 112, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.D.; Krömer, S.A.; Toschi, N.; Ebner, K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: Involvement of hypothalamic and limbic brain regions. Regul. Pept. 2000, 96, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Windle, R.J.; Shanks, N.; Lightman, S.L.; Ingram, C.D. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology 1997, 138, 2829–2834. [Google Scholar] [CrossRef]
- Georgiou, P.; Zanos, P.; Garcia-Carmona, J.A.; Hourani, S.; Kitchen, I.; Kieffer, B.L.; Laorden, M.L.; Bailey, A. The oxytocin analogue carbetocin prevents priming-induced reinstatement of morphine-seeking: Involvement of dopaminergic, noradrenergic and MOPr systems. Eur. Neuropsychopharmacol. 2015, 25, 2459–2464. [Google Scholar] [CrossRef]
- Zanos, P.; Georgiou, P.; Wright, S.R.; Hourani, S.M.; Kitchen, I.; Winsky-Sommerer, R.; Bailey, A. The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine-abstinent mice. Neuropsychopharmacology 2014, 39, 855–865. [Google Scholar] [CrossRef]
- Carrara-Nascimento, P.F.; Griffin III, W.C.; Pastrello, D.M.; Olive, M.F.; Camarini, R. Changes in extracellular levels of glutamate in the nucleus accumbens after ethanol-induced behavioral sensitization in adolescent and adult mice. Alcohol 2011, 45, 451–460. [Google Scholar] [CrossRef]
- Didne, V.; van Ingelgom, T.; Tirelli, E.; Quertemont, E. Long-term exposure to daily ethanol injections in DBA/2J and Swiss mice: Lessons for the interpretation of ethanol sensitization. PLoS ONE 2019, 14, e0214696. [Google Scholar] [CrossRef]
- Le, A.D.; Ko, J.; Chow, S.; Quan, B. Alcohol consumption by C57BL/6, BALB/c, and DBA/2 mice in a limited access paradigm. Pharmacol. Biochem. Behav. 1994, 47, 375–378. [Google Scholar] [CrossRef]
- Camarini, R.; Hodge, C.W. Ethanol preexposure increases ethanol self-administration in C57BL/6J and DBA/2J mice. Pharmacol. Biochem. Behav. 2004, 79, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Crabbe, J.C.; Phillips, T.J. Pharmacogenetic studies of alcohol self-administration and withdrawal. Psychopharmacology 2004, 174, 539–560. [Google Scholar] [CrossRef] [PubMed]
- Passoni, I.; Leonzino, M.; Gigliucci, V.; Chini, B.; Busnelli, M. Carbetocin is a Functional Selective Gq Agonist That Does Not Promote Oxytocin Receptor Recycling After Inducing β-Arrestin-Independent Internalisation. J. Neuroendocrinol. 2016, 28, 12363. [Google Scholar] [CrossRef] [PubMed]
- Caligioni, C.S. Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. 2009. Appendix 4: Appendix 4I. [Google Scholar] [CrossRef]
- Phillips, T.J.; Huson, M.; Gwiazdon, C.; Burkhart-Kasch, S.; Shen, E.H. Effects of acute and repeated ethanol exposures on the locomotor activity of BXD recombinant inbred mice. Alcohol. Clin. Exp. Res. 1995, 19, 269–278. [Google Scholar] [CrossRef]
- Legastelois, R.; Botia, B.; Naassila, M. Sensitization to the stimulant motor effects of ethanol is not dependent on tolerance to ataxic or sedative properties of ethanol in female mice. Drug Alcohol. Depend. 2015, 3, 4. [Google Scholar] [CrossRef]
- Rueda, A.V.L.; Teixeira, A.M.A.; Yonamine, M.; Camarini, R. Environmental enrichment blocks ethanol-induced locomotor sensitization and decreases BDNF levels in the prefrontal cortex in mice. Addict. Biol. 2012, 17, 736–745. [Google Scholar] [CrossRef]
- Rhodes, J.S.; Best, K.; Belknap, J.K.; Finn, D.A.; Crabbe, J.C. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav. 2005, 84, 53–63. [Google Scholar] [CrossRef]
- Marianno, P.; Abrahao, K.P.; Camarini, R. Environmental enrichment blunts ethanol consumption after restraint stress in C57BL/6 mice. PLoS ONE 2017, 12, e0170317. [Google Scholar] [CrossRef]
- Segal, D.S.; Mandell, A.J. Long-Term Administration of d-Amphetamine: Progressive Augmentation of Motor Activity and Stereotypy. Pharmacol. Biochem. Behav. 1974, 2, 249–255. [Google Scholar] [CrossRef]
- Segal, D.S.; Geyer, M.A.; Schuckit, M.A. Stimulant-induced psychosis: An evaluation of animal methods. Essays Neurochem. Neuropharmacol. 1981, 5, 95–129. [Google Scholar] [PubMed]
- Phillips, T.J.; Roberts, A.J.; Lessov, C.N. Behavioral sensitization to ethanol: Genetics and the effects of stress. Pharmacol. Biochem. Behav. 1997, 57, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Richtand, N. Behavioral Sensitization, Alternative Splicing, and D3 Dopamine Receptor-Mediated Inhibitory Function. Neuropsychopharmacollogy 2006, 31, 2368–2375. [Google Scholar] [CrossRef]
- Rae, M.B.; Zanos, P.; Georgiou, P.; Chivers, P.; Bailey, A.; Camarini, R. Environmental enrichment enhances conditioned place preference to ethanol via an oxytocinergic-dependent mechanism in male mice. Neuropharmacology 2018, 138, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, B.N.; Kalivas, P.W.; Bobadilla, A.C. Understanding Addiction Using Animal Models. Front. Behav. Neurosci. 2019, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.E.; Berridge, K.C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev. 1993, 18, 247–391. [Google Scholar] [CrossRef]
- Stephens, M.A.; Wand, G. Stress and the HPA axis: Role of glucocorticoids in alcohol dependence. Alcohol Res. 2012, 34, 468–483. [Google Scholar]
- McGinty, G.; Hyland, P.; Shevlin, M. Trauma Response and Psychosis: Investigating the Association between PTSD Symptomology and Psychotic Experiences. Eur. J. Psychotraumatology 2019, 10, 2–4. [Google Scholar]
- Mitchell, J.M.; Arcuni, P.A.; Weinstein, D.; Woolley, J.D. Intranasal Oxytocin Selectively Modulates Social Perception, Craving, and Approach Behavior in Subjects With Alcohol Use Disorder. J. Addict. Med. 2016, 10, 182–189. [Google Scholar] [CrossRef]
- Greaves, P. Chapter 12—Female Genital Tract. In Histopathology of Preclinical Toxicity Studies, 4th ed.; Academic Press: Boston, MA, USA, 2012; pp. 667–723. [Google Scholar]
- Wangikar, P.; Ahmed, T.; Vangala, S. Chapter 76: Toxicology Pathology of reproductive system. In Reproductive and Developmental Toxicology, 1st ed.; Gupta, R.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1003–1026. [Google Scholar]
- Seligowski, A.V.; Hurly, J.; Mellen, E.; Ressler, K.J.; Ramikie, T.S. Translational studies of estradiol and progesterone in fear and PTSD. Eur. J. Psychotraumatol. 2020, 11, 1723857. [Google Scholar] [CrossRef]
- Ycaza Herrera, A.; Mather, M. Actions and interactions of estradiol and glucocorticoids in cognition and the brain: Implications for aging women. Neurosci. Biobehav. Rev. 2015, 55, 36–52. [Google Scholar] [CrossRef]
- Seale, J.V.; Wood, S.A.; Atkinson, H.C.; Harbuz, M.S.; Lightman, S.L. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J. Neuroendocrinol. 2004, 16, 989–998. [Google Scholar] [CrossRef] [PubMed]
- King, C.E.; Griffin, W.C.; Lopez, M.F.; Becker, H.C. Activation of hypothalamic oxytocin neurons reduces binge-like alcohol drinking through signaling at central oxytocin receptors. Neuropsychopharmacology 2021, 46, 1950–1957. [Google Scholar] [CrossRef] [PubMed]
- Rae, M.; Lemos Duarte, M.; Gomes, I.; Camarini, R.; Devi, L.A. Oxytocin and vasopressin: Signalling, behavioural modulation and potential therapeutic effects. Br. J. Pharmacol. 2022, 179, 1544–1564. [Google Scholar] [CrossRef]
- Qi, J.; Yang, J.Y.; Song, M.; Li, Y.; Wang, F.; Wu, C.F. Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 376, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.L.; Sarnyai, Z.; Babarczi, E.; Szabo, G.; Telegdy, G. The role of oxytocin-dopamine interactions in cocaine-induced locomotor hyperactivity. Neuropharmacology 1990, 29, 365–366. [Google Scholar] [CrossRef]
- Abrahao, K.P.; Ariwodola, O.J.; Butler, T.R.; Rau, A.R.; Skelly, M.J.; Carter, E.; Alexander, N.P.; McCool, B.A.; Souza-Formigoni, M.L.; Weiner, J.L. Locomotor sensitization to ethanol impairs NMDA receptor-dependent synaptic plasticity in the nucleus accumbens and increases ethanol self-administration. J. Neurosci. 2013, 33, 4834–4842. [Google Scholar] [CrossRef]
- Ribeiro, A.F.; Pigatto, G.; Goeldner, F.O.; Lopes, J.F.; de Lacerda, R.B. Lack of relation between drug-seeking behavior in an addiction model and the expression of behavioral sensitization in response to ethanol challenge in mice. J. Neural Transm. 2008, 115, 43–54. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, B.Y.; Santos, L.G.; Marianno, P.; Rae, M.; de Almeida, M.G.; de Brito, M.C.; Eichler, R.; Camarini, R. Carbetocin Inhibits Behavioral Sensitization to Ethanol in Male and Female Mice, Independent of Corticosterone Levels. Toxics 2023, 11, 893. https://doi.org/10.3390/toxics11110893
Costa BY, Santos LG, Marianno P, Rae M, de Almeida MG, de Brito MC, Eichler R, Camarini R. Carbetocin Inhibits Behavioral Sensitization to Ethanol in Male and Female Mice, Independent of Corticosterone Levels. Toxics. 2023; 11(11):893. https://doi.org/10.3390/toxics11110893
Chicago/Turabian StyleCosta, Beatriz Yamada, Luana Gasparini Santos, Priscila Marianno, Mariana Rae, Marina Gomes de Almeida, Malcon Carneiro de Brito, Rosângela Eichler, and Rosana Camarini. 2023. "Carbetocin Inhibits Behavioral Sensitization to Ethanol in Male and Female Mice, Independent of Corticosterone Levels" Toxics 11, no. 11: 893. https://doi.org/10.3390/toxics11110893
APA StyleCosta, B. Y., Santos, L. G., Marianno, P., Rae, M., de Almeida, M. G., de Brito, M. C., Eichler, R., & Camarini, R. (2023). Carbetocin Inhibits Behavioral Sensitization to Ethanol in Male and Female Mice, Independent of Corticosterone Levels. Toxics, 11(11), 893. https://doi.org/10.3390/toxics11110893





