Can Maternal Exposure to Air Pollution Affect Post-Natal Liver Development?
Abstract
1. Introduction
2. Materials and Methods
2.1. Particles and Animal Exposure
2.2. Protein Assay
2.3. Hepatic Metabolism
2.4. Oxidative Stress and Inflammation
2.5. DNA Damage
2.6. RNA Isolation and qPCR
2.7. Statistical Analysis
3. Results
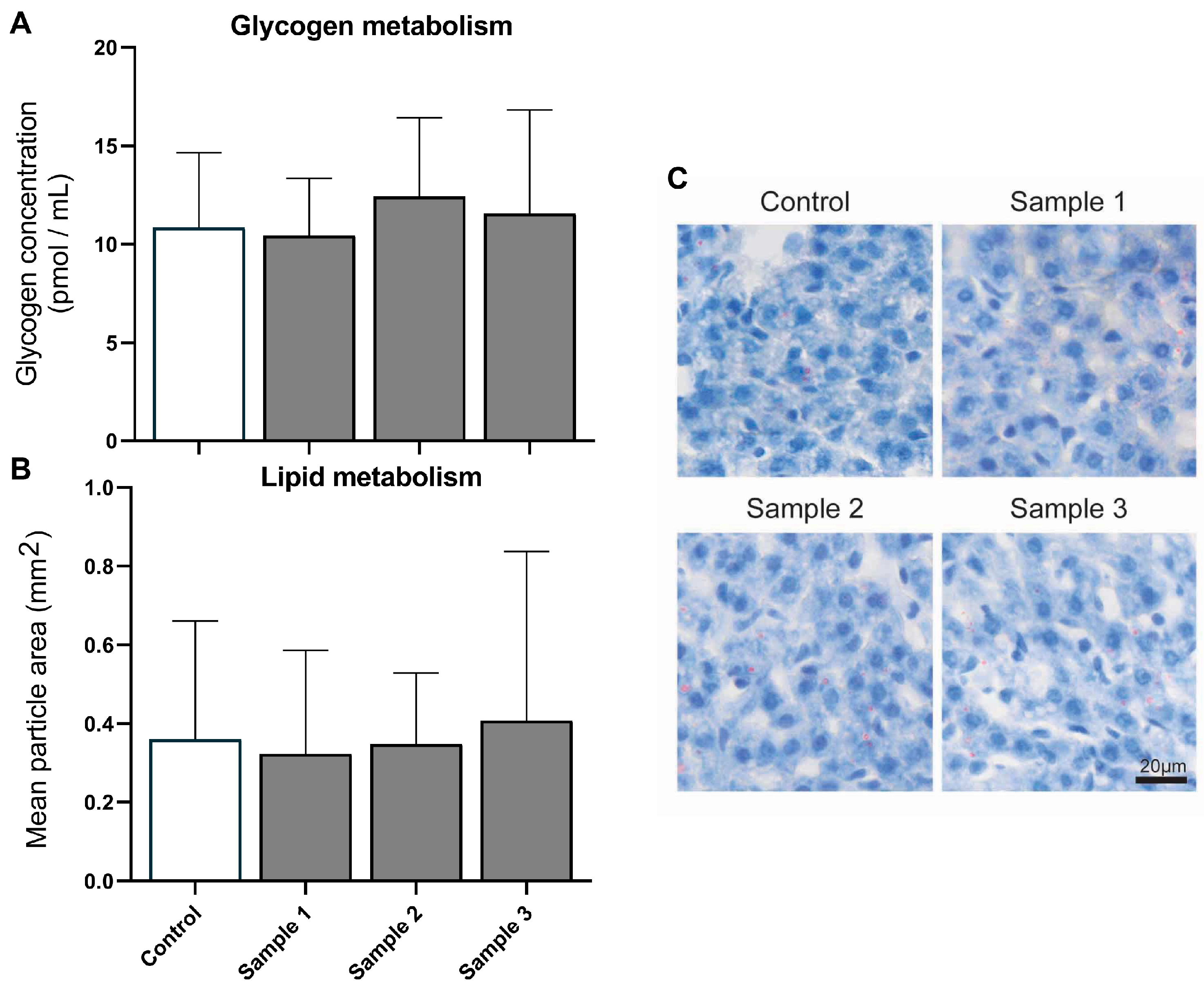
3.1. Liver Metabolism
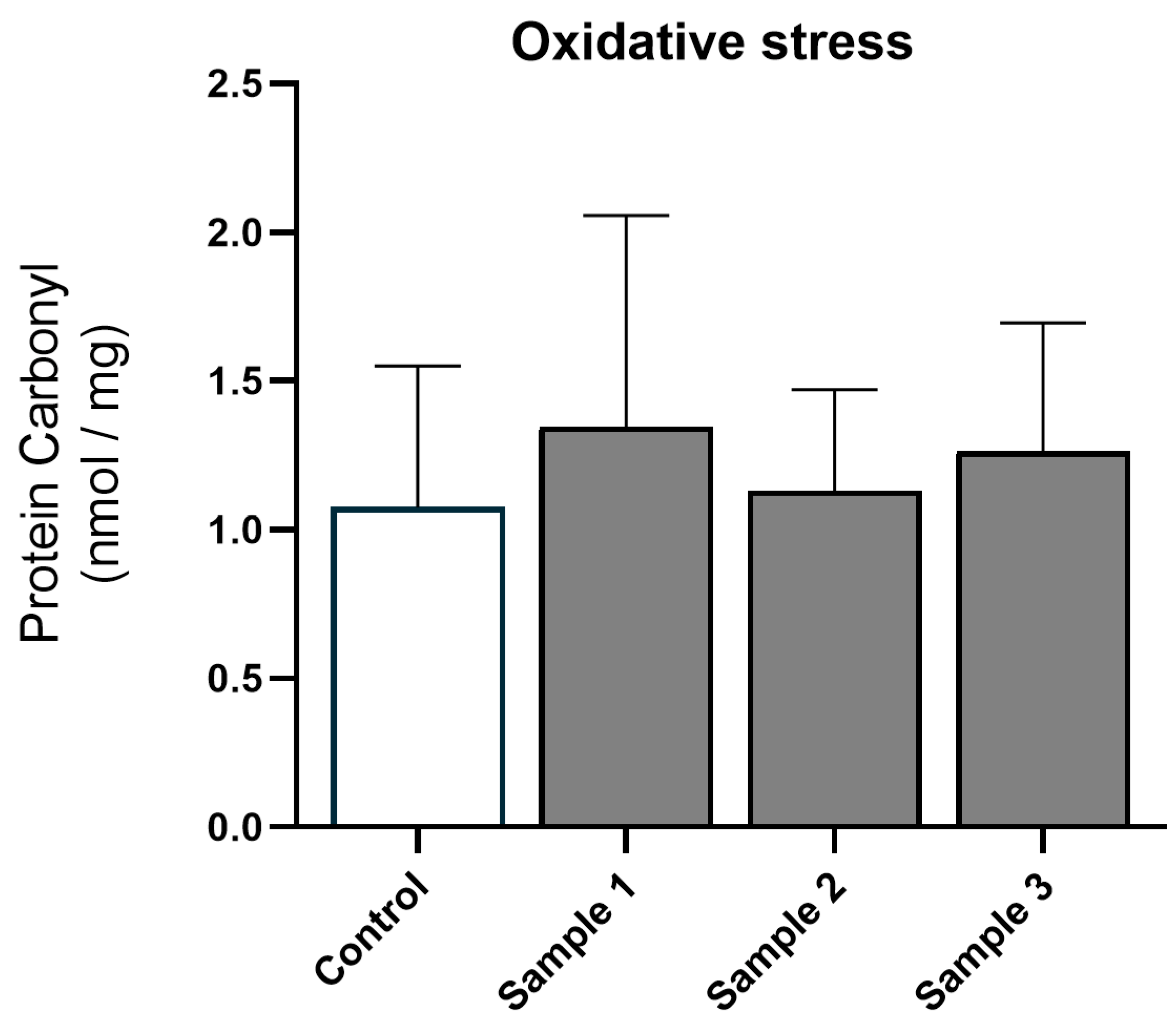
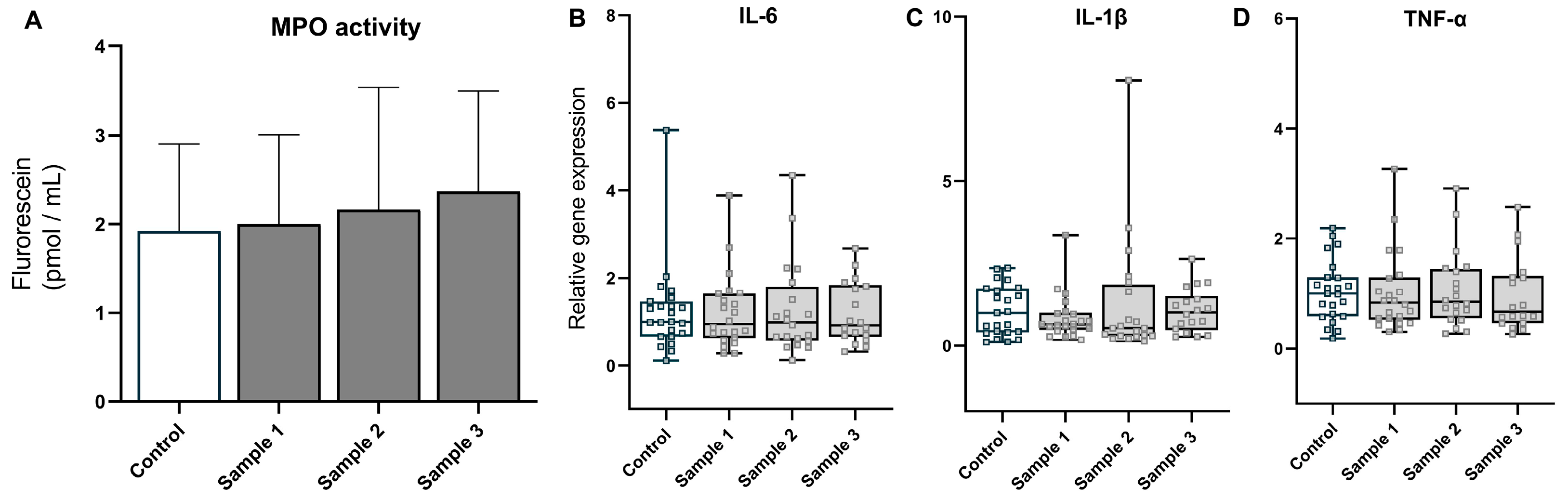
3.2. Oxidative Stress and Inflammation
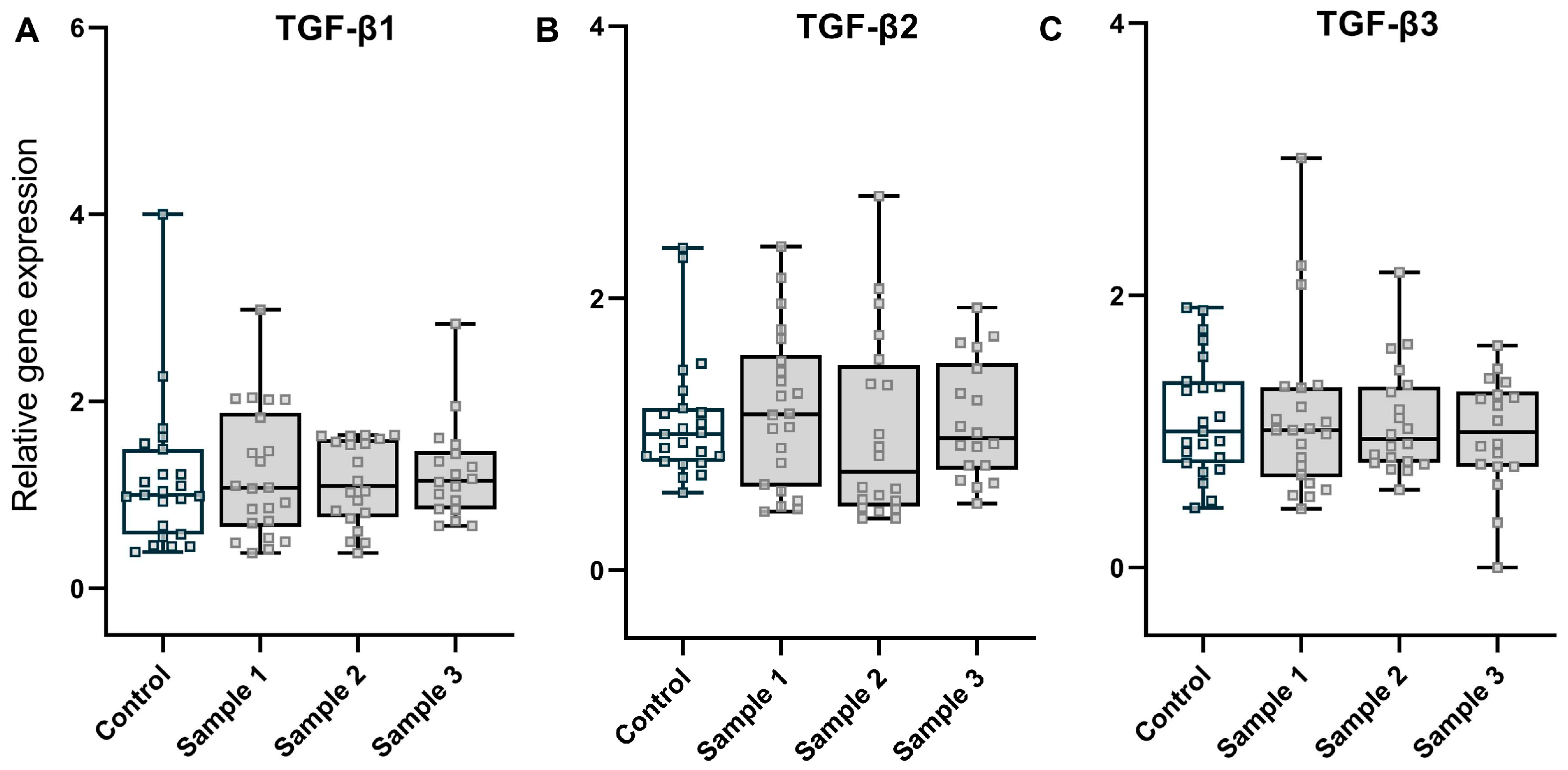
3.3. Fibrosis
3.4. Genotoxicity
3.5. Sex-Stratified Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and health impacts of air pollution: A review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Shahi, A.M.; Omraninava, A.; Goli, M.; Soheilarezoomand, H.R.; Mirzaei, N. The effects of air pollution on cardiovascular and respiratory causes of emergency admission. Emergency 2014, 2, 107–114. [Google Scholar] [PubMed]
- Backes, C.H.; Nelin, T.; Gorr, M.W.; Wold, L.E. Early life exposure to air pollution: How bad is it? Toxicol. Lett. 2013, 216, 47–53. [Google Scholar] [CrossRef]
- Chen, L.; Bennett, E.; Wheeler, A.J.; Lyons, A.B.; Woods, G.M.; Johnston, F.; Zosky, G.R. Maternal exposure to particulate matter alters early post-natal lung function and immune cell development. Environ. Res. 2018, 164, 625–635. [Google Scholar] [CrossRef]
- Song, Y.; Southam, K.; Bennett, E.; Johnston, F.; Foa, L.; Wheeler, A.J.; Zosky, G.R. Adverse effects of prenatal exposure to residential dust on post-natal brain development. Environ. Res. 2021, 198, 110489. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.; Hougaard, K.S.; Boisen, A.M.; Jacobsen, N.R.; Jensen, K.A.; Moller, P.; Brunborg, G.; Gutzkow, K.B.; Andersen, O.; Loft, S.; et al. Pulmonary exposure to carbon black by inhalation or instillation in pregnant mice: Effects on liver DNA strand breaks in dams and offspring. Nanotoxicology 2012, 6, 486–500. [Google Scholar] [CrossRef]
- Guo, B.; Guo, Y.; Nima, Q.; Feng, Y.; Wang, Z.; Lu, R.; Baimayangji; Ma, Y.; Zhou, J.; Xu, H.; et al. Exposure to air pollution is associated with an increased risk of metabolic dysfunction-associated fatty liver disease. J. Hepatol. 2022, 76, 518–525. [Google Scholar] [CrossRef]
- So, R.; Chen, J.; Mehta, A.J.; Liu, S.; Strak, M.; Wolf, K.; Hvidtfeldt, U.A.; Rodopoulou, S.; Stafoggia, M.; Klompmaker, J.O.; et al. Long-term exposure to air pollution and liver cancer incidence in six European cohorts. Int. J. Cancer 2021, 149, 1887–1897. [Google Scholar] [CrossRef]
- VoPham, T.; Bertrand, K.A.; Tamimi, R.M.; Laden, F.; Hart, J.E. Ambient PM2.5 air pollution exposure and hepatocellular carcinoma incidence in the United States. Cancer Causes Control 2018, 29, 563–572. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, S.; Lim, C.W.; Lee, K.; Kim, B. The role of air pollutants in initiating liver disease. Toxicol. Res. 2014, 30, 65–70. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, X.; Zhang, X.; Wang, A.; Zhang, C.; Huttemann, M.; Grossman, L.I.; Chen, L.C.; Rajagopalan, S.; Sun, Q.; et al. Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J. Hepatol. 2013, 58, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhang, X.; Wang, J.; Dandekar, A.; Kim, H.; Qiu, Y.; Xu, X.; Cui, Y.; Wang, A.; Chen, L.C.; et al. Exposure to fine airborne particulate matters induces hepatic fibrosis in murine models. J. Hepatol. 2015, 63, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, P.H.; Loft, S.; Jacobsen, N.R.; Jensen, K.A.; Autrup, H.; Ravanat, J.L.; Wallin, H.; Moller, P. Oxidative stress, inflammation, and DNA damage in rats after intratracheal instillation or oral exposure to ambient air and wood smoke particulate matter. Toxicol. Sci. 2010, 118, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Matz, C.J.; Stieb, D.M.; Davis, K.; Egyed, M.; Rose, A.; Chou, B.; Brion, O. Effects of age, season, gender and urban-rural status on time-activity: Canadian Human Activity Pattern Survey 2 (CHAPS 2). Int. J. Environ. Res. Public Health 2014, 11, 2108–2124. [Google Scholar] [CrossRef]
- Johnson, M.; Shin, H.H.; Roberts, E.; Sun, L.; Fisher, M.; Hystad, P.; Van Donkelaar, A.; Martin, R.V.; Fraser, W.D.; Lavigne, E.; et al. Critical time windows for air pollution exposure and birth weight in a multicity Canadian pregnancy cohort. Epidemiology 2022, 33, 7–16. [Google Scholar] [CrossRef]
- Lee, A.G.; Le Grand, B.; Hsu, H.L.; Chiu, Y.M.; Brennan, K.J.; Bose, S.; Rosa, M.J.; Brunst, K.J.; Kloog, I.; Wilson, A.; et al. Prenatal fine particulate exposure associated with reduced childhood lung function and nasal epithelia GSTP1 hypermethylation: Sex-specific effects. Respir. Res. 2018, 19, 76. [Google Scholar] [CrossRef]
- Rosa, M.J.; Hair, G.M.; Just, A.C.; Kloog, I.; Svensson, K.; Pizano-Zarate, M.L.; Pantic, I.; Schnaas, L.; Tamayo-Ortiz, M.; Baccarelli, A.A.; et al. Identifying critical windows of prenatal particulate matter (PM(2.5)) exposure and early childhood blood pressure. Environ. Res. 2020, 182, 109073. [Google Scholar] [CrossRef]
- Sunyer, J.; Dadvand, P. Pre-natal brain development as a target for urban air pollution. Basic Clin. Pharmacol. Toxicol. 2019, 125 (Suppl. S3), 81–88. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Kurisu, S.; Miya, T.; Terato, H.; Masaoka, A.; Ohyama, Y.; Kubo, K.; Ide, H. Quantitation of DNA damage by an aldehyde reactive probe (ARP). Nucleic Acids Res. Suppl. 2001, 1, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yue, P.; Deiuliis, J.A.; Lumeng, C.N.; Kampfrath, T.; Mikolaj, M.B.; Cai, Y.; Ostrowski, M.C.; Lu, B.; Parthasarathy, S.; et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 2009, 119, 538–546. [Google Scholar] [CrossRef]
- Xu, X.; Yavar, Z.; Verdin, M.; Ying, Z.; Mihai, G.; Kampfrath, T.; Wang, A.; Zhong, M.; Lippmann, M.; Chen, L.C.; et al. Effect of early particulate air pollution exposure on obesity in mice: Role of p47phox. Arter. Thromb. Vasc. Biol. 2010, 30, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.H.; Fiel, M.I.; Sun, Q.; Guo, J.; Gordon, R.E.; Chen, L.C.; Friedman, S.L.; Odin, J.A.; Allina, J. Kupffer cell activation by ambient air particulate matter exposure may exacerbate non-alcoholic fatty liver disease. J. Immunotoxicol. 2009, 6, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.X.; Ge, C.X.; Qin, Y.T.; Gu, T.T.; Lou, D.S.; Li, Q.; Hu, L.F.; Feng, J.; Huang, P.; Tan, J. Prolonged PM2.5 exposure elevates risk of oxidative stress-driven nonalcoholic fatty liver disease by triggering increase of dyslipidemia. Free Radic. Biol. Med. 2019, 130, 542–556. [Google Scholar] [CrossRef]
- Barton, H.A.; Cogliano, V.J.; Flowers, L.; Valcovic, L.; Setzer, R.W.; Woodruff, T.J. Assessing susceptibility from early-life exposure to carcinogens. Environ. Health Perspect. 2005, 113, 1125–1133. [Google Scholar] [CrossRef]
- Moller, P.; Danielsen, P.H.; Karottki, D.G.; Jantzen, K.; Roursgaard, M.; Klingberg, H.; Jensen, D.M.; Christophersen, D.V.; Hemmingsen, J.G.; Cao, Y.; et al. Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat. Res. Rev. Mutat. Res. 2014, 762, 133–166. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Chen, L.; Bennett, E.; Wheeler, A.J.; Southam, K.; Yen, S.; Johnston, F.; Zosky, G.R. Can Maternal Exposure to Air Pollution Affect Post-Natal Liver Development? Toxics 2023, 11, 61. https://doi.org/10.3390/toxics11010061
Song Y, Chen L, Bennett E, Wheeler AJ, Southam K, Yen S, Johnston F, Zosky GR. Can Maternal Exposure to Air Pollution Affect Post-Natal Liver Development? Toxics. 2023; 11(1):61. https://doi.org/10.3390/toxics11010061
Chicago/Turabian StyleSong, Yong, Ling Chen, Ellen Bennett, Amanda J. Wheeler, Katherine Southam, Seiha Yen, Fay Johnston, and Graeme R. Zosky. 2023. "Can Maternal Exposure to Air Pollution Affect Post-Natal Liver Development?" Toxics 11, no. 1: 61. https://doi.org/10.3390/toxics11010061
APA StyleSong, Y., Chen, L., Bennett, E., Wheeler, A. J., Southam, K., Yen, S., Johnston, F., & Zosky, G. R. (2023). Can Maternal Exposure to Air Pollution Affect Post-Natal Liver Development? Toxics, 11(1), 61. https://doi.org/10.3390/toxics11010061





