Vascular Response of Tetrabromobisphenol a in Rat Aorta: Calcium Channels Inhibition and Potassium Channels Activation
Abstract
:1. Introduction
2. Methods
2.1. Drugs and Chemicals
2.2. Ex Vivo Studies
Contractility Experiments in Isolated Rat Thoracic Aorta Rings
- -
- Nif (0.001 and 1 μM), an inhibitor of voltage-gated calcium channels (VGCC);
- -
- TEA (1000 μM), an inhibitor of conductance Ca2+-activated K+ (BKCa) channel;
- -
- 4-AP (1000 μM), an inhibitor of voltage-gated potassium channels (Kv);
- -
- Gly (10 μM), an inhibitor of ATP-sensitive potassium (KATP).
2.3. In Vitro Studies
2.3.1. Culture of A7r5 Cells
2.3.2. Cell Viability
2.3.3. Electrophysiology Experiments
2.3.4. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.4. Statistical Analysis
3. Results
3.1. Ex Vivo Studies
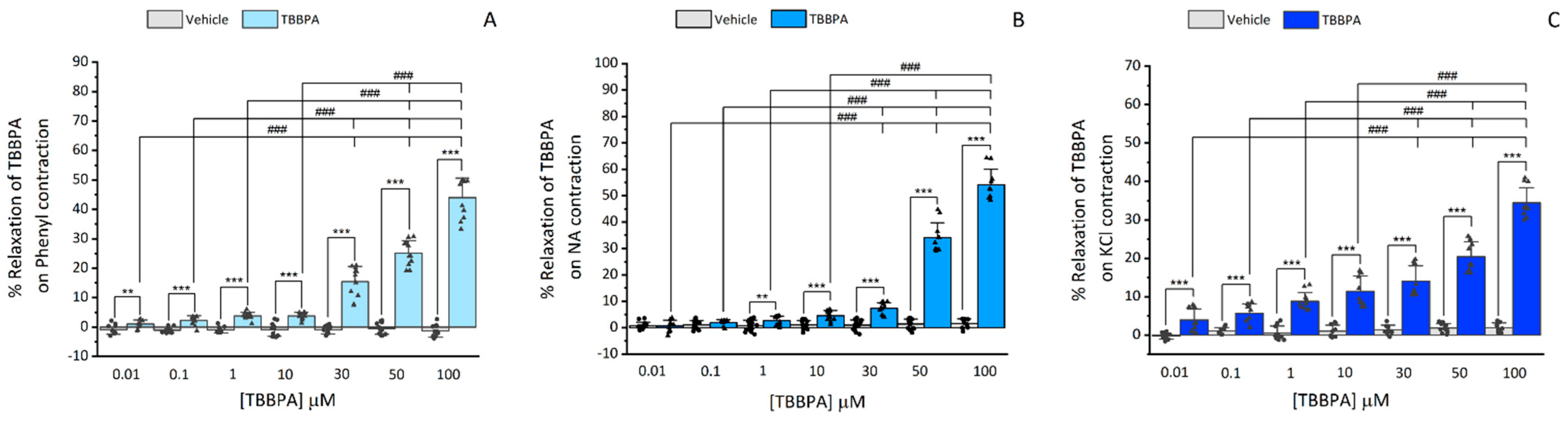
3.1.1. Effects of TBBPA on Isolated Rat Aorta
3.1.2. Influence of L-type Ca2+ Channels on TBBPA-Induced Vasorelaxation on Isolated Rat Aorta
3.1.3. Influence of K+ Channels on TBBPA-Induced Vasorelaxation on Isolated Rat Aorta
3.2. In Vitro Studies
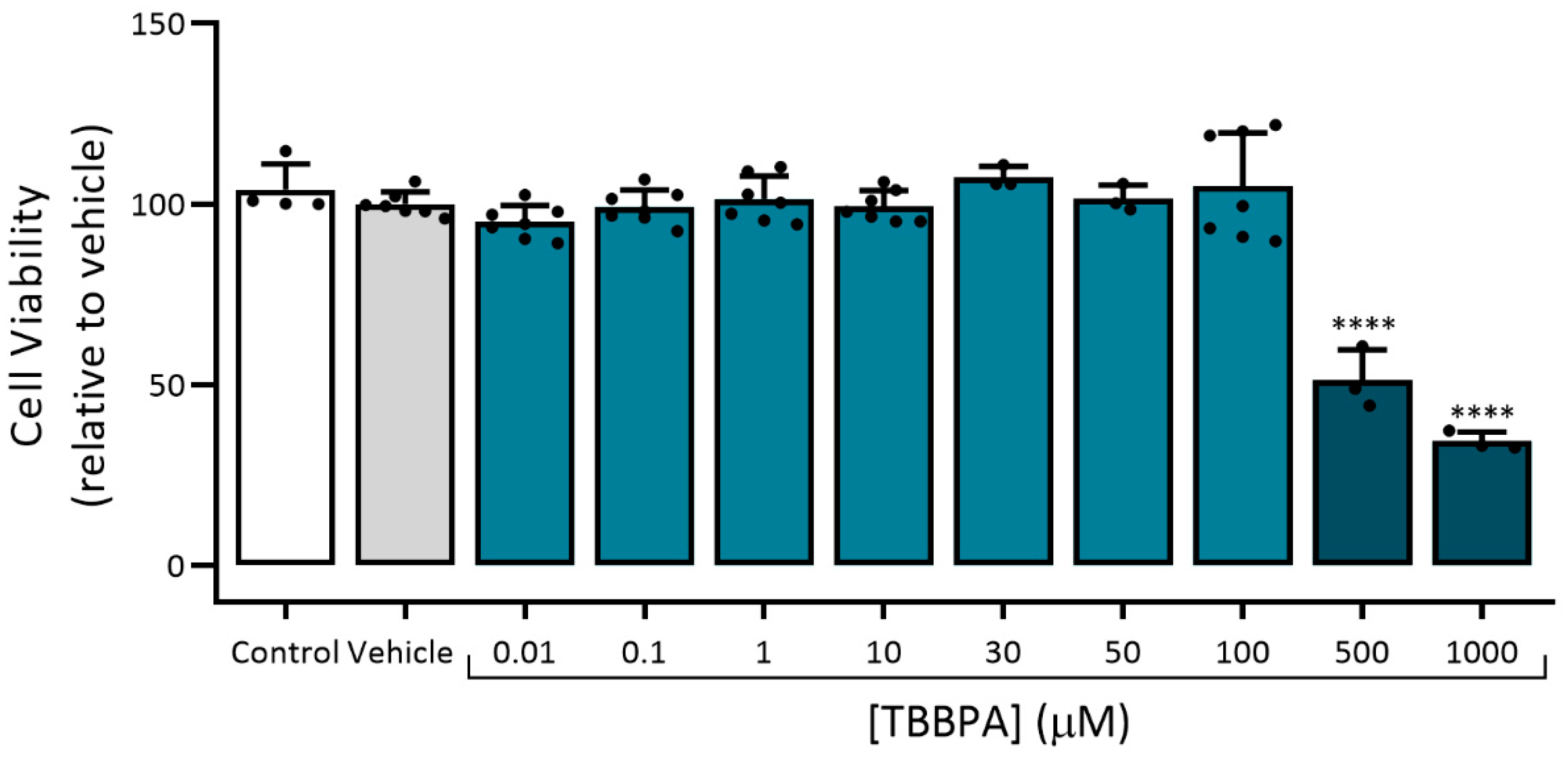
3.2.1. Assessment of Viability (MTT Assay)
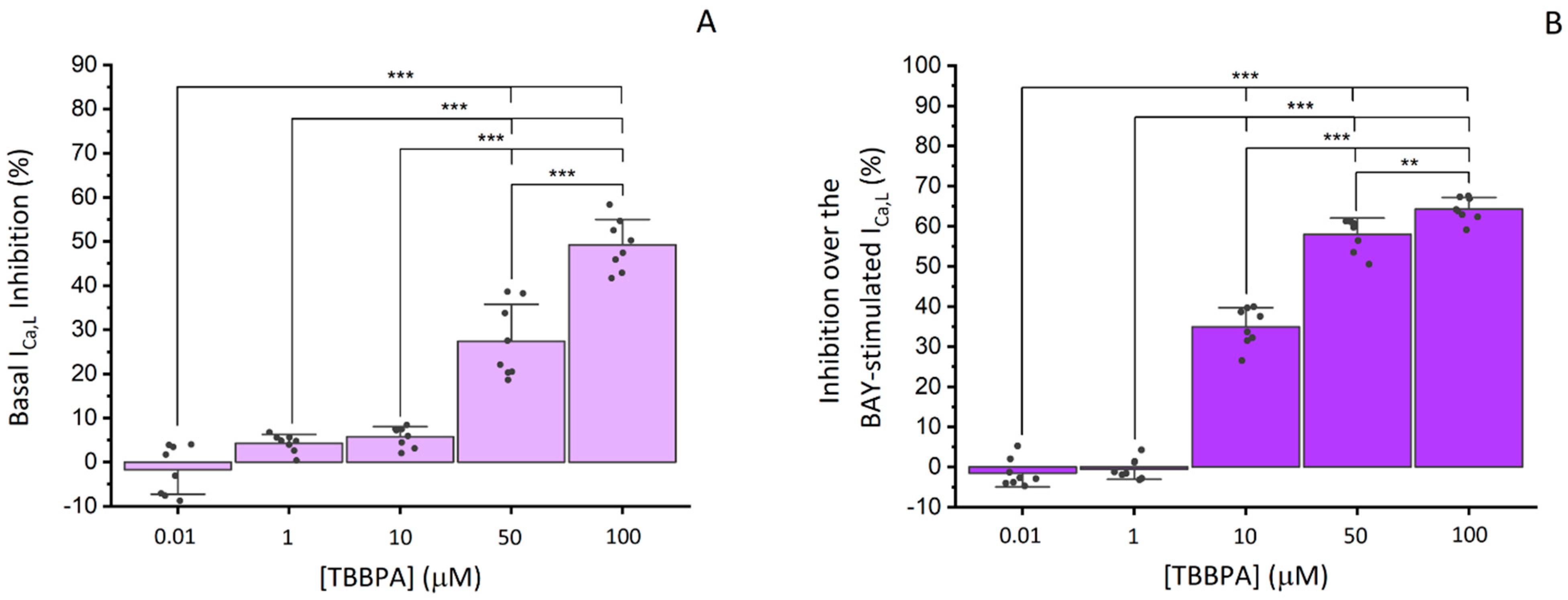
3.2.2. Effects of TBBPA on ICa,L in A7r5 Cells
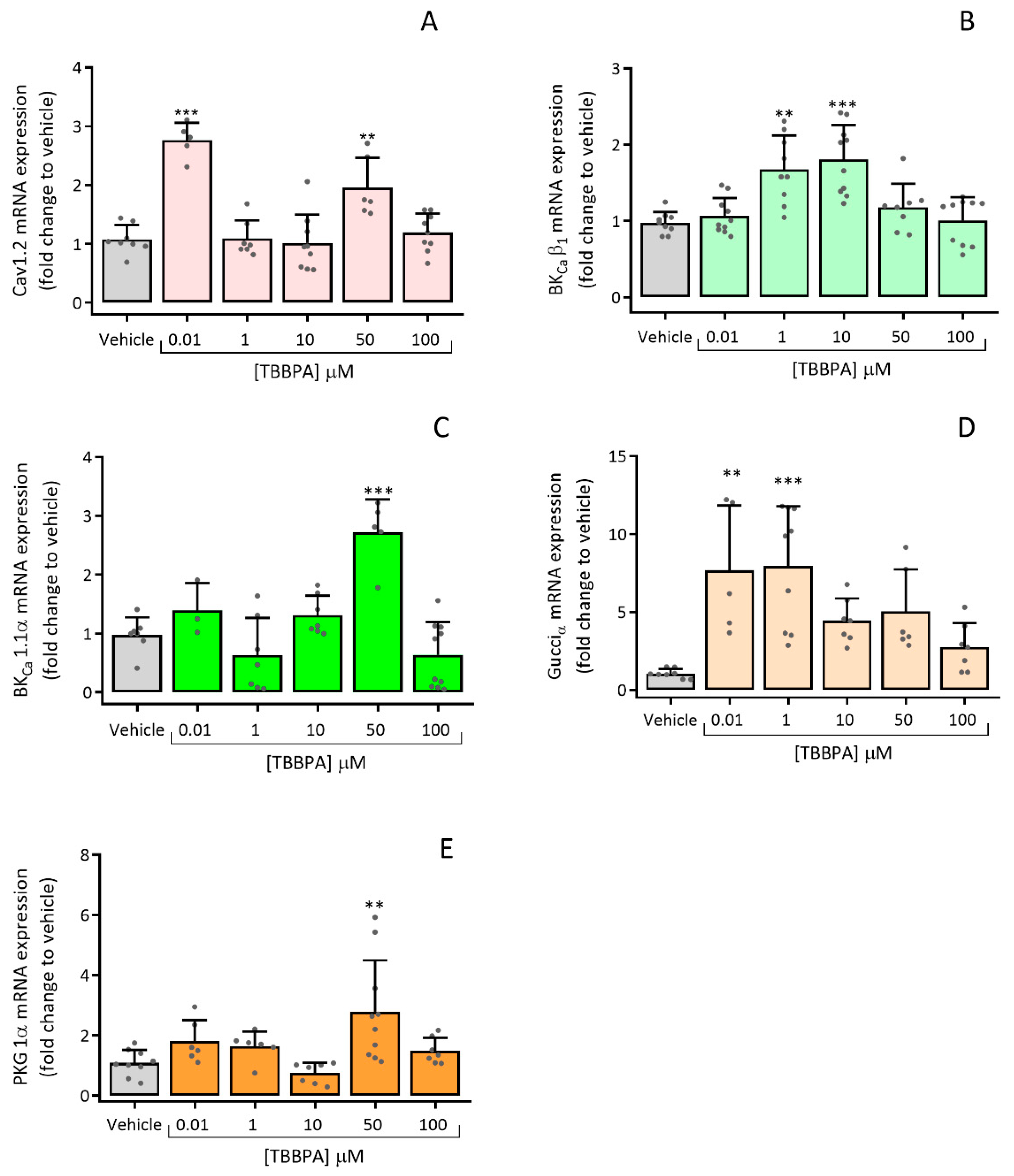
3.2.3. Effects of TBBPA on the Expression of Cav1.2, BKCa β1, BKCa 1.1α, Gucciα and PRKG 1α
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feiteiro, J.; Mariana, M.; Cairrao, E. Health toxicity effects of brominated flame retardants: From environmental to human exposure. Environ. Pollut. 2021, 285, 117475. [Google Scholar] [CrossRef] [PubMed]
- Altmann, L.; Welge, P.; Mensing, T.; Lilienthal, H.; Voss, B.; Wilhelm, M. Chronic exposure to trichloroethylene affects neuronal plasticity in rat hippocampal slices. Environ. Toxicol. Pharmacol. 2002, 12, 157–167. [Google Scholar] [CrossRef]
- Lilienthal, H.; Verwer, C.M.; van der Ven, L.T.; Piersma, A.H.; Vos, J.G. Exposure to tetrabromobisphenol A (TBBPA) in Wistar rats: Neurobehavioral effects in offspring from a one-generation reproduction study. Toxicology 2008, 246, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, Y.; Fujimoto, H.; Woo, G.H.; Inoue, K.; Takahashi, M.; Mitsumori, K.; Hirose, M.; Nishikawa, A.; Shibutani, M. Developmental toxicity of brominated flame retardants, tetrabromobisphenol A and 1,2,5,6,9,10-hexabromocyclododecane, in rat offspring after maternal exposure from mid-gestation through lactation. Reprod. Toxicol. 2009, 28, 456–467. [Google Scholar] [CrossRef]
- Kitamura, S.; Kato, T.; Iida, M.; Jinno, N.; Suzuki, T.; Ohta, S.; Fujimoto, N.; Hanada, H.; Kashiwagi, K.; Kashiwagi, A. Anti-thyroid hormonal activity of tetrabromobisphenol A, a flame retardant, and related compounds: Affinity to the mammalian thyroid hormone receptor, and effect on tadpole metamorphosis. Life Sci. 2005, 76, 1589–1601. [Google Scholar] [CrossRef]
- Sun, H.; Shen, O.X.; Wang, X.R.; Zhou, L.; Zhen, S.Q.; Chen, X.D. Anti-thyroid hormone activity of bisphenol A, tetrabromobisphenol A and tetrachlorobisphenol A in an improved reporter gene assay. Toxicol. In Vitro 2009, 23, 950–954. [Google Scholar] [CrossRef]
- Kitamura, S.; Jinno, N.; Ohta, S.; Kuroki, H.; Fujimoto, N. Thyroid hormonal activity of the flame retardants tetrabromobisphenol A and tetrachlorobisphenol A. Biochem. Biophys. Res. Commun. 2002, 293, 554–559. [Google Scholar] [CrossRef]
- Fukuda, N.; Ito, Y.; Yamaguchi, M.; Mitumori, K.; Koizumi, M.; Hasegawa, R.; Kamata, E.; Ema, M. Unexpected nephrotoxicity induced by tetrabromobisphenol A in newborn rats. Toxicol. Lett. 2004, 150, 145–155. [Google Scholar] [CrossRef]
- Ghisari, M.; Bonefeld-Jorgensen, E.C. Impact of environmental chemicals on the thyroid hormone function in pituitary rat GH3 cells. Mol. Cell Endocrinol. 2005, 244, 31–41. [Google Scholar] [CrossRef]
- Reistad, T.; Mariussen, E.; Ring, A.; Fonnum, F. In vitro toxicity of tetrabromobisphenol-A on cerebellar granule cells: Cell death, free radical formation, calcium influx and extracellular glutamate. Toxicol. Sci. 2007, 96, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Suh, K.S.; Choi, E.M.; Rhee, S.Y.; Oh, S.; Kim, S.W.; Pak, Y.K.; Choe, W.; Ha, J.; Chon, S. Tetrabromobisphenol A induces cellular damages in pancreatic beta-cells in vitro. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2017, 52, 624–631. [Google Scholar] [CrossRef]
- Tykocki, N.R.; Boerman, E.M.; Jackson, W.F. Smooth Muscle Ion Channels and Regulation of Vascular Tone in Resistance Arteries and Arterioles. Compr. Physiol. 2017, 7, 485–581. [Google Scholar] [CrossRef]
- Lorigo, M.; Mariana, M.; Feiteiro, J.; Cairrao, E. How is the human umbilical artery regulated? J. Obstet. Gynaecol. Res. 2018, 44, 1193–1201. [Google Scholar] [CrossRef]
- Weidelt, T.; Boldt, W.; Markwardt, F.J.T.J.o.P. Acetylcholin induced K+ currents in smooth muscle cells of intact rat small arteries. J. Physiol. 1997, 500, 617–630. [Google Scholar] [CrossRef]
- Jewell, R.P.; Saundry, C.M.; Bonev, A.D.; Tranmer, B.I.; Wellman, G.C. Inhibition of Ca++ sparks by oxyhemoglobin in rabbit cerebral arteries. J. Neurosurg. 2004, 100, 295–302. [Google Scholar] [CrossRef]
- Mariana, M.; Feiteiro, J.; Cairrao, E. Cardiovascular Response of Rat Aorta to Di-(2-ethylhexyl) Phthalate (DEHP) Exposure. Cardiovasc. Toxicol. 2018, 18, 356–364. [Google Scholar] [CrossRef]
- Feiteiro, J.; Mariana, M.; Gloria, S.; Cairrao, E. Inhibition of L-type calcium channels by Bisphenol A in rat aorta smooth muscle. J. Toxicol. Sci. 2018, 43, 579–586. [Google Scholar] [CrossRef]
- Feiteiro, J.; Rocha, S.M.; Mariana, M.; Maia, C.J.; Cairrão, E. Pathways involved in the human vascular Tetrabromobisphenol A response: Calcium and potassium channels and nitric oxide donors. Toxicology 2022, 470, 153158. [Google Scholar] [CrossRef]
- Cairrao, E.; Alvarez, E.; Carvas, J.M.; Santos-Silva, A.J.; Verde, I. Non-genomic vasorelaxant effects of 17beta-estradiol and progesterone in rat aorta are mediated by L-type Ca2+ current inhibition. Acta Pharmacol. Sin. 2012, 33, 615–624. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Feiteiro, J.; Mariana, M.; Verde, I.; Cairrão, E. Tributyltin Affects Rat Vascular Contractility Through L-Type Calcium Channels. Int. J. Environ. Res. 2018, 12, 215–221. [Google Scholar] [CrossRef]
- Lorigo, M.; Cairrao, E. UV-B filter octylmethoxycinnamate-induced vascular endothelial disruption on rat aorta: In silico and in vitro approach. Chemosphere 2022, 307, 135807. [Google Scholar] [CrossRef]
- ECHA. Opinion of the Committee for Risk Assessment on a Dossier Proposing Harmonised Classification and Labelling at EU Level. 2021. Available online: https://echa.europa.eu/documents/10162/4d29288d-d6a5-4aa0-f3a2-40741237c2b6 (accessed on 20 May 2022).
- ECHA. 2,2′,6,6′-Tetrabromo-4,4′-Isopropylidenediphenol. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.001.125 (accessed on 24 August 2022).
- Choi, E.M.; Suh, K.S.; Rhee, S.Y.; Oh, S.; Kim, S.W.; Pak, Y.K.; Choe, W.; Ha, J.; Chon, S. Exposure to tetrabromobisphenol A induces cellular dysfunction in osteoblastic MC3T3-E1 cells. J. Environ. Sci. Health Part A 2017, 52, 561–570. [Google Scholar] [CrossRef]
- Godfrey, A.; Abdel-Moneim, A.; Sepulveda, M.S. Acute mixture toxicity of halogenated chemicals and their next generation counterparts on zebrafish embryos. Chemosphere 2017, 181, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Laboratories, S. The subchronic toxicity of sediment-sorbed tetrabromobisphenol A to Chironomus tentans under flow-through conditions. Inc. Rep. 1989, 1989, 89–108. [Google Scholar]
- Pittinger, C.A.; Pecquet, A.M. Review of historical aquatic toxicity and bioconcentration data for the brominated flame retardant tetrabromobisphenol A (TBBPA): Effects to fish, invertebrates, algae, and microbial communities. Environ. Sci. Pollut. Res. Int. 2018, 25, 14361–14372. [Google Scholar] [CrossRef]
- Chen, S.J.; Ma, Y.J.; Wang, J.; Chen, D.; Luo, X.J.; Mai, B.X. Brominated flame retardants in children’s toys: Concentration, composition, and children’s exposure and risk assessment. Environ. Sci. Technol. 2009, 43, 4200–4206. [Google Scholar] [CrossRef]
- Covaci, A.; Voorspoels, S.; Abdallah, M.A.; Geens, T.; Harrad, S.; Law, R.J. Analytical and environmental aspects of the flame retardant tetrabromobisphenol-A and its derivatives. J. Chromatogr. A 2009, 1216, 346–363. [Google Scholar] [CrossRef]
- Guimaraes, S.; Moura, D. Vascular adrenoceptors: An update. Pharmacol. Rev. 2001, 53, 319–356. [Google Scholar]
- Xiao, R.-P. The Adrenergic Receptors in the 21st Century. Circulation 2006, 113, e750. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, R.; Li, P.; Li, J.; Yan, J.; Yin, F.; Han, C.; Zhang, Y. Gene expression profiles in response to the activation of adrenoceptors in A7r5 aortic smooth muscle cells. Clin. Exp. Pharmacol. Physiol. 2004, 31, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Carbajal-Garcia, A.; Reyes-Garcia, J.; Montano, L.M. Androgen Effects on the Adrenergic System of the Vascular, Airway, and Cardiac Myocytes and Their Relevance in Pathological Processes. Int. J. Endocrinol. 2020, 2020, 8849641. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, S.C.; Grimm, S.A.; Wu, S.P.; DeMayo, F.J.; Korach, K.S. Estrogen receptor alpha (ERalpha)-binding super-enhancers drive key mediators that control uterine estrogen responses in mice. J. Biol. Chem. 2020, 295, 8387–8400. [Google Scholar] [CrossRef] [PubMed]
- Weihua, Z.; Saji, S.; Makinen, S.; Cheng, G.; Jensen, E.V.; Warner, M.; Gustafsson, J.A. Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc. Natl. Acad. Sci. USA 2000, 97, 5936–5941. [Google Scholar] [CrossRef]
- Traupe, T.; Stettler, C.D.; Li, H.; Haas, E.; Bhattacharya, I.; Minotti, R.; Barton, M. Distinct roles of estrogen receptors alpha and beta mediating acute vasodilation of epicardial coronary arteries. Hypertension 2007, 49, 1364–1370. [Google Scholar] [CrossRef]
- Perusquia, M.; Hernandez, R.; Morales, M.A.; Campos, M.G.; Villalon, C.M. Role of endothelium in the vasodilating effect of progestins and androgens on the rat thoracic aorta. Gen. Pharmacol. 1996, 27, 181–185. [Google Scholar] [CrossRef]
- Sedin, J.; Dahlgren, D.; Sjoblom, M.; Nylander, O. The Impact of alpha-Adrenoceptors in the Regulation of the Hypotonicity-Induced Increase in Duodenal Mucosal Permeability In Vivo. Pharmaceutics 2021, 13, 96. [Google Scholar] [CrossRef]
- Bojic, M.G.; Todorovic, L.; Santrac, A.; Mian, M.Y.; Sharmin, D.; Cook, J.M.; Savic, M.M. Vasodilatory effects of a variety of positive allosteric modulators of GABAA receptors on rat thoracic aorta. Eur. J. Pharmacol. 2021, 899, 174023. [Google Scholar] [CrossRef]
- Kuo, I.Y.; Wolfle, S.E.; Hill, C.E. T-type calcium channels and vascular function: The new kid on the block? J. Physiol. 2011, 589, 783–795. [Google Scholar] [CrossRef]
- Lorigo, M.; Oliveira, N.; Cairrao, E. Clinical Importance of the Human Umbilical Artery Potassium Channels. Cells 2020, 9, 1956. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, S.; Sun, F.; Zhang, M.; Wu, F.; Xu, F.; Ding, Z. Protective effects of puerarin against tetrabromobisphenol a-induced apoptosis and cardiac developmental toxicity in zebrafish embryo-larvae. Environ. Toxicol. 2015, 30, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ji, G.; Liu, J.; Zhang, S.; Gong, Y.; Shi, L. TBBPA induces developmental toxicity, oxidative stress, and apoptosis in embryos and zebrafish larvae (Danio rerio). Environ. Toxicol. 2016, 31, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.B.; Raad, M.; Sebag, I.A.; Chalifour, L.E. Lifelong exposure to bisphenol a alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicol. Sci. 2013, 133, 174–185. [Google Scholar] [CrossRef]
- Saura, M.; Marquez, S.; Reventun, P.; Olea-Herrero, N.; Arenas, M.I.; Moreno-Gomez-Toledano, R.; Gomez-Parrizas, M.; Munoz-Moreno, C.; Gonzalez-Santander, M.; Zaragoza, C.; et al. Oral administration of bisphenol A induces high blood pressure through angiotensin II/CaMKII-dependent uncoupling of eNOS. FASEB J. 2014, 28, 4719–4728. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Moon, M.K.; Kang, G.H.; Lee, K.J.; Choi, S.H.; Lim, S.; Oh, B.C.; Park, D.J.; Park, K.S.; Jang, H.C.; et al. Chronic exposure to bisphenol A can accelerate atherosclerosis in high-fat-fed apolipoprotein E knockout mice. Cardiovasc. Toxicol. 2014, 14, 120–128. [Google Scholar] [CrossRef]
- Belcher, S.M.; Gear, R.B.; Kendig, E.L. Bisphenol A alters autonomic tone and extracellular matrix structure and induces sex-specific effects on cardiovascular function in male and female CD-1 mice. Endocrinology 2015, 156, 882–895. [Google Scholar] [CrossRef]
- Reventun, P.; Sanchez-Esteban, S.; Cook, A.; Cuadrado, I.; Roza, C.; Moreno-Gomez-Toledano, R.; Munoz, C.; Zaragoza, C.; Bosch, R.J.; Saura, M. Bisphenol A induces coronary endothelial cell necroptosis by activating RIP3/CamKII dependent pathway. Sci. Rep. 2020, 10, 4190. [Google Scholar] [CrossRef]
- Brown, A.R.; Green, J.M.; Moreman, J.; Gunnarsson, L.M.; Mourabit, S.; Ball, J.; Winter, M.J.; Trznadel, M.; Correia, A.; Hacker, C.; et al. Cardiovascular Effects and Molecular Mechanisms of Bisphenol A and Its Metabolite MBP in Zebrafish. Environ. Sci. Technol. 2019, 53, 463–474. [Google Scholar] [CrossRef]
- Sui, Y.; Park, S.H.; Wang, F.; Zhou, C. Perinatal Bisphenol A Exposure Increases Atherosclerosis in Adult Male PXR-Humanized Mice. Endocrinology 2018, 159, 1595–1608. [Google Scholar] [CrossRef] [Green Version]


| Gene | GenBank Accession No. | Primer (5′-3′) | Concentration (μM) |
|---|---|---|---|
| Cyc A | NM_017090.2 | Fw: 5- CAA GAC TGA GTG GCT GGA TGG -3 Rv: 5- GCC CGC AAG TCA AAG AAA TTA GAG -3 | 0.3 |
| Cav1.2 | NM_012517.2 | Fw: 5- CTC GAA GTT GGG AGA ACA GC -3 Rv: 5- GAC GAA ACC CAC GAA GAT GT -3 | 0.4 |
| BKCa β1 | NM_019273.1 | Fw: 5- CCA GGA ATC CAC CTG TCA CT -3 Rv: 5- TCA CAT CAA CCA AGG CTG TC -3 | 0.3 |
| BKCa 1.1α | NM_031828.1 | Fw: 5- GTC TGC ATC TTT GGG GAT GT -3 Rv: 5- GGG GAA GTT GTG CAG TGT TT -3 | 0.3 |
| Gucciα | NM_017090.2 | Fw: 5- GTG TGC CTC GGA AAA TCA AT -3 Rv: 5- ATC TCG GGG TGA ACA CAA AG -3 | 0.3 |
| PRKG 1α | NM_001105731.3 | Fw: 5- CGT GAG GCT ATA CCG GAC AT -3 Rv: 5- GCA AAC GCT TCT ACC ACA CA -3 | 0.3 |
| [TBBPA] μM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 | 0.1 | 1 | 10 | 30 | 50 | 100 | ||||
| Contraction with Phenylephrine (1 μM) | TBBPA (Ct1) | VS | Nif 1 μM + TBBPA | *** | *** | *** | *** | *** | *** | *** |
| K+ channels inhibitors + TBBPA | * | * | *** | *** | *** | *** | *** | |||
| K+ channels inhibitors + Nif 1 μM + TBBPA | *** | *** | *** | *** | *** | *** | * | |||
| Nif 1 μM (Ct2) | VS | Nif 1 μM + TBBPA | n.s | n.s | n.s | n.s | n.s | n.s | n.s | |
| K+ channels inhibitors + TBBPA | *** | *** | *** | *** | *** | *** | *** | |||
| K+ channels inhibitors + Nif 1 μM + TBBPA | n.s | n.s | n.s | n.s | n.s | n.s | n.s | |||
| K+ channels inhibitors + Nif 1 μM (Ct3) | VS | Nif 1 μM + TBBPA | * | * | * | * | n.s | n.s | n.s | |
| K+ channels inhibitors + TBBPA | *** | *** | *** | *** | *** | *** | *** | |||
| K+ channels inhibitors + Nif 1 μM + TBBPA | n.s | n.s | n.s | n.s | n.s | n.s | n.s | |||
| [TBBPA] μM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 | 0.1 | 1 | 10 | 30 | 50 | 100 | ||||
| Contraction with Noradrenaline (1 μM) | TBBPA (Ct1) | VS | Nif 1 μM + TBBPA | *** | *** | *** | *** | *** | *** | *** |
| K+ channels blockage + TBBPA | n.s | n.s | n.s | n.s | ** | *** | *** | |||
| K+ channels blockage + Nif 1 μM + TBBPA | *** | *** | *** | *** | ** | *** | *** | |||
| Nif 1 μM (Ct2) | VS | Nif 1 μM + TBBPA | n.s | n.s | n.s | n.s | n.s | n.s | n.s | |
| K+ channels blockage + TBBPA | *** | *** | *** | *** | *** | *** | *** | |||
| K+ channels blockage + Nif 1 μM + TBBPA | *** | *** | *** | *** | *** | *** | *** | |||
| K+ channels + Nif 1 μM (Ct3) | VS | Nif 1 μM + TBBPA | *** | *** | *** | *** | *** | *** | *** | |
| K+ channels blockage + TBBPA | *** | *** | *** | *** | *** | *** | *** | |||
| K+ channels blockage + Nif 1 μM + TBBPA | * | * | n.s | n.s | n.s | n.s | n.s | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feiteiro, J.; Rocha, S.M.; Mariana, M.; Maia, C.J.; Cairrao, E. Vascular Response of Tetrabromobisphenol a in Rat Aorta: Calcium Channels Inhibition and Potassium Channels Activation. Toxics 2022, 10, 529. https://doi.org/10.3390/toxics10090529
Feiteiro J, Rocha SM, Mariana M, Maia CJ, Cairrao E. Vascular Response of Tetrabromobisphenol a in Rat Aorta: Calcium Channels Inhibition and Potassium Channels Activation. Toxics. 2022; 10(9):529. https://doi.org/10.3390/toxics10090529
Chicago/Turabian StyleFeiteiro, Joana, Sandra M. Rocha, Melissa Mariana, Cláudio J. Maia, and Elisa Cairrao. 2022. "Vascular Response of Tetrabromobisphenol a in Rat Aorta: Calcium Channels Inhibition and Potassium Channels Activation" Toxics 10, no. 9: 529. https://doi.org/10.3390/toxics10090529
APA StyleFeiteiro, J., Rocha, S. M., Mariana, M., Maia, C. J., & Cairrao, E. (2022). Vascular Response of Tetrabromobisphenol a in Rat Aorta: Calcium Channels Inhibition and Potassium Channels Activation. Toxics, 10(9), 529. https://doi.org/10.3390/toxics10090529







