Health Risk Assessment Attributed to Consumption of Fish Contaminated with Mercury in the Rio Branco Basin, Roraima, Amazon, Brazil
Abstract
1. Introduction
2. Materials and Methods
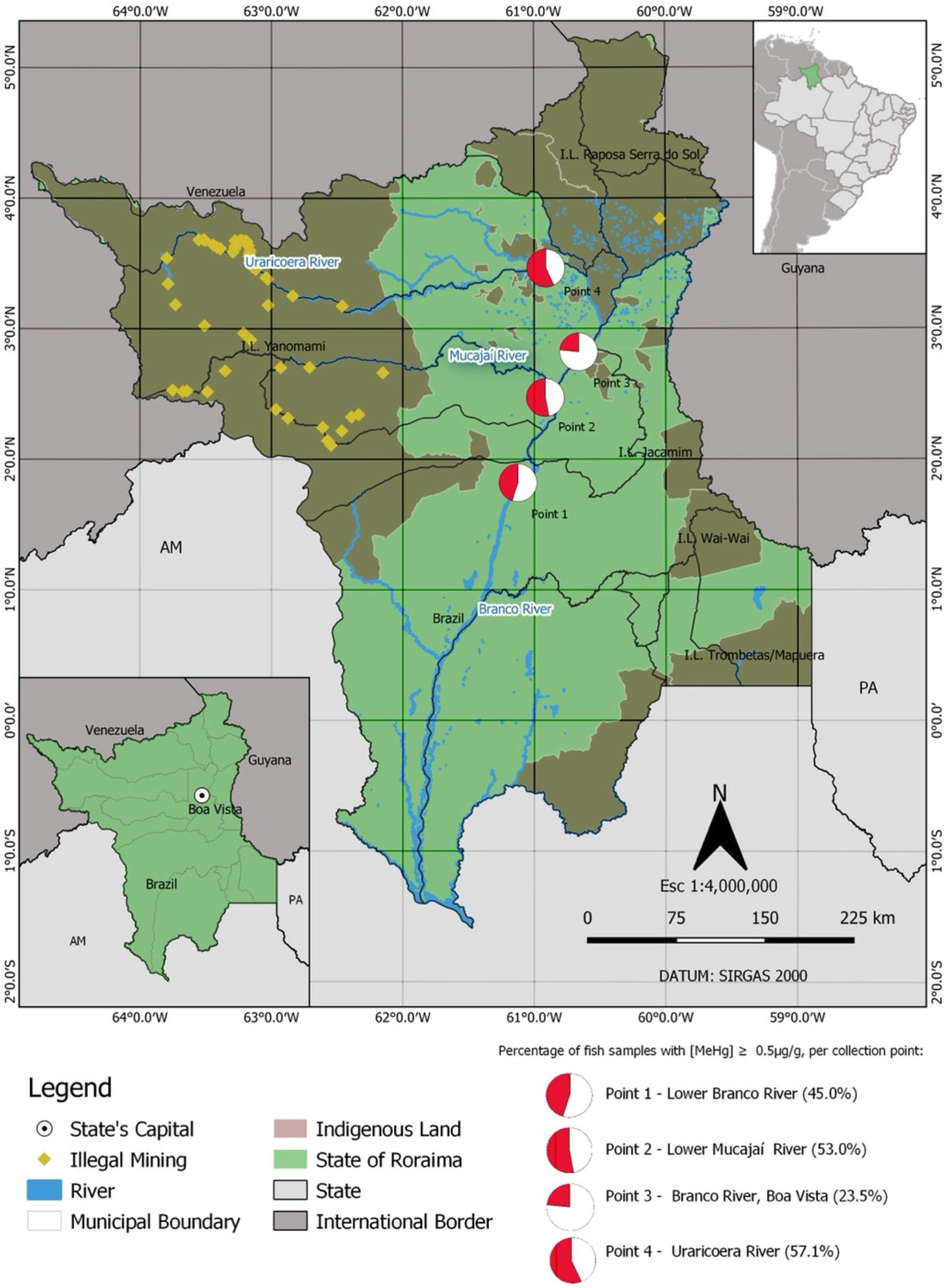
2.1. Study Area
2.2. Health Risk Assessment
- (i)
- The first step is descriptive and consists, basically, of the characterization of the population studied (i.e., the definition of the population subgroups and their respective average body weights) to determine the mercury levels in fish consumed and estimate the quantity of fish ingested daily.
- (ii)
- The second step concerns the calculations used to estimate the average mercury quantity ingested daily by the studied population subgroups. In other words, in this step, we calculated the daily dose of mercury ingested.
- (iii)
- (iv)
- The final step refers to the creation of distinct exposure scenarios for a counterfactual analysis. The counterfactual analysis is designed to investigate the impact of hypothetical situations that could potentially reflect real exposures. In the context of this approach, the counterfactual analysis evaluates the impact of different levels of consumption of mercury-contaminated fish on the selected population subgroups’ health.
2.2.1. First Step in the Risk Assessment
Collection and Storage of Fish Samples
Mercury Analysis
Average Concentration of Methylmercury in Fish
Definition and Characterization of Population Subgroups
Average Amount of Fish Ingested
2.2.2. Second Step in the Risk Assessment
Estimated Methylmercury Ingestion (Ingestion Dose)
2.2.3. Third Step in the Risk Assessment
Risk Ratio Calculation
2.2.4. Fourth Step in the Risk Assessment
Creation of Exposure Scenarios and Counterfactual Analysis
2.3. Maximum Safe Consumption of Fish (MSC)
3. Results
3.1. Fish Collection and Contamination with Methylmercury
3.2. Health Risk Assessment
3.3. Maximum Safe Fish Consumption
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Domingo, J.L. Omega-3 fatty acids and the benefits of fish consumption: Is all that glitters gold? Environ. Int. 2007, 33, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, K.S. Health benefits and potential risks related to consumption of fish or fish oil. Regul. Toxicol. Pharmacol. 2003, 38, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Crespo-López, M.E.; Augusto-Oliveira, M.; Lopes-Araújo, A.; Santos-Sacramento, L.; Yuki Takeda, P.; Macchi, B.M.; Do Nascimento, J.L.M.; Maia, C.S.F.; Lima, R.R.; Arrifano, G.P. Mercury: What can we learn from the Amazon? Environ. Int. 2021, 146, 106223. [Google Scholar] [CrossRef] [PubMed]
- Basta, P.C.; Viana, P.V.S.; Vasconcellos, A.; Périssé, A.; Hofer, C.; Paiva, N.S.; Kempton, J.W.; Ciampi de Andrade, D.; Oliveira, R.A.A.; Achatz, R.W.; et al. Mercury exposure in Munduruku indigenous communities from Brazilian Amazon: Methodological background and an overview of the principal results. Int. J. Environ. Res. Public Health 2021, 18, 9222. [Google Scholar] [CrossRef] [PubMed]
- Morel, F.M.; Kraepiel, A.M.; Amyot, M. The chemical cycle and bioaccumulation of mercury. Annu. Rev. Ecol. Syst. 1998, 29, 543–566. [Google Scholar] [CrossRef]
- Mason, R.P.; Fitzgerald, W.F.; Morel, F.M. The biogeochemical cycling of elemental mercury: Anthropogenic influences. Geochim. Cosmochim. Acta 1994, 58, 3191–3198. [Google Scholar] [CrossRef]
- Guimaraes, J.R.D.; Roulet, M.; Lucotte, M.; Mergler, D. Mercury methylation along a lake-forest transect in the Tapajós river floodplain, Brazilian Amazon: Seasonal and vertical variations. Sci. Total Environ. 2000, 261, 91–98. [Google Scholar] [CrossRef]
- Azevedo, L.S.; Pestana, I.A.; Almeida, M.G.; Ferreira da Costa Nery, A.; Bastos, W.R.; Magalhães Souza, C.M. Mercury biomagnification in an ichthyic food chain of an amazon floodplain lake (Puruzinho Lake): Influence of seasonality and food chain modeling. Ecotoxicol. Environ. Saf. 2021, 207, 111249. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Pfeiffer, W.C. Mercury from gold mining in the Amazon environment: An overview. Quim. Nova 1992, 15, 155–160. [Google Scholar]
- Pfeiffer, W.C.; Lacerda, L.D.; Salomons, W.; Malm, O. Environmental fate of mercury from gold mining in the Brazilian Amazon. Environ. Rev. 1993, 1, 26–37. [Google Scholar] [CrossRef]
- Passos, C.J.; Mergler, D. Human mercury exposure and adverse health effects in the Amazon: A review. Cad. Saude Publica 2008, 24, s503–s520. [Google Scholar] [CrossRef] [PubMed]
- Clifton, J.C., II. Mercury exposure and public health. Pediatr. Clin. N. Am. 2007, 54, 237–269. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, A.C.S.D.; Hallwass, G.; Bezerra, J.G.; Aciole, A.N.S.; Meneses, H.N.D.M.; Lima, M.D.O.; Jesus, I.M.D.; Hacon, S.D.S.; Basta, P.C. Health Risk Assessment of Mercury Exposure from Fish Consumption in Munduruku Indigenous Communities in the Brazilian Amazon. Int. J. Environ. Res. Public Health 2021, 18, 7940. [Google Scholar] [CrossRef] [PubMed]
- Lechler, P.J.; Miller, J.R.; Lacerda, L.D.; Vinson, D.; Bonzongo, J.C.; Lyons, W.B.; Warwick, J.J. Elevated mercury concentrations in soils, sediments, water, and fish of the Madeira River basin, Brazilian Amazon: A function of natural enrichments? Sci. Total Environ. 2000, 260, 87–96. [Google Scholar] [CrossRef]
- Rodríguez Martín-Doimeadios, R.C.; Berzas Nevado, J.J.; Guzmán Bernardo, F.J.; Jiménez Moreno, M.; Arrifano, G.P.F.; Herculano, A.M.; Nascimento, J.L.M.D.; Crespo-López, M.E. Comparative study of mercury speciation in commercial fishes of the Brazilian Amazon. Environ. Sci. Pollut. Res. 2014, 21, 7466–7479. [Google Scholar] [CrossRef]
- Souza-Araujo, J.; Giarrizzo, T.; Lima, M.O.; Souza, M.B.G. Mercury and methyl mercury in fishes from Bacajá River (Brazilian Amazon): Evidence for bioaccumulation and biomagnification. J. Fish Biol. 2016, 89, 249–263. [Google Scholar] [CrossRef]
- Lino, A.S.; Kasper, D.; Guida, Y.S.; Thomaz, J.R.; Malm, O. Mercury and selenium in fishes from the Tapajós River in the Brazilian Amazon: An evaluation of human exposure. J. Trace Elem. Med. Biol. 2018, 48, 196–201. [Google Scholar] [CrossRef]
- Albuquerque, F.E.A.; Minervino, A.H.H.; Miranda, M.; Herrero-Latorre, C.; Barrêto Júnior, R.A.; Oliveira, F.L.C.; Sucupira, M.C.A.; Ortolani, E.L.; López-Alonso, M. Toxic and essential trace element concentrations in fish species in the Lower Amazon, Brazil. Sci. Total Environ. 2020, 732, 138983. [Google Scholar] [CrossRef]
- Hacon, S.D.S.; Oliveira-Da-Costa, M.; Gama, C.D.S.; Ferreira, R.; Basta, P.C.; Schramm, A.; Yokota, D. Mercury exposure through fish consumption in traditional communities in the Brazilian Northern Amazon. Int. J. Environ. Res. Public Health 2020, 17, 5269. [Google Scholar] [CrossRef]
- Lebel, J.; Roulet, M.; Mergler, D.; Lucotte, M.; Larribe, F. Fish diet and mercury exposure in a riparian Amazonian population. Water Air Soil Pollut. 1997, 97, 31–44. [Google Scholar] [CrossRef]
- de Souza Lima, A.P.; Müller, R.C.S.; de Souza Sarkis, J.E.; Alves, C.N.; da Silva Bentes, M.H.; Brabo, E.; de Oliveira Santos, E. Mercury contamination in fish from Santarém, Pará, Brazil. Environ. Res. 2000, 83, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Passos, C.J.S.; Da Silva, D.S.; Lemire, M.; Fillion, M.; Guimaraes, J.R.D.; Lucotte, M.; Mergler, D. Daily mercury intake in fish-eating populations in the Brazilian Amazon. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, T.C.; Penteado, J.O.; dos Santos, M.; da Silva Júnior, F.M.R. Methylmercury in Fish from the Amazon Region-A Review Focused on Eating Habits. Water Air Soil Pollut. 2021, 232, 1–9. [Google Scholar] [CrossRef]
- Castilhos, Z.C.; Rodrigues-Filho, S.; Rodrigues, A.P.; Villas-Boas, R.C.; Siegel, S.; Veiga, M.M.; Beinhoff, C. Mercury contamination in fish from gold mining areas in Indonesia and human health risk assessment. Sci. Total Environ. 2006, 368, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.M.; Zakeel, M.C.M.; Zavahir, J.S.; Marikar, F.M.M.T.; Jahan, I. Heavy Metal Accumulation in Rice and Aquatic Plants Used as Human Food: A General Review. Toxics 2021, 9, 360. [Google Scholar] [CrossRef]
- FAO/WHO. Joint FAO/WHO Expert Committee on Food Additives (JECFA); Report of the Tenth Section; FAO/WHO: Rotterdam, The Netherlands, 2016; Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-735-10%252FReport%252FREP16_CFe.pdf (accessed on 22 June 2021).
- FAO/WHO. Evaluation of certain food additives and contaminants: Sixty-first report of the Joint FAO/WHO Expert Committee on Food Additives. In Proceedings of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), Rome, Italy, 10–19 June 2003. [Google Scholar]
- U.S.-EPA. Reference Dose for Methylmercury; U.S. Environmental Protection Agency: Washington, DC, USA, 2000. [Google Scholar]
- Santos Serrão de Castro, N.; de Oliveira Lima, M. Hair as a biomarker of long-term mercury exposure in Brazilian Amazon: A systematic review. Int. J. Environ. Res. Public Health 2018, 15, 500. [Google Scholar] [CrossRef] [PubMed]
- Berzas Nevado, J.J.; Rodríguez Martín-Doimeadios, R.C.; Guzmán Bernardo, F.J.; Jiménez Moreno, M.; Herculano, A.M.; do Nascimento, J.L.M.; Crespo-López, M.E. Mercury in the Tapajós River basin, Brazilian Amazon: A review. Environ. Int. 2010, 36, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Frischtak, H.; Berky, A.; Ortiz, E.J.; Morales, A.M.; Hsu-Kim, H.; Pendergast, L.L.; Pan, W.K. Elevated Hair Mercury Levels Are Associated With Neurodevelopmental Deficits in Children Living Near Artisanal and Small-Scale Gold Mining in Peru. GeoHealth 2020, 4, e2019GH000222. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.C.; Bernardi, J.V.E.; Abreu, L.; Dórea, J.G. Neurodevelopment Outcomes in Children Exposed to Organic Mercury from Multiple Sources in a Tin-Ore Mine Environment in Brazil. Arch. Environ. Contam. Toxicol. 2015, 68, 432–441. [Google Scholar] [CrossRef]
- Marques, R.C.; Bernardi, J.V.E.; Cunha, M.P.L.; Dórea, J.G. Impact of organic mercury exposure and home delivery on neurodevelopment of Amazonian children. Int. J. Hyg. Environ. Health 2016, 219, 498–502. [Google Scholar] [CrossRef]
- Junior, J.M.F.C.; Da Silva Lima, A.A.; Junior, D.R.; Khoury, E.D.T.; Da Silva Souza, G.; De Lima Silveira, L.C.; Pinheiro, M.D.C.N. Manifestações emocionais e motoras de ribeirinhos expostos ao mercúrio na Amazônia. Rev. Bras. Epidemiol. 2017, 20, 212–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lacerda, E.M.D.C.B.; Souza, G.D.S.; Cortes, M.I.T.; Rodrigues, A.R.; Pinheiro, M.C.N.; Silveira, L.C.D.L.; Ventura, D.F. Comparison of Visual Functions of Two Amazonian Populations: Possible Consequences of Different Mercury Exposure. Front. Neurosci. 2020, 13, 1428. [Google Scholar] [CrossRef] [PubMed]
- Arrifano, G.P.; Martín-Doimeadios, R.C.; Jiménez-Moreno, M.; Ramírez-Mateos, V.; da Silva, N.F.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Paraense, R.S.; Macchi, B.M.; do Nascimento, J.L.; et al. Large-scale projects in the amazon and human exposure to mercury: The case-study of the Tucuruí Dam. Ecotoxicol. Environ. Saf. 2018, 147, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.A.; Rudd, J.W.M.; Bodaly, R.A.; Roulet, N.P.; StLouis, V.L.; Heyes, A.; Moore, T.R.; Schiff, S.; Aravena, R.; Scott, K.J.; et al. Increases in fluxes of greenhouse gases and methyl mercury following flooding of an experimental reservoir. Environ. Sci. Technol. 1997, 31, 1334–1344. [Google Scholar] [CrossRef]
- Gomes, V.M.; dos Santos, A.; Zara, L.F.; Ramos, D.D.; Forti, J.C.; Ramos, D.D.; Santos, F.A. Study on mercury methylation in the Amazonian rivers in flooded areas for hydroelectric use. Water Air Soil Pollut. 2019, 230, 211. [Google Scholar] [CrossRef]
- Palermo, E.F.A.; Kasper, D.; Reis, T.S.; Nogueira, S.; Branco, C.W.C.; Malm, O. Mercury level increase in fish tissues downstream the Tucuruí reservoir, Brazil. RMZ-M&G 2004, 51, 1292–1294. [Google Scholar]
- Forsberg, B.R.; Melack, J.M.; Dunne, T.; Barthem, R.B.; Goulding, M.; Paiva, R.C.D.; Sorribas, M.V.; Silva, U.L., Jr.; Weisser, S. The potential impact of new Andean dams on Amazon fluvial ecosystems. PLoS ONE 2017, 12, e0182254. [Google Scholar] [CrossRef]
- Arruda, M.C.F. Avaliação Dos Indicadores da Política de Pesca do Programa Zona Franca Verde: Perspectivas Econômicas e Ambientais. Master’s thesis, Universidade Federal do Amazonas, Manaus, Brasil, 2017. [Google Scholar]
- Barone, G.; Storelli, A.; Meleleo, D.; Dambrosio, A.; Garofalo, R.; Busco, A.; Storelli, M.M. Levels of Mercury, Methylmercury and Selenium in Fish: Insights into Children Food Safety. Toxics 2021, 9, 39. [Google Scholar] [CrossRef]
- Vega, C.M.; Orellana, J.D.Y.; Oliveira, M.W.; Hacon, S.S.; Basta, P.C. Human Mercury Exposure in Yanomami Indigenous Villages from the Brazilian Amazon. Int. J. Environ. Res. Public Health 2018, 15, 1051. [Google Scholar] [CrossRef]
- Hacon, S.; Rochedo, E.R.; Campos, R.R.; Lacerda, L.D. Mercury exposure through fish consumption in the urban area of Alta Floresta in the Amazon Basin. J. Geochem. Explor. 1997, 58, 209–216. [Google Scholar] [CrossRef]
- Dutra, M.D.S.; Jesus, I.M.D.; Santos, E.C.; Lima, M.D.O.; Medeiros, R.L.F.D.; Cavadas, M.; Luiz, R.R.; Câmara, V.D.M. Longitudinal assessment of mercury exposure in schoolchildren in an urban area of the Brazilian Amazon. Cad. Saude Publica 2012, 28, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.M.; de Almeida, R.; Holanda, I.B.; Mussy, M.H.; Galvão, R.C.; Crispim, P.T.; Dórea, J.G.; Bastos, W.R. Total and methyl-mercury in hair and milk of mothers living in the city of Porto Velho and in villages along the Rio Madeira, Amazon, Brazil. Int. J. Hyg. Environ. Health 2013, 216, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.M.; Morais, R.P. Aspectos hidrogeomorfológicos do sistema fluvial do baixo rio Uraricoera e alto rio Branco como subsídio na gestão de terras. Rev. Geogr. 2014, 10, 118–135. [Google Scholar]
- Sing, K.A.; Hryhorczuk, D.; Saffirio, G.; Sinks, T.; Paschal, D.C.; Sorensen, J.; Chen, E.H. Organic Mercury levels among the Yanomama of the Brazilian Amazon Basin. Ambio 2003, 32, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.A.; Hryhorczuk, D.O.; Saffirio, G.; Sinks, T.; Paschal, D.C.; Chen, E.H. Environmental exposure to organic mercury among the Makuxi in the Amazon Basin. Int. J. Occup. Environ. Health 1996, 2, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.B.; Albert, B.; Pfeiffer, W.C. Mercury levels in Yanomami Indians hair from Roraima-Brazil. In Heavy Metals in the Environment, Proceedings of the International Conference on Heavy Metals in the Environment, Edinbourgh, UK, 16–20 September 1991; CEP Consultants: Edinbourg, Scotland, 1991; pp. 367–370. [Google Scholar]
- Hutukara Associação Yanomami. Cicatrizes na Floresta: Evolução do Garimpo Ilegal na TI Yanomami em 2020. Realização: Hutukara Associação Yanomami e Associação Wanasseduume Ye’kwana; Assessoria técnica; Instituto Socioambiental: Boa Vista, Brasil, 2020. [Google Scholar]
- Hutukara Associação Yanomami, Yanomami sob ataque. Realização: Hutukara Associação Yanomami e Associação Wanasseduume Ye’kwana; Assessoria Técnica; Instituto Socioambiental: Boa Vista, Brasil, 2022. [Google Scholar]
- World Health Organization. Guidance for Identifying Populations at Risk from Mercury Exposure; Mercury Publications: Geneva, Switzerland, 2008. [Google Scholar]
- Matos, L.S.; Correa, A.S.A.S.; da Silva, S.A.A.; Muniz, C.C.; Ignacio, A.R.A. Mercury concentrations in fish and human health assessment in preflood phase of a hydro dam in Teles Pires River, Southern Brazilian Amazon. Elem. Sci. Anthrop. 2021, 9, 20. [Google Scholar] [CrossRef]
- Akagi, H.; Suzuki, T.; Arimura, K.; Ando, T.; Sakamoto, M.; Satoh, H.; Matsuyama, A. Mercury Analysis Manual, 105th ed.; Ministry of the Environmental: Tokyo, Japan, 2004. Available online: http://nimd.env.go.jp/kenkyu/docs/march_mercury_analysis_manual(e).pdf (accessed on 21 November 2019).
- Malm, O.; Branches, F.J.; Akagi, H.; Castro, M.B.; Pfeiffer, W.C.; Harada, M.; Bastos, W.R.; Kato, H. Mercury and methylmercury in fish and human hair from the Tapajos river basin, Brazil. Sci. Total Environ. 1995, 175, 141–150. [Google Scholar] [CrossRef]
- Harris, H.H.; Pickering, I.J.; George, G.N. The chemical form of mercury in fish. Science 2003, 301, 1203. [Google Scholar] [CrossRef]
- POF Pesquisa de Orçamento Familiar; Instituto Brasileiro de Geografia e Estatística (IBGE): Brasília, Brasil, 2008. Available online: https://sidra.ibge.gov.br/pesquisa/pof/tabelas (accessed on 3 June 2022).
- Ruxton, C.H.S. The benefits of fish consumption. Nutr. Bull. 2011, 36, 6–19. [Google Scholar] [CrossRef]
- Torpy, J.M.; Lynm, C.; Glass, R.M. Eating fish: Health benefits and risks. JAMA 2006, 296, 1926. [Google Scholar] [CrossRef]
- Egeland, G.M.; Middaugh, J.P. Balancing fish consumption benefits with mercury exposure. Science 1997, 278, 1904–1905. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Resolução RDC nº42. Dispõe Sobre o Regulamento Técnico MERCOSUL sobre Limites Máximos de Contaminantes Inorgânicos em Alimentos; Diário Oficial da União da República Federativa do Brasil: Braília, Brasil, 2013. [Google Scholar]
- Marrugo-Madrid, S.; Pinedo-Hernández, J.; Paternina-Uribe, R.; Marrugo-Negrete, J.; Díez, S. Health risk assessment for human exposure to mercury species and arsenic via consumption of local food in a gold mining area in Colombia. Environ. Res. 2022, 113950. [Google Scholar] [CrossRef] [PubMed]
- Crump, K.S.; Kjellstrøm, T.; Shipp, A.M.; Silvers, A.; Stewart, A. Influence of prenatal mercury exposure upon scholastic and psychological test performance: Benchmark analysis of a New Zealand cohort. Risk Anal. 1998, 18, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.J.; Thurston, S.W.; Pearson, A.T.; Davidson, P.W.; Cox, C.; Shamlaye, C.F.; Cernichiari, E.; Clarkson, T.W. Postnatal exposure to methyl mercury from fish consumption: A review and new data from the Seychelles Child Development Study. Neurotoxicology 2009, 30, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.W.; Myers, G.J.; Weiss, B.; Shamlaye, C.F.; Cox, C. Prenatal methyl mercury exposure from fish consumption and child development: A review of evidence and perspectives from the Seychelles Child Development Study. Neurotoxicology 2006, 27, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Weihe, P.; Araki, S.; Budtz-Jüòrgensen, E.; Grandjean, P. Evoked potentials in Faroese children prenatally exposed to methylmercury. Neurotoxicol. Teratol. 1999, 21, 471–472. [Google Scholar] [CrossRef]
- Fujiki, M.; Tajima, S. The pollution of Minamata Bay by mercury. Water Sci. Technol. 1992, 25, 133–140. [Google Scholar] [CrossRef]
- Aaseth, J.; Wallace, D.R.; Vejrup, K.; Alexander, J. Methylmercury and developmental neurotoxicity: A global concern. Curr. Opin. Toxicol. 2020, 19, 80–87. [Google Scholar] [CrossRef]
- Kishi, R. Impacts of Developmental Exposure to Environmental Chemicals on Human Health with Global Perspectives. In Health Impacts of Developmental Exposure to Environmental Chemicals; Springer: Singapore, 2020; pp. 3–22. [Google Scholar]
- Oliveira, R.A.A.D.; Pinto, B.D.; Rebouças, B.H.; Ciampi de Andrade, D.; Vasconcellos, A.C.S.D.; Basta, P.C. Neurological Impacts of Chronic Methylmercury Exposure in Munduruku Indigenous Adults: Somatosensory, Motor, and Cognitive Abnormalities. Int. J. Environ. Res. Public Health 2021, 18, 10270. [Google Scholar] [CrossRef]
- Achatz, R.W.; de Vasconcellos, A.C.S.; Pereira, L.; Viana, P.V.D.S.; Basta, P.C. Impacts of the Goldmining and Chronic Methylmercury Exposure on the Good-Living and Mental Health of Munduruku Native Communities in the Amazon Basin. Int. J. Environ. Res. Public Health 2021, 18, 8994. [Google Scholar] [CrossRef]
- Kempton, J.W.; Périssé, A.R.S.; Hofer, C.B.; de Vasconcellos, A.C.S.; de Sousa Viana, P.V.; de Oliveira Lima, M.; de Jesus, I.M.; de Souza Hacon, S.; Basta, P.C. An assessment of health outcomes and methylmercury exposure in Munduruku indigenous women of childbearing age and their children under 2 years old. Int. J. Environ. Res. Public Health 2021, 18, 10091. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.F.; Lowe, M.; Chan, H.M. Mercury exposure, cardiovascular disease, and mortality: A systematic review and dose-response meta-analysis. Environ. Res. 2021, 193, 110538. [Google Scholar] [CrossRef] [PubMed]
- Fillion, M.; Mergler, D.; Passos CJ, S.; Larribe, F.; Lemire, M.; Guimarães, J.R.D. A preliminary study of mercury exposure and blood pressure in the Brazilian Amazon. Environ. Health 2006, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Salonen, J.T.; Seppønen, K.; Nyyssønen, K.; Korpela, H.; Kauhanen, J.; Kantola, M.; Tuomilehto, J.; Esterbauer, H.; Tatzber, F.; Salonen, R. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation 1995, 91, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Bakker, L.B.; Gasparinetti, P.; de Queiroz, J.M.; Vasconcellos, A.C.S. Economic Impacts on Human Health Resulting from the Use of Mercury in the Illegal Gold Mining in the Brazilian Amazon: A Methodological Assessment. Int. J. Environ. Res. Public Health 2021, 18, 11869. [Google Scholar] [CrossRef]
- Gutiérrez-Mosquera, H.; Marrugo-Negrete, J.; Díez, S.; Morales-Mira, G.; Montoya-Jaramillo, L.J.; Jonathan, M.P. Mercury distribution in different environmental matrices in aquatic systems of abandoned gold mines, Western Colombia: Focus on human health. J. Hazard. Mat. 2021, 404, 124080. [Google Scholar] [CrossRef]
- Wells, E.M.; Kopylev, L.; Nachman, R.; Radke, E.G.; Congleton, J.; Segal, D. Total Blood Mercury Predicts Methylmercury Exposure in Fish and Shellfish Consumers. Biol. T. Elem. Res. 2022, 200, 3867–3875. [Google Scholar] [CrossRef]
- Bradley, M.A.; Barst, B.D.; Basu, N. A review of mercury bioavailability in humans and fish. Int. J. Environ. Res. Public Health 2017, 14, 169. [Google Scholar] [CrossRef]
- Hacon, S.; Farias, R.A.D.; Barrocas, P.; Campos, R.C.; Wasserman, J.; Argento, R. Avaliação da exposição humana ao mercúrio na região norte de Mato Grosso, Amazônia Legal. Cad. Saude Colet. 2005, 13, 837–854. [Google Scholar]
- Perez, T.D. Avaliação da Contaminação de Hoplias Malabaricus (Traíra) Como Bioindicador de Saúde Ambiental em Pisciculturas em Áreas de Garimpo. Estudo de Caso Município de Paranaíta-MT. 54 f. Dissertação (Mestrado em Saúde Pública e Meio Ambiente). Master’s Thesis, Escola Nacional de Saúde Pública Sergio Arouca, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil, 2008. [Google Scholar]
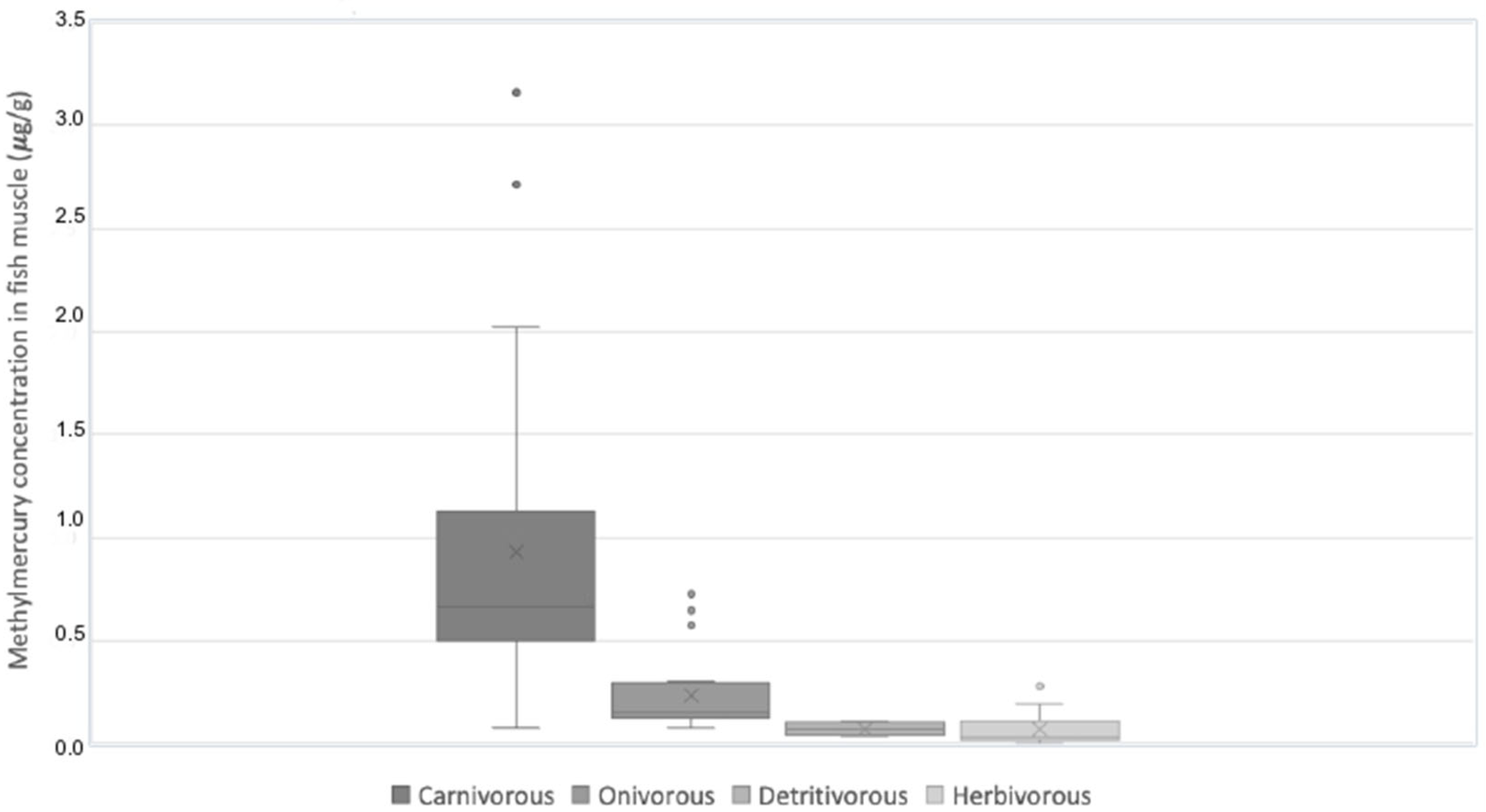
| Common Name (n) | Average (Hg) μg/g | Standard Deviation | Min–Max Hg | Average Weight (g) | Min–Max Weight (g) | Average Total Length (cm) | Min–Max Total Length | Trophic Level |
|---|---|---|---|---|---|---|---|---|
| Aracu (05) | 0.14 | 0.11 | 0.01–0.28 | 336 | 153–500 | 29.7 | 25–32.8 | Herbivorous |
| Barba Chata (03) | 2.07 | 0.57 | 1.60–2.72 | 868 | 555–1120 | 47.3 | 41.5–51 | Carnivorous |
| Cará-açú (05) | 0.40 | 0.20 | 0.17–0.65 | 694 | 511–963 | 36.3 | 27–50 | Omnivorous |
| Coroataí (04) | 2.01 | 0.9 | 0.96–3.16 | 1378 | 422–4085 | 51.8 | 39–84 | Carnivorous |
| Curimatã (03) | 0.09 | 0.02 | 0.07–0.11 | 358 | 265–405 | 28.1 | 26–29.7 | Detritivorous |
| Dourada (02) | 0.66 | 0.04 | 0.63–0.69 | 1280 | 955–1605 | 80.5 | 61–100 | Carnivorous |
| Filhote/Piraíba (03) | 1.17 | 0.31 | 0.87–1.49 | 18,348 | 3045–32,000 | 99 | 68–115 | Carnivorous |
| Jandiá (02) | 0.09 | 0.03 | 0.07–0.11 | 1111 | 1090–1132 | 49.8 | 49.5–50 | Carnivorous |
| Jaraqui (02) | 0.04 | 0.01 | 0.04–0.04 | 380 | 380 | 29 | 28.5–29.5 | Detritivorous |
| Liro (01) | 0.43 | N.A. | N.A. | 354 | N.A. | 38 | N.A. | Carnivorous |
| Mandii (02) | 0.44 | 0.40 | 0.15–0.72 | 67 | 41–92 | 21 | 18–24 | Omnivorous |
| Mandubé (01) | 0.62 | N.A. | N.A. | 580 | N.A. | 39 | N.A. | Carnivorous |
| Matrinxã (12) | 0.13 | 0.03 | 0.07–0.2 | 358 | 232–490 | 27.9 | 25–30 | Omnivorous |
| Pacú (08) | 0.03 | 0.02 | 0.00–0.07 | 334 | 131–670 | 23.4 | 19–30.1 | Herbivorous |
| Pescada (07) | 0.61 | 0.22 | 0.47–1.08 | 564 | 395–680 | 36.4 | 30.5–39 | Carnivorous |
| Piracatinga (01) | 1.47 | N.A. | N.A. | 770 | N.A. | 45.6 | N.A. | Carnivorous |
| Pirandirá (01) | 1.06 | N.A. | N.A. | 2025 | N.A. | 56.6 | N.A. | Carnivorous |
| Piranha-preta (02) | 0.40 | 0.03 | 0.38–0.42 | 227 | 176–278 | 21.8 | 20.5–23 | Carnivorous |
| Surubim (06) | 0.65 | 0.18 | 0.43–0.95 | 1163 | 885–1905 | 54.2 | 50–63.3 | Carnivorous |
| Tucunaré (05) | 0.71 | 0.26 | 0.49–1.12 | 950 | 615–1605 | 37.4 | 34.3–52.5 | Carnivorous |
| Dose of Hg Ingested (µg/g) | |||||
|---|---|---|---|---|---|
| Average (Hg) in Fish (µg/g) | Level of Consumption | Mass of Hg Ingested (µg) | Population Subset | Urban Population | Non-Urban Population |
| 0.545 µg/g | Low consumption (50 g/day) | 27.26 | Women of childbearing age | 0.53 | 0.54 |
| Adult Men | 0.40 | 0.42 | |||
| Children aged 5 to 12 | 0.95 | 1.01 | |||
| Children aged 2 to 4 | 1.84 | 1.88 | |||
| Moderate consumption (100 g/day) | 54.52 | Women of childbearing age | 1.06 | 1.08 | |
| Adult Men | 0.79 | 0.84 | |||
| Children aged 5 to 12 | 1.89 | 2.02 | |||
| Children aged 2 to 4 | 3.67 | 3.77 | |||
| High consumption (200 g/day) | 109.04 | Women of childbearing age | 2.13 | 2.15 | |
| Adult Men | 1.59 | 1.67 | |||
| Children aged 5 to 12 | 3.78 | 4.03 | |||
| Children aged 2 to 4 | 7.34 | 7.53 | |||
| Risk Ratio U.S. EPA | Risk Ratio FAO/WHO | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Consumption of Urban Population | Consumption of Non-Urban Population | Consumption of Urban Population | Consumption of Non-Urban Population | |||||||||
| Low | Moderate | High | Low | Moderate | High | Low | Moderate | High | Low | Moderate | High | |
| Women of childbearing age | 5.32 | 10.63 | 21.27 | 5.38 | 10.77 | 21.53 | 2.31 | 4.62 | 9.25 | 2.34 | 4.68 | 9.36 |
| Adult men | 3.97 | 7.95 | 15.90 | 4.18 | 8.37 | 16.73 | 0.88 | 1.77 | 3.53 | 0.93 | 1.86 | 3.72 |
| Children aged 5 to 12 | 9.46 | 18.92 | 37.84 | 10.09 | 20.17 | 40.34 | 4.11 | 8.23 | 16.45 | 4.38 | 8.77 | 17.54 |
| Children aged 2 to 4 | 18.36 | 36.71 | 73.43 | 18.83 | 37.65 | 75.30 | 7.98 | 15.96 | 31.93 | 8.19 | 16.37 | 32.74 |
| Maximum Safe Fish Consumption (in Grams per Day) According to U.S. EPA (2000) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Women of Childbearing Age | Adult Men | Children Aged 5 to 12 | Children Aged 2 to 4 | ||||||
| Common Name | Average (Hg) μg/g | Urban | Non-Urban | Urban | Non-Urban | Urban | Non-Urban | Urban | Non-Urban |
| Aracu | 0.137 | 37.42 | 36.96 | 50.07 | 47.57 | 21.04 | 19.73 | 10.84 | 10.57 |
| Barba Chata | 2.075 | 2.47 | 2.44 | 3.31 | 3.14 | 1.39 | 1.30 | 0.72 | 0.70 |
| Cará-açú | 0.397 | 12.91 | 12.76 | 17.28 | 16.42 | 7.26 | 6.81 | 3.74 | 3.65 |
| Coroataí | 2.014 | 2.55 | 2.51 | 3.41 | 3.24 | 1.43 | 1.34 | 0.74 | 0.72 |
| Curimatã | 0.091 | 56.34 | 55.65 | 75.37 | 71.62 | 31.67 | 29.70 | 16.32 | 15.91 |
| Dourada | 0.665 | 7.71 | 7.62 | 10.31 | 9.80 | 4.33 | 4.06 | 2.23 | 2.18 |
| Filhote/Piraíba | 1.172 | 4.37 | 4.32 | 5.85 | 5.56 | 2.46 | 2.31 | 1.27 | 1.24 |
| Jandiá | 0.092 | 55.73 | 55.04 | 74.55 | 70.84 | 31.33 | 29.38 | 16.14 | 15.74 |
| Jaraqui | 0.039 | 131.46 | 129.85 | 175.87 | 167.10 | 73.90 | 69.31 | 38.08 | 37.13 |
| Liro | 0.426 | 12.04 | 11.89 | 16.10 | 15.30 | 6.77 | 6.35 | 3.49 | 3.40 |
| Mandii | 0.439 | 11.68 | 11.54 | 15.62 | 14.85 | 6.56 | 6.16 | 3.38 | 3.30 |
| Mandubé | 0.621 | 8.26 | 8.15 | 11.05 | 10.49 | 4.64 | 4.35 | 2.39 | 2.33 |
| Matrinxã | 0.13 | 39.44 | 38.95 | 52.76 | 50.13 | 22.17 | 20.79 | 11.42 | 11.14 |
| Pacú | 0.03 | 170.90 | 168.80 | 228.63 | 217.23 | 96.07 | 90.10 | 49.50 | 48.27 |
| Pescada | 0.61 | 8.40 | 8.30 | 11.24 | 10.68 | 4.72 | 4.43 | 2.43 | 2.37 |
| Piracatinga | 1.472 | 3.48 | 3.44 | 4.66 | 4.43 | 1.96 | 1.84 | 1.01 | 0.98 |
| Pirandirá | 1.062 | 4.83 | 4.77 | 6.46 | 6.14 | 2.71 | 2.55 | 1.40 | 1.36 |
| Piranha-preta | 0.401 | 12.79 | 12.63 | 17.10 | 16.25 | 7.19 | 6.74 | 3.70 | 3.61 |
| Surubim | 0.646 | 7.94 | 7.84 | 10.62 | 10.09 | 4.46 | 4.18 | 2.30 | 2.24 |
| Tucunaré | 0.706 | 7.26 | 7.17 | 9.72 | 9.23 | 4.08 | 3.83 | 2.10 | 2.05 |
| Maximum Safe Fish Consumption (in Grams per Day) According to FAO/WHO (2003) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Women of Childbearing Age | Adult Men | Children Aged 5 to 12 | Children aged 2 to 4 | ||||||
| Common Name | Average (Hg) μg/g | Urban | Non-Urban | Urban | Non-Urban | Urban | Non-Urban | Urban | Non-Urban |
| Aracu | 0.137 | 86.07 | 85.02 | 225.30 | 214.06 | 48.38 | 45.38 | 24.93 | 24.31 |
| Barba Chata | 2.075 | 5.68 | 5.61 | 14.87 | 14.13 | 3.19 | 3.00 | 1.65 | 1.61 |
| Cará-açú | 0.397 | 29.70 | 29.34 | 77.75 | 73.87 | 16.70 | 15.66 | 8.60 | 8.39 |
| Coroataí | 2.014 | 5.86 | 5.78 | 15.33 | 14.56 | 3.29 | 3.09 | 1.70 | 1.65 |
| Curimatã | 0.091 | 129.58 | 127.99 | 339.18 | 322.27 | 72.84 | 68.32 | 37.53 | 36.60 |
| Dourada | 0.665 | 17.73 | 17.51 | 46.41 | 44.10 | 9.97 | 9.35 | 5.14 | 5.01 |
| Filhote/Piraíba | 1.172 | 10.06 | 9.94 | 26.34 | 25.02 | 5.66 | 5.30 | 2.91 | 2.84 |
| Jandiá | 0.092 | 128.18 | 126.60 | 335.49 | 318.77 | 72.05 | 67.58 | 37.13 | 36.20 |
| Jaraqui | 0.039 | 302.36 | 298.65 | 791.42 | 751.96 | 169.96 | 159.41 | 87.58 | 85.39 |
| Liro | 0.426 | 27.68 | 27.34 | 72.45 | 68.84 | 15.56 | 14.59 | 8.02 | 7.82 |
| Mandii | 0.439 | 26.86 | 26.53 | 70.31 | 66.80 | 15.10 | 14.16 | 7.78 | 7.59 |
| Mandubé | 0.621 | 18.99 | 18.76 | 49.70 | 47.22 | 10.67 | 10.01 | 5.50 | 5.36 |
| Matrinxã | 0.13 | 90.71 | 89.59 | 237.43 | 225.59 | 50.99 | 47.82 | 26.27 | 25.62 |
| Pacú | 0.03 | 393.07 | 388.24 | 1028.85 | 977.55 | 220.95 | 207.23 | 113.85 | 111.01 |
| Pescada | 0.61 | 19.33 | 19.09 | 50.60 | 48.08 | 10.87 | 10.19 | 5.60 | 5.46 |
| Piracatinga | 1.472 | 8.01 | 7.91 | 20.97 | 19.92 | 4.50 | 4.22 | 2.32 | 2.26 |
| Pirandirá | 1.062 | 11.10 | 10.97 | 29.06 | 27.61 | 6.24 | 5.85 | 3.22 | 3.14 |
| Piranha-preta | 0.401 | 29.41 | 29.05 | 76.97 | 73.13 | 16.53 | 15.50 | 8.52 | 8.31 |
| Surubim | 0.646 | 18.25 | 18.03 | 47.78 | 45.40 | 10.26 | 9.62 | 5.29 | 5.16 |
| Tucunaré | 0.706 | 16.70 | 16.50 | 43.72 | 41.54 | 9.39 | 8.81 | 4.84 | 4.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Vasconcellos, A.C.S.; Ferreira, S.R.B.; de Sousa, C.C.; de Oliveira, M.W.; de Oliveira Lima, M.; Basta, P.C. Health Risk Assessment Attributed to Consumption of Fish Contaminated with Mercury in the Rio Branco Basin, Roraima, Amazon, Brazil. Toxics 2022, 10, 516. https://doi.org/10.3390/toxics10090516
de Vasconcellos ACS, Ferreira SRB, de Sousa CC, de Oliveira MW, de Oliveira Lima M, Basta PC. Health Risk Assessment Attributed to Consumption of Fish Contaminated with Mercury in the Rio Branco Basin, Roraima, Amazon, Brazil. Toxics. 2022; 10(9):516. https://doi.org/10.3390/toxics10090516
Chicago/Turabian Stylede Vasconcellos, Ana Claudia Santiago, Sylvio Romério Briglia Ferreira, Ciro Campos de Sousa, Marcos Wesley de Oliveira, Marcelo de Oliveira Lima, and Paulo Cesar Basta. 2022. "Health Risk Assessment Attributed to Consumption of Fish Contaminated with Mercury in the Rio Branco Basin, Roraima, Amazon, Brazil" Toxics 10, no. 9: 516. https://doi.org/10.3390/toxics10090516
APA Stylede Vasconcellos, A. C. S., Ferreira, S. R. B., de Sousa, C. C., de Oliveira, M. W., de Oliveira Lima, M., & Basta, P. C. (2022). Health Risk Assessment Attributed to Consumption of Fish Contaminated with Mercury in the Rio Branco Basin, Roraima, Amazon, Brazil. Toxics, 10(9), 516. https://doi.org/10.3390/toxics10090516








