Thermal Remediation of Soil Contaminated with Polycyclic Aromatic Hydrocarbons: Pollutant Removal Process and Influence on Soil Functionality
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment
2.3. Soil Properties Analyses
2.4. Data Analysis
3. Results and Discussion
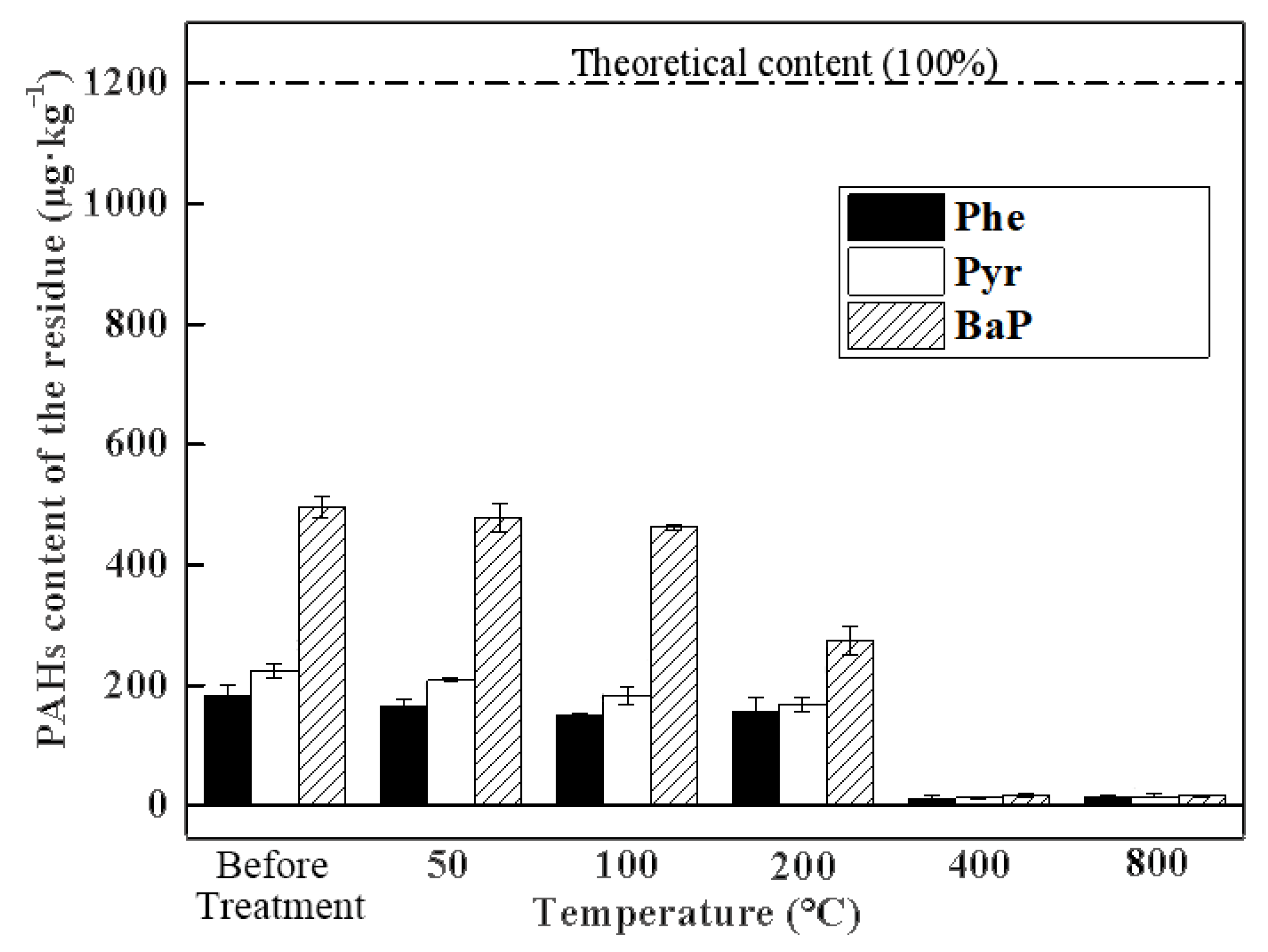
3.1. Efficiency of PAH Removal from Soil by Thermal Remediation
3.2. Thermal Kinetics of PAH-Contaminated Soil
3.3. Changes in Soil Properties after Thermal Remediation
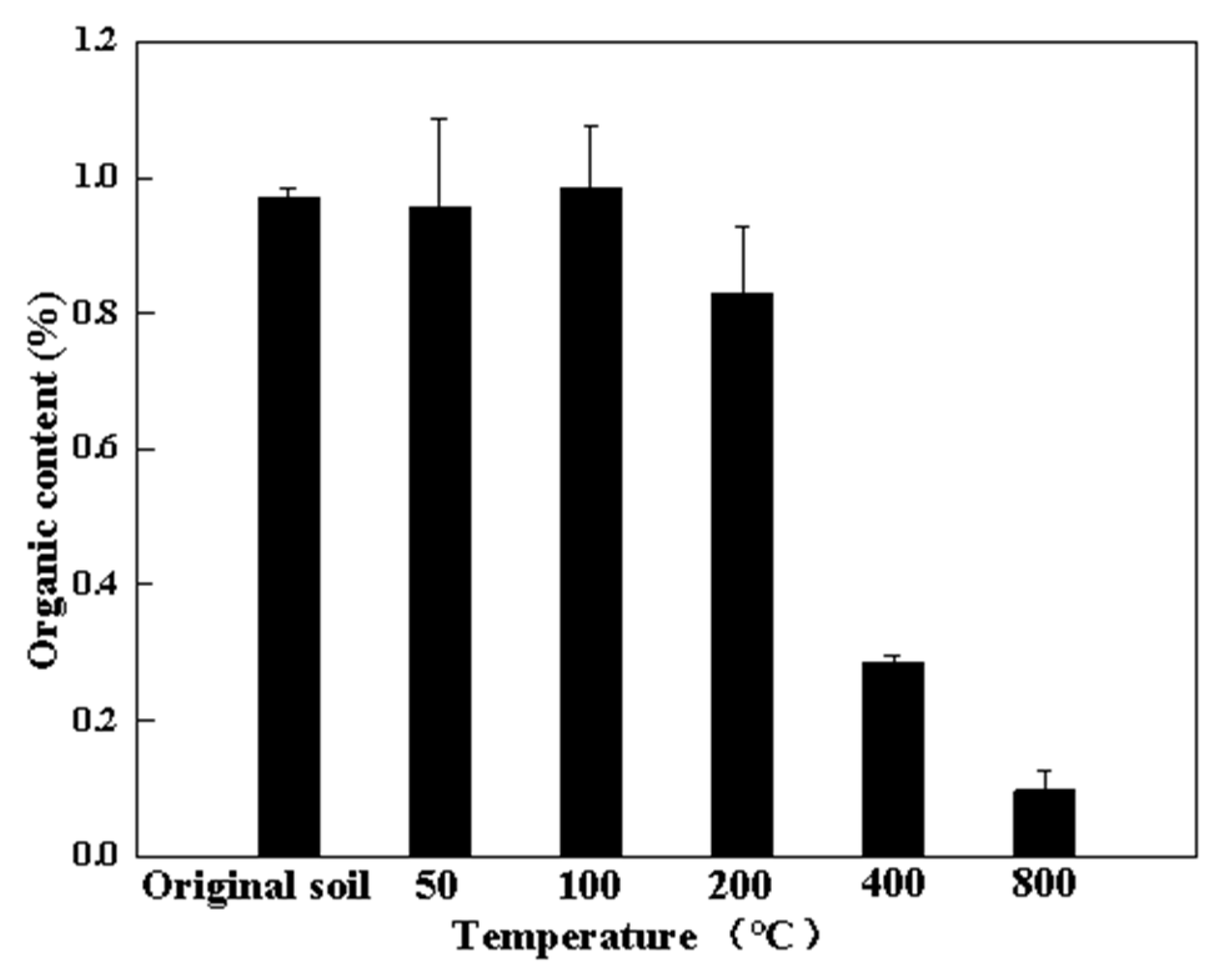
3.3.1. Organic Matter
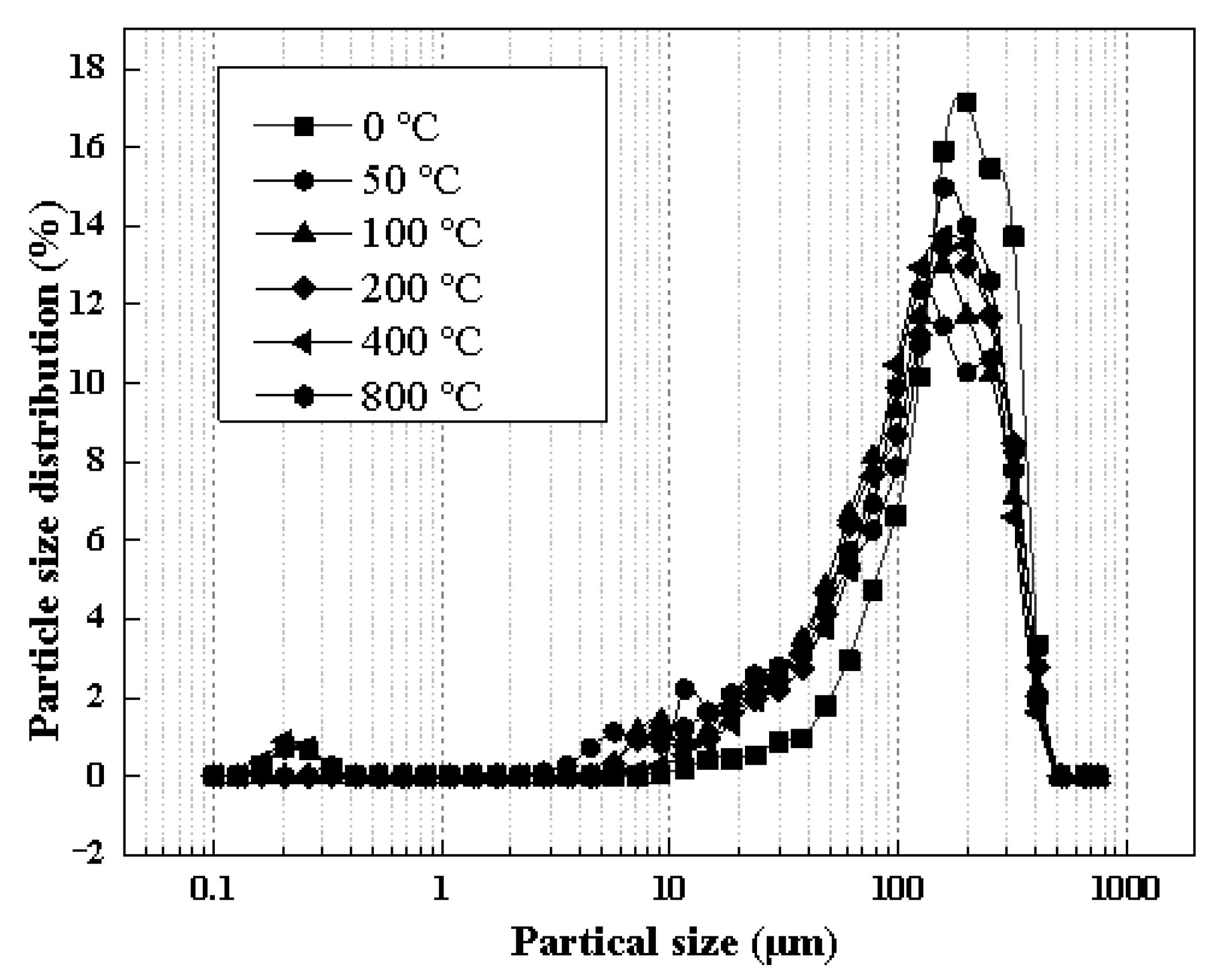
3.3.2. Physical Properties
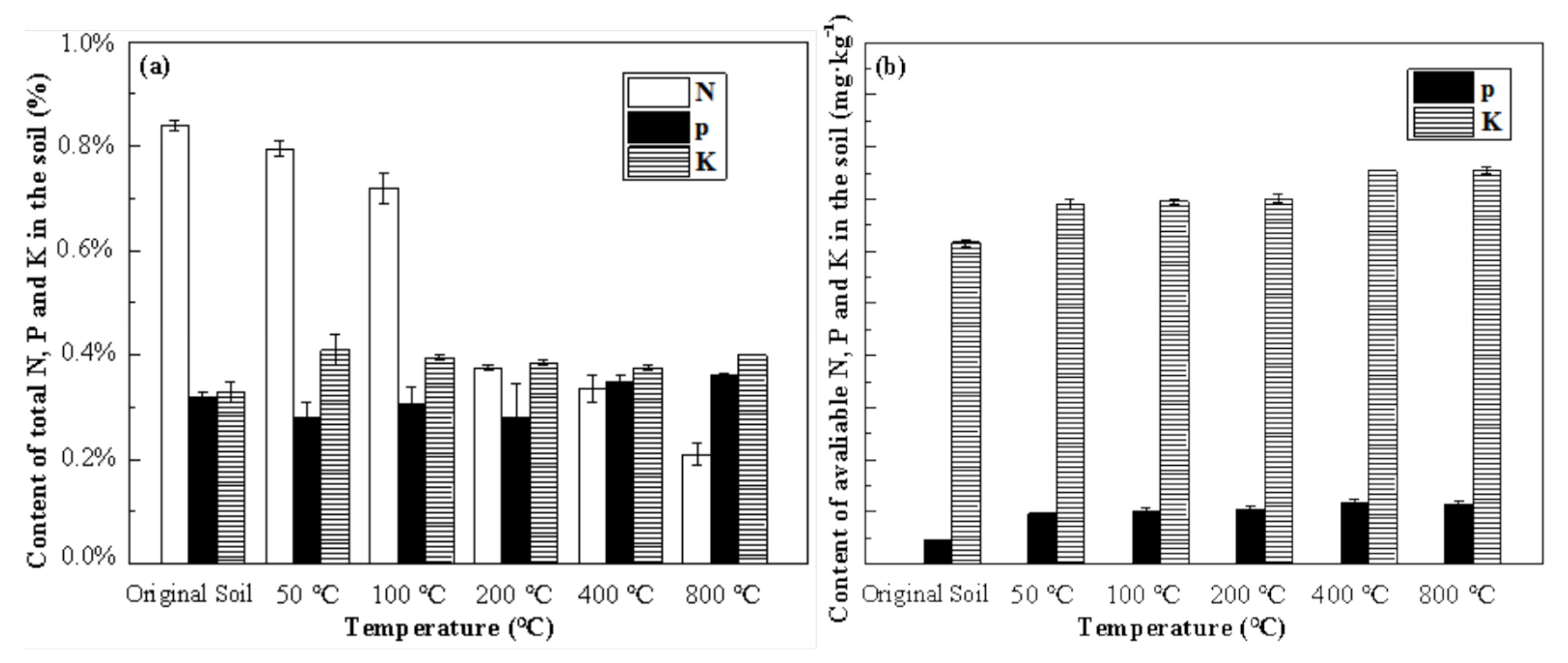
3.3.3. Bioavailability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mastral, A.M.; Callén, M.S. A Review on Polycyclic Aromatic Hydrocarbon (PAH) Emissions fm Energy Generation. Environ. Sci. Technol. 2000, 34, 3051–3057. [Google Scholar] [CrossRef]
- Kamiya, Y.; Kameda, T.; Ohura, T.; Tohno, S. Determination of Particle-Associated PAH Derivatives (ClPAHs, NPAHs, OPAHs) in Ambient Air and Automobile Exhaust by Gas Chromatography/Mass Spectrometry with Negative Chemical Ionization. Polycycl. Aromat. Compd. 2016, 37, 128–140. [Google Scholar] [CrossRef]
- Kwon, E.; Castaldi, M.J. Investigation of mechanisms of polycyclic aromatic hydrocarbons (PAHs) initiated from the thermal degradation of styrene butadiene rubber (SBR) in N2 atmosphere. Environ. Sci. Technol. 2008, 42, 2175–2180. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, B.; Guo, P.; Wang, S.; Guo, S. Bioremediation of polycyclic aromatic hydrocarbons contaminated soil under the superimposed electric field condition. Chemosphere 2020, 273, 128723. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.V.; Gan, S.; Ng, H.K.; EongPoh, P. Extraction agents for the removal of polycyclic aromatic hydrocarbons (PAHs) from soil in soil washing technologies. Environ. Pollut. 2014, 184, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Lau, E.; Ng, H. Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. 2009, 172, 532–549. [Google Scholar] [CrossRef]
- Ranc, B.; Faure, P.; Croze, V.; Lorgeoux, C.; Simonnot, M.-O. Comparison of the effectiveness of soil heating prior or during in situ chemical oxidation (ISCO) of aged PAH-contaminated soils. Environ. Sci. Pollut. Res. 2017, 24, 11265–11278. [Google Scholar] [CrossRef]
- Chou, J.D.; Wey, M.Y.; Chang, S.-H. Study on Pb and PAHs Emission Levels of Heavy Metals and PAHs-Contaminated Soil during Thermal Treatment Process. J. Environ. Eng. 2010, 136, 112–118. [Google Scholar] [CrossRef]
- Qi, Z.; Tong, C.; Ba, S.; Yan, M.; Lu, S.; Buekens, A.; Yan, J.; Bulmău, C.; Li, X. Effect of temperature and particle size on the thermal desorption of PCBs from contaminated soil. Environ. Sci. Pollut. Res. 2014, 21, 4697–4704. [Google Scholar] [CrossRef]
- Yi, Y.M.; Park, S.; Munster, C.; Kim, G.; Sung, K. Changes in Ecological Properties of Petroleum Oil-Contaminated Soil after Low-Temperature Thermal Desorption Treatment. Water Air Soil Pollut. 2016, 227, 108. [Google Scholar] [CrossRef]
- Qiu, R.; Zhang, J.; Dong, Z.; Yu, Z.; Feng, H.; Lai, L. Low-temperature Thermal Desorption of Farmland Soil Contaminated by Mercury. Environ. Sci. Technol. 2014, 37, 48–52. [Google Scholar] [CrossRef]
- Usman, M.; Chaudhary, A.; Biache, C.; Faure, P.; Hanna, K. Effect of thermal pre-treatment on the availability of PAHs for successive chemical oxidation in contaminated soils. Environ. Sci. Pollut. Res. 2015, 23, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, X.; Lv, Z.; Ren, J.; Ding, D.; Lin, N.; Wei, C.; Fu, H. Feasibility of thermal remediation of soil contaminated with PAHs. Chin. J. Environ. Eng. 2018, 12, 2833–2844. [Google Scholar] [CrossRef]
- Vidonish, E.; Zygourakis, K.; Masielloc, C.A.; Sabadelld, G.; Alvarez, P.J.J. Thermal Treatment of Hydrocarbon-Impacted Soils: A Review of Technology Innovation for Sustainable Remediation. Engineering 2016, 2, 426–437. [Google Scholar] [CrossRef]
- Dai, D.W.; Li, C.P.; Tian, L.Z. Thermal Desorption Experiment of Polycyclic Aromatic Hydrocarbons (PAHs) Contaminated Soil Used as Cement Raw Meal. Environ. Eng. 2015, 587–589, 860–864. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Changes in Physical, Chemical, and Microbiological Properties During the Two-Stage Composting of Green Waste due to the Addition of β-cyclodextrin. Compost. Sci. Util. 2019, 27, 41–60. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Giustra, M.G.; Vagliasindi, F. Low-temperature thermal desorption of diesel polluted soil: Influence of temperature and soil texture on contaminant removal kinetics. J. Hazard. Mater. 2011, 185, 392–400. [Google Scholar] [CrossRef]
- Zivder, Z.; Heidarzadeh, N.; Asadollahfardi, G. Remediation of diesel-contaminated soil by low-temperature thermal Remediation of diesel-contaminated soil by low-temperature thermal desorption. Int. J. Environ. Sci. Technol. 2019, 16, 113–6124. [Google Scholar] [CrossRef]
- Ziyu, H.; Jiao, W.; Tian, Y.; Hu, J.; Han, D. Lab-scale removal of PAHs in contaminated soil using electrical resistance heating: Removal efficiency and alteration of soil properties. Chemosphere 2020, 239, 124496. [Google Scholar] [CrossRef]
- Jiao, F.; Shi, X.-R.; Han, F.-P.; Yuan, Z.-Y. Increasing aridity, temperature and soil pH induce soil C-N-P imbalance in grasslands. Sci. Rep. 2016, 6, 19601. [Google Scholar] [CrossRef]
- Han, Z.; Guo, Z.; Zhang, Y.; Xiao, X.; Xu, Z.; Sun, Y. Pyrolysis Characteristics of Biomass Impregnated with Cadmium, Copper and Lead: Influence and Distribution. Waste Biomass Valoriz. 2017, 9, 1223–1230. [Google Scholar] [CrossRef]
- Luo, L.; Lin, S.; Huang, H.; Zhang, S. Relationships between aging of PAHs and soil properties. Environ. Pollut. 2012, 170, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.L.; Zhang, Y.; Selin, N.E. Climate change and emissions impacts on atmospheric PAH transport to the Arctic. Environ. Sci. Technol. 2014, 48, 429–437. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.L.; DeSutter, T.M.; Casey, F.X.M.; Khan, E.; Wick, A.F. Thermal remediation alters soil properties—A review-science direct. J. Environ. Manag. 2018, 206, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.W.; Fu, J.; Suuberg, E.M. Thermodynamics of Multicomponent PAH Mixtures and Development of Tar-Like Behavior. Ind. Eng. Chem. Res. 2011, 50, 3613–3620. [Google Scholar] [CrossRef] [PubMed]
- Piña, J.; Merino, J.; Errazu, A.F.; Bucalá, V. Thermal treatment of soils contaminated with gas oil: Influence of soil composition and treatment temperature. J. Hazard. Mater. 2002, 94, 273–290. [Google Scholar] [CrossRef]
- Schaumann, G.E.; Antelmann, O. Thermal characteristics of soil organic matter measured by DSC: A hint on a glass transition. J. Plant Nutr. Soil Sci. 2015, 163, 179–181. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, L. Effect of soil components on the surfactant-enhanced soil sorption of PAHs. J. Soils Sediments 2012, 12, 161–168. [Google Scholar] [CrossRef]
- Wei, M.; Xia, T.; Jiang, L.; Yao, Y.; Jia, X.; Liu, H. Occurrence characteristics of PAHs in different particle size of soil from a coking plant. Ecol. Environ. Sci. 2013, 22, 863–869. [Google Scholar] [CrossRef]
- Esfandbod, M.; Forghani, A.; Adhami, E.; Rashti, M.R. The Role of CEC and pH in Cd Retention from Soils of North of Iran. J. Soil Contam. 2011, 20, 908–920. [Google Scholar] [CrossRef][Green Version]
- Guppy, C.N.; Menzies, N.W.; Moody, P.W.; Blamey, F.P.C. Competitive sorption reactions between phosphorus and organic matter in soil: A review. Aust. J. Soil Res. 2005, 43, 189–202. [Google Scholar] [CrossRef]
- Jalali, M.; Ranjbar, F. Rates of decomposition and phosphorus release from organic residues related to residue composition. J. Plant Nutr. Soil Sci. 2010, 172, 353–359. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, J.; Gao, Z.; Chen, S. Study on Quantitative Distribution of Soil Microorganism and Relationship with Enzyme Activity and Physical, Chemical Property of Shelter-forest in Rocky Coastal Area. For. Res. 2002, 15, 88–95. [Google Scholar] [CrossRef]



| Properties | Organic Matter (%) | pH | CEC (cmol·kg−1) | Urease Activity (NH3-Nmg·g−1) | Nutrient (%) | Particle Size (μm) | Specific Surface Area (m3·kg−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 h | 24 h | N | P | K | D90 | D75 | D50 | |||||
| Soil | 0.93 | 7.7 | 3.05 | 61.25 | 364.89 | 0.0084 | 0.0032 | 0.0033 | 312.42 | 242.411 | 168.247 | 496.43 |
| Compound | Molecular Weight (g·mol−1) | Boiling Point (°C) | Heat of Vaporization (kJ·mol−1) | Chemical Structure |
|---|---|---|---|---|
| Phenanthrene (Phe) | 178 | 340 | 52.7 | 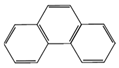 |
| Pyrene (Pyr) | 202 | 404 | 65.8 | 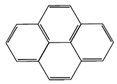 |
| Benzo(a)pyrene (BaP) | 252 | 495 | 71.1 | 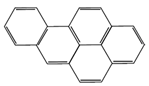 |
| Experimental Group | CK | UC | Phe | Pyr | BaP |
|---|---|---|---|---|---|
| Treatment | Original soil after drying and removing impurities | Impregnated by 5% acetonitrile solution, and aged in cool dry place for two weeks before thermal treatment. | Impregnated by Phe solution and aged | Impregnated by Pyre solution and aged | Impregnated by BaP solution and aged |
| Stage | Duration | Phenomenon and Mechanism |
|---|---|---|
| Aging | Until heating | The PAH content was much lower than the original value, especially for Phe and Pyr due to the semi-volatile properties and smaller molecular size. |
| Desorption | Under 200 °C | The content of Phe and Pyr decreased slightly, while more than 15% of the BaP lost in this stage can be regarded as continuing of aging of persistent pollutant. |
| Decomposition | 200 °C to 400 °C | Boiling point of the PAHs was approximately 400 °C, and the majority of the organic matter was decomposed into low molecular weight matter, which can promote volatilization of the pollutants. Therefore, the main loss of the heating process occurred in this stage. |
| Ashing | Over 400 °C | The color of the soil become gray due to ashing of the organic carbon, whereas the PAH content did not obviously change. |
| Soil | Correlation Coefficient | Activation Energy (kJ·mol−1) |
|---|---|---|
| CK | 98.56 | 1337.02 |
| UC | 98.33 | 1324.12 |
| Phe | 98.78 | 1217.33 |
| Pyr | 96.12 | 1101.24 |
| BaP | 99.54 | 1231.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Shi, H.; Wang, C.; Fei, Y.; Han, Z. Thermal Remediation of Soil Contaminated with Polycyclic Aromatic Hydrocarbons: Pollutant Removal Process and Influence on Soil Functionality. Toxics 2022, 10, 474. https://doi.org/10.3390/toxics10080474
Liu C, Shi H, Wang C, Fei Y, Han Z. Thermal Remediation of Soil Contaminated with Polycyclic Aromatic Hydrocarbons: Pollutant Removal Process and Influence on Soil Functionality. Toxics. 2022; 10(8):474. https://doi.org/10.3390/toxics10080474
Chicago/Turabian StyleLiu, Chenfeng, Huading Shi, Chen Wang, Yang Fei, and Ziyu Han. 2022. "Thermal Remediation of Soil Contaminated with Polycyclic Aromatic Hydrocarbons: Pollutant Removal Process and Influence on Soil Functionality" Toxics 10, no. 8: 474. https://doi.org/10.3390/toxics10080474
APA StyleLiu, C., Shi, H., Wang, C., Fei, Y., & Han, Z. (2022). Thermal Remediation of Soil Contaminated with Polycyclic Aromatic Hydrocarbons: Pollutant Removal Process and Influence on Soil Functionality. Toxics, 10(8), 474. https://doi.org/10.3390/toxics10080474





