Histological Identification and Quantification of Eosinophils and Ascites in Leghorn Chickens Treated with High Oral Concentrations of NaCl–Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals for Experimentation
2.2. Preparation of Food and Drinking Water with NaCl
- − Food with 3.3% NaCl: To the commercial feed (J. Baker Laboratory, Edo. Mex.), 3% NaCl was added to obtain a total final concentration of 3.3%.
- − Drinking water with 1% NaCl: NaCl (1%) was added to the drinking water.
2.3. Design of the Two Experiments
2.4. Obtaining the Samples
2.5. Histology
2.6. Identification of Granulocytes
2.7. Leukogram
2.8. Evaluation of the Tissue Samples
Histological Evaluation
2.9. Leukogram Evaluation
2.10. Hypertrophy of the Right Ventricle Index (HRVI)
2.11. Statistical Analysis
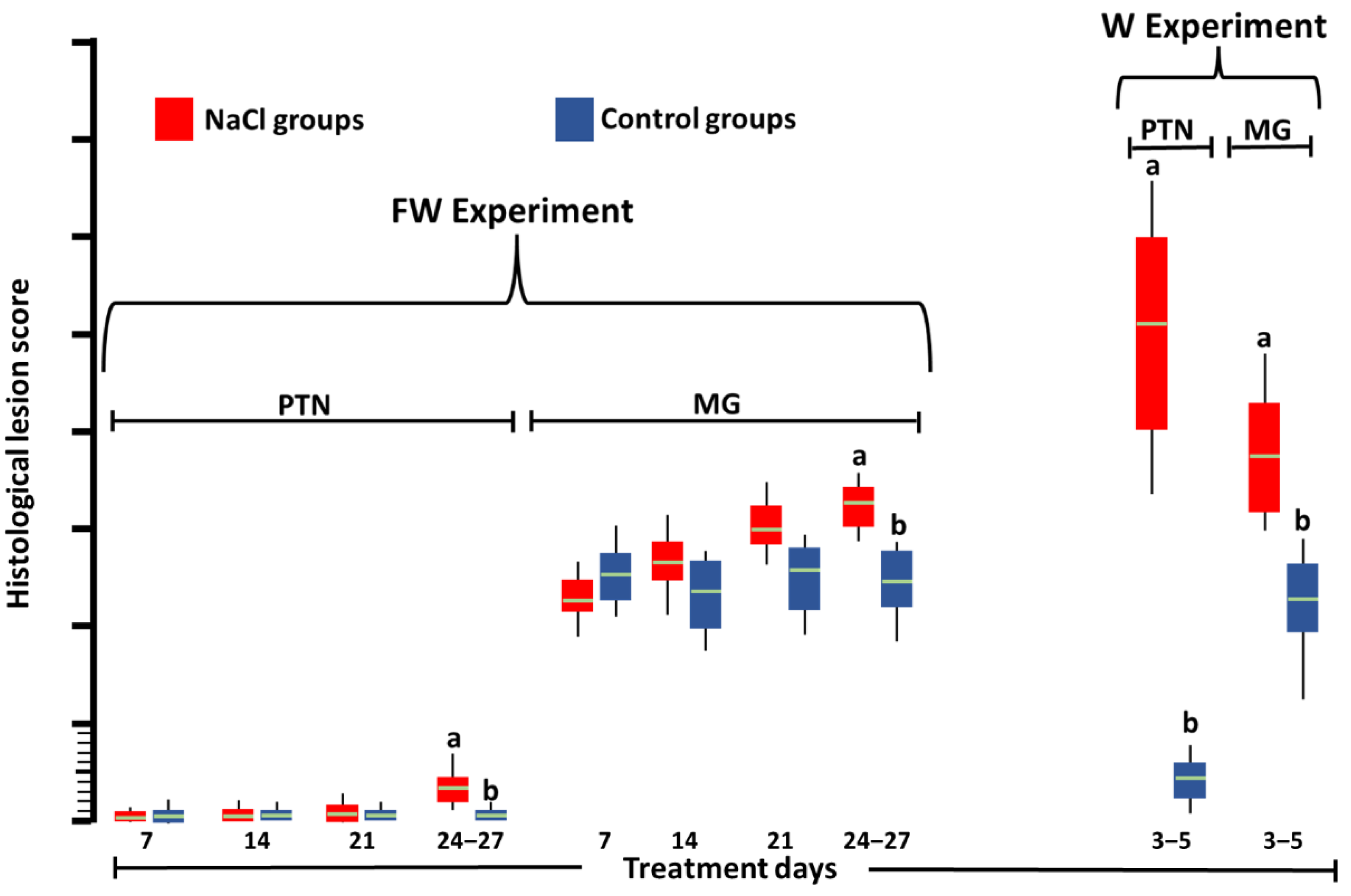
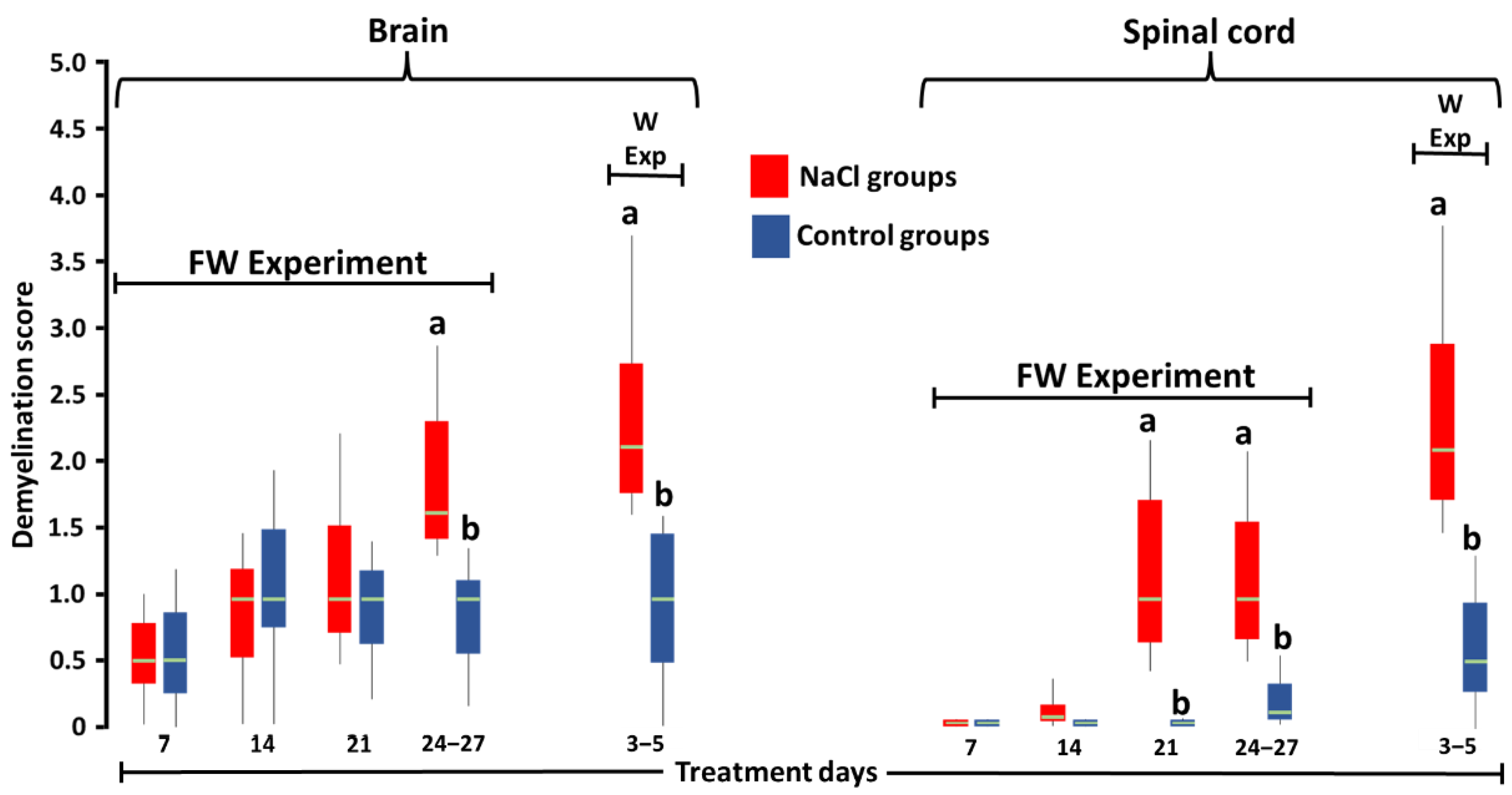
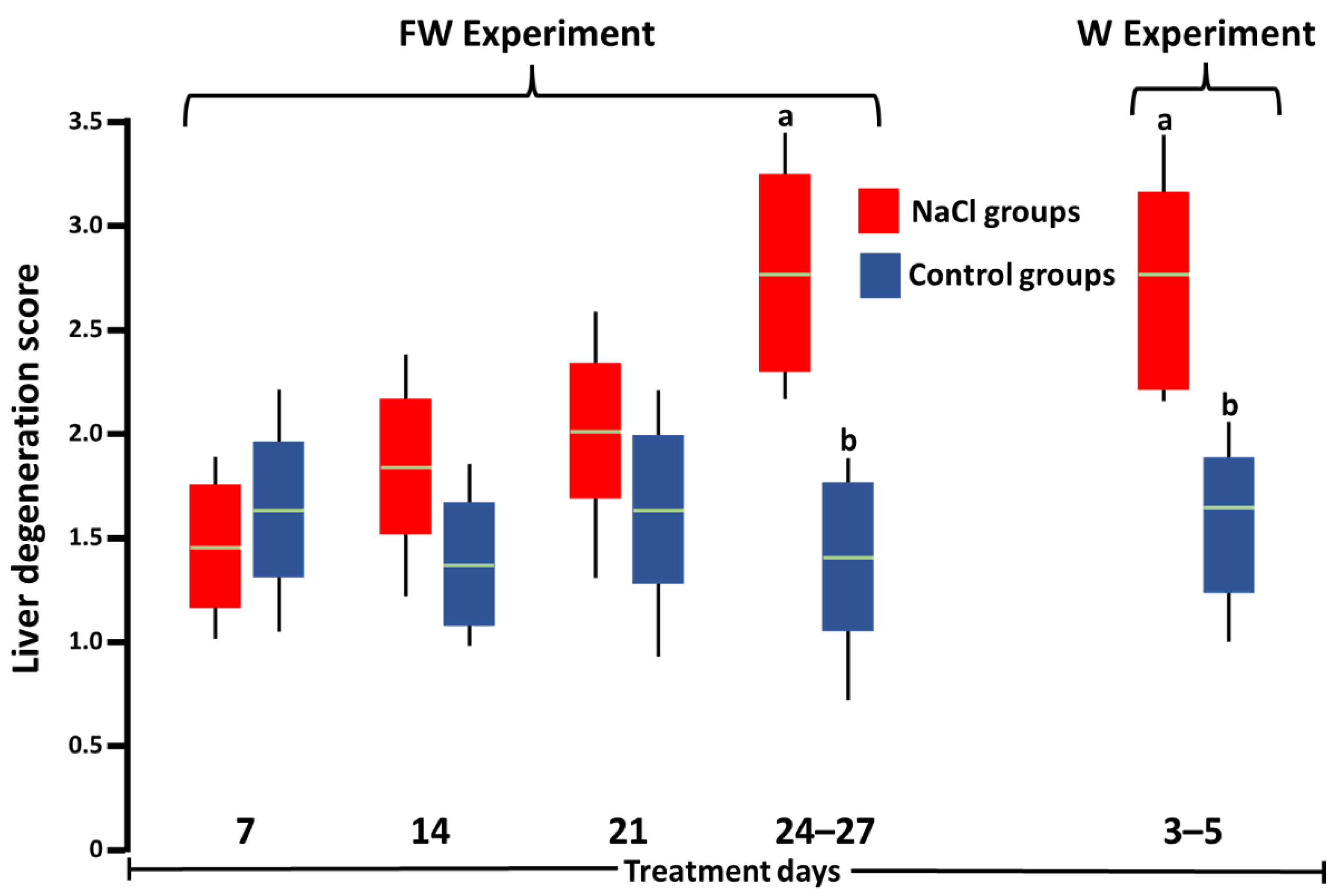
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Constable, P.D.; Hinchcliff, K.W.; Done, S.H.; Grünberg, W. Diseases of the Nervous System. In Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs, and Goats; Elsevier: St. Louis, MO, USA, 2017; pp. 1155–1370. ISBN 978-0-7020-7057-0. [Google Scholar]
- Madson, D.M.; Arruda, P.H.E.; Arruda, B.L. Nervous and Locomotor System. In Diseases of Swine; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Jianqiang, Z., Eds.; Wiley-Blackwell/American Association of Swine Veterinarians: Hoboken, NJ, USA, 2019; pp. 339–372. ISBN 978-1-119-35090-3. [Google Scholar]
- Patson, C.; Sladky, K.K. Sodium intoxication in a domestic rabbit. J. Exot. Pet Med. 2020, 35, 114–116. [Google Scholar] [CrossRef]
- Fulton, R.M. Toxins and Poisons. In Diseases of Poultry; Swayne, D.E., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 1349–1382. ISBN 978-1-119-37115-1. [Google Scholar]
- Carlberg, D.J.; Borek, H.A.; Syverud, S.A.; Holstege, C.P. Survival of Acute Hypernatremia Due to Massive Soy Sauce Ingestion. J. Emerg. Med. 2013, 45, 228–231. [Google Scholar] [CrossRef]
- Metheny, N.A.; Krieger, M.M. Salt Toxicity: A Systematic Review and Case Reports. J. Emerg. Nurs. 2020, 46, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Khan, M.Z.; Zaman, Q.; Muhammad, G. Effect of Concurrent Feeding of Furazolidone and Sodium Chloride upon some Clinical, Pathological and Cardiac Morphometric Parameters in Broiler Chicks. J. Vet. Med. Ser. A 1995, 42, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Julian, R.J. The effect of increased sodium in the drinking water on right ventricular hypertrophy, right ventricular failure and ascites in broiler chickens. Avian Pathol. 1987, 16, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Montali, R.J. Comparative pathology of inflammation in the higher vertebrates (reptiles, birds and mammals). J. Comp. Pathol. 1988, 99, 1–26. [Google Scholar] [CrossRef]
- Petrone, V.M.; Constantino, C.F.; Pradal-Roa, P. Identification and quantification of granulocytes in caecal mucosa and submucosa of chickens experimentally infected with Eimeria tenella and Salmonella Enteritidis. Br. Poult. Sci. 2002, 43, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.J.; Fletcher, O.J. Hemic System. In Avian Histopathology; Abdul-Aziz, T., Fletcher, O.J., Barnes, H.J., Eds.; American Association of Avian Pathologists, Inc.: Jacksonville, FL, USA, 2016; pp. 1–16. [Google Scholar]
- Baze, W.B. Available online: https://www.proquest.com/openview/9fc87a4a172c5dba9225f80e20b5f9c7/1?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 20 May 2022).
- Wages, D.P.; Ficken, M.D.; Cook, M.E.; Mitchell, J. Salt Toxicosis in Commercial Turkeys. Avian Dis. 1995, 39, 158. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, G.C.; West, J.L. Pathologic Features of Experimental Sodium Chloride Poisoning in Chicks. Avian Dis. 1969, 13, 762. [Google Scholar] [CrossRef]
- Osweiler, G.D.; Carr, T.F.; Sanderson, T.P.; Carson, T.L.; Kinker, J.A. Water Deprivation-Sodium Ion Toxicosis in Cattle. J. Vet. Diagn. Investig. 1995, 7, 583–585. [Google Scholar] [CrossRef] [Green Version]
- Puette, M.; Crowell, W.A. Histologic and Morphometric Examination of Avian Glomeruli from Normal and Swollen Kidneys of Broilers at Slaughter. Avian Dis. 1993, 37, 874. [Google Scholar] [CrossRef] [PubMed]
- Siller, W.G. Renal pathology of the fowl—A review. Avian Pathol. 1981, 10, 187–262. [Google Scholar] [CrossRef] [PubMed]
- Cantile, C.; Youssef, S. Nervous System. In Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals; Maxie, M.G., Ed.; Elsevier: St. Louis, MO, USA, 2016; Volume 1, pp. 251–406. ISBN 978-0-7020-5322-1. [Google Scholar]
- Trainer, D.O.; Karstad, L. Salt poisoning in Wisconsin wildlife. J. Am. Vet. Med. Assoc. 1960, 136, 14–17. [Google Scholar] [PubMed]
- Julian, R.J. Ascites in poultry. Avian Pathol. 1993, 22, 419–454. [Google Scholar] [CrossRef]
- Mirsalimi, S.M.; O’Brien, P.J.; Julian, R.J. Changes in erythrocyte deformability in NaCl-induced right-sided cardiac failure in broiler chickens. Am. J. Vet. Res. 1992, 53, 2359–2363. [Google Scholar]
- Salguero Cruz, S.C.; de Lima, M.R.; Vieira Gonçalves, D. Requerimientos Nutricionales de las Aves. In Tablas Brasileñas para Aves y Cerdos; Rostagno, H.S., Ed.; Universidad Federal de Viçosa: Viçosa, Brazil, 2017; p. 488. ISBN 978-85-8179-122-7. [Google Scholar]
- Leary, S.L.; Underwood, W.; Anthony, R.; Grandin, T.; Greenacre, C.; Gwaltney-Brant, S.; McCrackin, M.A.; Meyer, R.; Miller, D.; Shearer, J.; et al. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition; American Veterinary Medical Association: Schaumburg, IL, USA, 2020; ISBN 978-1-882691-09-8. [Google Scholar]
- Luna, G.L. Manual of Histologic Staining Methods of the Armed Forces. Institute of Pathology, 3rd ed.; Mc Graw-Hill Co.: New York, NY, USA, 1968. [Google Scholar]
- Hanker, J.S.; Yates, P.E.; Metz, C.B.; Rustioni, A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem. J. 1977, 9, 789–792. [Google Scholar] [CrossRef]
- Campbell, T.W. Avian Hematology and Cytology, 2nd ed.; Iowa State University Press: Ames, IA, USA, 1995; ISBN 978-0-8138-2970-8. [Google Scholar]
- Martindale, L. The effect of high dietary sodium chloride on renal function in chicks. Br. Poult. Sci. 1975, 16, 577–581. [Google Scholar] [CrossRef]
- Sokkar, S.M.; Hussein, B.M.; Mohammed, M.A. Renal lesions in baby chicks due to sodium chloride poisoning. Avian Pathol. 1983, 12, 277–285. [Google Scholar] [CrossRef]
- Nishimura, H.; Koseki, C.; Imai, M.; Braun, E.J. Sodium chloride and water transport in the thin descending limb of Henle of the quail. Am. J. Physiol. -Ren. Physiol. 1989, 257, F994–F1002. [Google Scholar] [CrossRef]
- Sinovek, Z.; Petrovic, R.; Javanovic, N. Kryna slika japanskih prepelica hranjenih smesama sa povecanim sadrzajem soli. Peradarstvo 1992, 27, 37–41. [Google Scholar]
- Ilhan, N.; Daglioglu, M.C.; Ilhan, O.; Coskun, M.; Tuzcu, E.A.; Kahraman, H.; Keskin, U. Assessment of Neutrophil/Lymphocyte Ratio in Patients with Age-related Macular Degeneration. Ocul. Immunol. Inflamm. 2015, 23, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Brigotti, M. The Interactions of Human Neutrophils with Shiga Toxins and Related Plant Toxins: Danger or Safety? Toxins 2012, 4, 157–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huchzermeyer, F.W.; De Ruyck, A.C.; Van Ark, H. Broiler pulmonary hypertension syndrome. III. Commercial broiler strains differ in their susceptibility. Onderstepoort J. Vet. Res. 1988, 55, 5–9. [Google Scholar] [PubMed]
- Lubritz, D.L.; Smith, J.L.; Mcpherson, B.N. Heritability of Ascites and the Ratio of Right to Total Ventricle Weight in Broiler Breeder Male Lines. Poult. Sci. 1995, 74, 1237–1241. [Google Scholar] [CrossRef]

| Sampling Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| (Treatment Day) | |||||||||
| Experiment | Group | Treatment | 3 | 4 | 5 | 7 | 14 | 21 | 24–27 |
| FW | 1 | NaCl (food, water) 1 | - | - | - | 45 n | 45 | 45 | 21 2 |
| 2 (control group) | Drinking water and 0.3% NaCl in food 3 | - | - | - | 45 | 45 | 45 | 21 | |
| W | 1 | 1% NaCl in water 4 | 9 5 | 12 | 24 | - | - | - | - |
| 2 (control group) | Drinking water and 0.3% NaCl in food 3 | 9 | 12 | 24 | - | - | - | - | |
| Groups | Total | Lymphocytes | Monocytes | Heterophiles | Eosinophils | Basophils |
|---|---|---|---|---|---|---|
| FW experiment (27 days td) | 12522.08 ± 9192.40 (100%) | 6003.77 ± 5201.13 (47.95%) | 1000.21 ± 686.64 (7.99%) | 5029.37 ± 3266.48 (40.16%) a | 255.11 ± 353.86 (2.04%) | 233.62 ± 198.19 (1.87%) a |
| Control | 7892.19 ± 4428.99 (100%) | 5060.03 ± 2603.80 (64.11%) | 596.97 ± 363.44 (7.56%) | 2138.13 ± 1197.72 (27.09%) b | 45.76 ± 51.93 (0.58%) | 51.3 ± 88.11 (0.65%) b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrone-Garcia, V.M.; Castellanos-Huerta, I.A.; El-Ashram, S.; Juárez-Estrada, M.A.; Fuente-Martínez, B.; Graham, D.B.; Tellez-Isaias, G. Histological Identification and Quantification of Eosinophils and Ascites in Leghorn Chickens Treated with High Oral Concentrations of NaCl–Pilot Study. Toxics 2022, 10, 381. https://doi.org/10.3390/toxics10070381
Petrone-Garcia VM, Castellanos-Huerta IA, El-Ashram S, Juárez-Estrada MA, Fuente-Martínez B, Graham DB, Tellez-Isaias G. Histological Identification and Quantification of Eosinophils and Ascites in Leghorn Chickens Treated with High Oral Concentrations of NaCl–Pilot Study. Toxics. 2022; 10(7):381. https://doi.org/10.3390/toxics10070381
Chicago/Turabian StylePetrone-Garcia, Victor M., Inkar Alejandro Castellanos-Huerta, Saeed El-Ashram, Marco A. Juárez-Estrada, Benjamin Fuente-Martínez, Danielle B. Graham, and Guillermo Tellez-Isaias. 2022. "Histological Identification and Quantification of Eosinophils and Ascites in Leghorn Chickens Treated with High Oral Concentrations of NaCl–Pilot Study" Toxics 10, no. 7: 381. https://doi.org/10.3390/toxics10070381
APA StylePetrone-Garcia, V. M., Castellanos-Huerta, I. A., El-Ashram, S., Juárez-Estrada, M. A., Fuente-Martínez, B., Graham, D. B., & Tellez-Isaias, G. (2022). Histological Identification and Quantification of Eosinophils and Ascites in Leghorn Chickens Treated with High Oral Concentrations of NaCl–Pilot Study. Toxics, 10(7), 381. https://doi.org/10.3390/toxics10070381









