Is Illicit Substance Use Gender-Specific? The Basic Points of Mental and Health Disorders
Abstract
:1. Introduction
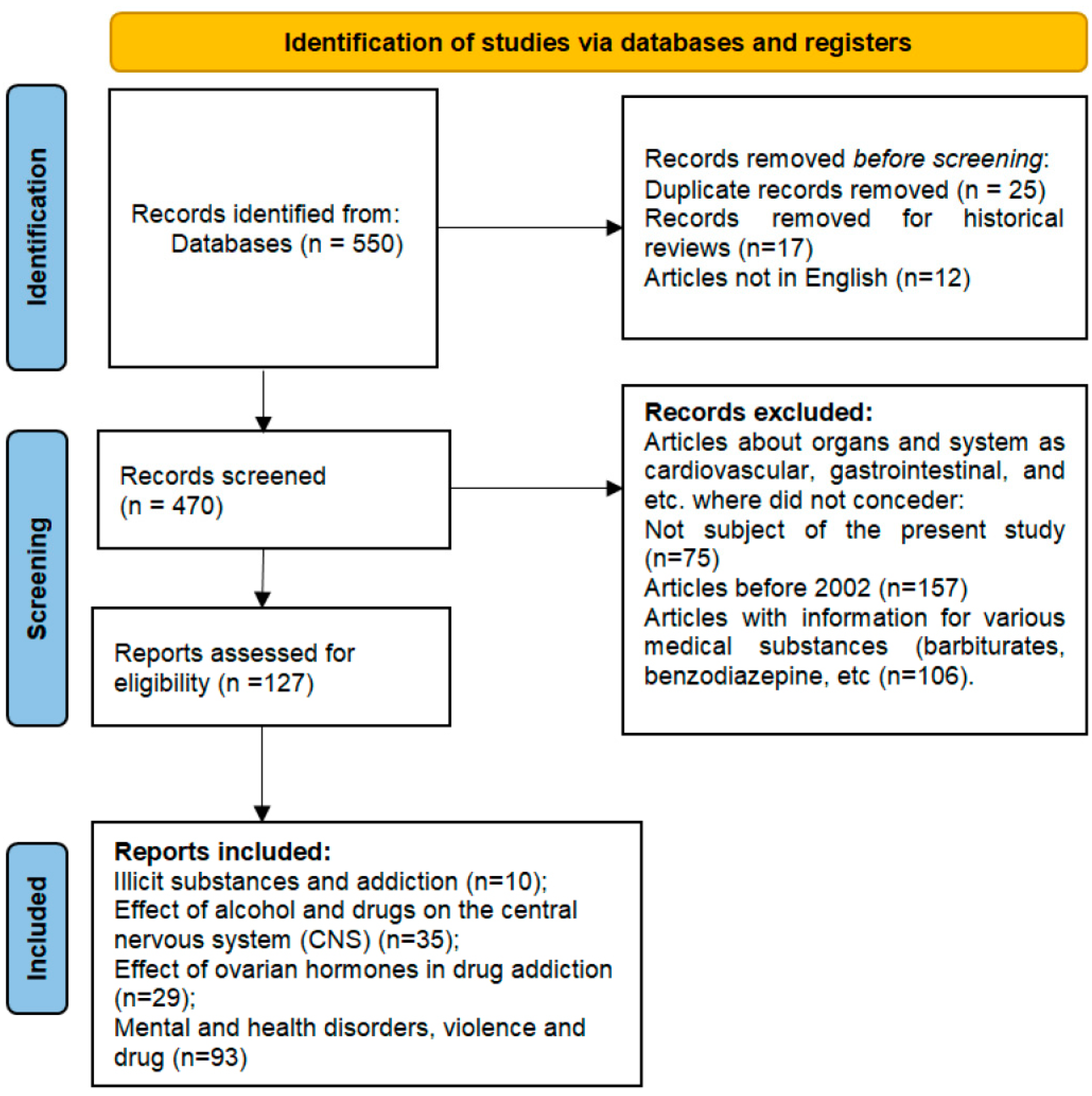
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
3. The Early Childhood Trauma as the Start Points of Mental Disorders and Drugs Use
3.1. Mental and Health Disorders, Violence, and Drugs
3.2. Drugs Use and the Transmission of Infectious Diseases
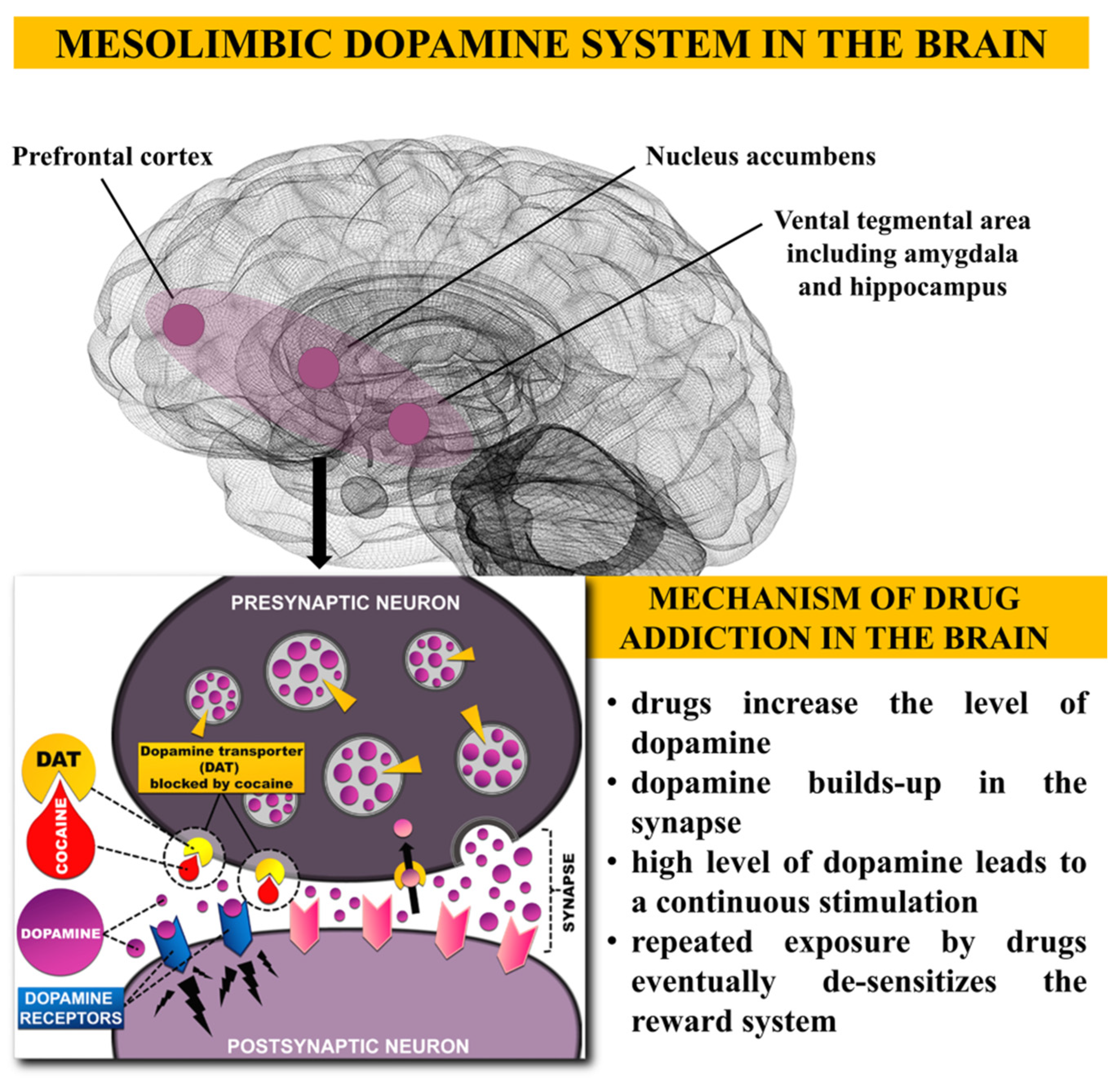
4. Illicit Substances Use and Their Effects on Dopamine Receptors and Brain
4.1. What does Happen in Addicted Human Brain?
4.2. Drug and Alcohol Use, Addiction and Changes in Brain
4.3. Dopamine Neurotransmission
D1- and D2-Like Family of Dopamine Receptors
4.4. Neuroplasticity Changes and Other Pathways in Drug and Alcohol Addiction
5. Does the Choice of Drugs Depend on Gender Biology?
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Degenhardt, L.; Bucello, C.; Calabria, B.; Nelson, P.; Roberts, A.; Hall, W.; Lynskey, M.; Wiessing, L.; GBD; Illicit Drug Use Writing Group. What data are available on the extent of illicit drug use and dependence globally? Results of four systematic reviews. Drug Alcohol Depend. 2011, 117, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Van de Baan, F.C.; Montanari, L.; Royuela, L.; Lemmens, P.H. Prevalence of illicit drug use before imprisonment in Europe: Results from a comprehensive literature review. Drugs Educ. Prev. Pol. 2021, 29, 1–12. [Google Scholar] [CrossRef]
- Murphy, A.; Taylor, E.; Elliott, R. The detrimental effects of emotional process dysregulation on decision-making in substance dependence. Front. Integr. Neurosci. 2012, 6, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, J.B.; McClellan, M.L.; Reed, B.G. Sex differences, gender and addiction. J. Neurosci. Res. 2017, 95, 136–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, A.L.; Becker, L.G.; Huber, A.M.; Catalano, R.F. Review of risk and protective factors of substance use and problem use in emerging adulthood. Addict Behav. 2012, 37, 747–775. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, D.; Asch, S.M.; Curtin, C.; Hah, J.; McDonald, K.M.; Fantini, M.P.; Hernandez-Boussard, T. Opioid abuse and poisoning: Trends in inpatient and emergency department discharges. Health Aff. 2017, 36, 1748–1753. [Google Scholar] [CrossRef]
- Potenza, M.N. Biological contributions to addictions in adolescents and adults: Prevention, treatment, and policy implications. J. Adolesc. Health Off. Publ. Soc. Adolesc. Med. 2013, 52, S22–S32. [Google Scholar] [CrossRef] [Green Version]
- Kroencke, L.; Kuper, N.; Bleidorn, W.; Denissen, J. How Does Substance Use Affect Personality Development? Disentangling Between- and Within-Person Effects. Soc. Psychol. Pers. Sci. 2021, 12, 517–527. [Google Scholar] [CrossRef]
- Cicchetti, D.; Toth, S.L. Child maltreatment. Annu. Rev. Clin. Psychol. 2005, 1, 409–438. [Google Scholar] [CrossRef]
- Capusan, A.J.; Gustafsson, P.A.; Kuja-Halkola, R.; Igelström, K.; Mayo, L.M.; Heilig, M. Re-examining the link between childhood maltreatment and substance use disorder: A prospective, genetically informative study. Mol. Psychiatry 2021, 26, 3201–3209. [Google Scholar] [CrossRef]
- Cloitre, M.; Cohen, L.R.; Ortigo, K.M.; Jackson, C.; Koenen, K.C. Treating Survivors of Childhood Abuse and Interpersonal Trauma: STAIR Narrative Therapy; Guilford Publications: New York, NY, USA, 2020. [Google Scholar]
- Sinha, R. New findings on biological factors predicting addiction relapse vulnerability. Curr. Psychiatry Rep. 2011, 13, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, M.R.; Anderson-White, E.; Ryan, L.J.; Ricardo, M.M.; Krembuszewski, B.A.; Sze, C.; Henderson, C.E. Substance Use Disorders. In Developmental Psychopathology; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 279–310. [Google Scholar] [CrossRef]
- Hyman, S.M.; Paliwal, P.; Chaplin, T.M.; Mazure, C.M.; Rounsaville, B.J.; Sinha, R. Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug Alcohol Depend. 2008, 92, 208–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, N.; Aizpurua, E.; Sánchez, F.C.; Peng, D. Childhood abuse classes for incarcerated men and women: Are there unique gender patterns in abuse classes? J. Interpers. Violence 2020, 37, NP6355–NP6383. [Google Scholar] [CrossRef] [PubMed]
- Giarratano, P.; Ford, J.D.; Nochajski, T.H. Gender differences in complex posttraumatic stress symptoms, and their relationship to mental health and substance abuse outcomes in incarcerated adults. J. Interpers. Violence 2020, 35, 1133–1157. [Google Scholar] [CrossRef]
- Sejdiu, A.; Pereira, K.N.; Joundi, H.; Patel, Y.R.; Basith, S.A.; Ayala, V.; Mathialagan, K.; Majumder, P. Demographic Pattern and Mortality Risk Factors for Prescription Opioid Overdose Hospitalizations: Results from Nationwide Inpatient Sample Analysis. Cureus 2021, 13, 6. [Google Scholar] [CrossRef]
- Hernandez-Avila, C.A.; Rounsaville, B.J.; Kranzler, H.R. Opioid-, cannabis-and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004, 74, 265–272. [Google Scholar] [CrossRef]
- Zanni, G.; DeSalle, M.J.; Deutsch, H.M.; Abagyan, R. Female and male rats readily consume and prefer oxycodone to water in a chronic, continuous access, two-bottle oral voluntary paradigm. Neuropharmacology 2020, 167, 107978. [Google Scholar] [CrossRef]
- Knouse, M.C.; Briand, L.A. Behavioral Sex Differences in Cocaine and Opioid Use Disorders: The Role of Gonadal Hormones. Neurosci. Biobehav. Rev. 2021, 128, 358–366. [Google Scholar] [CrossRef]
- Becker, J.B.; Chartoff, E. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 2019, 44, 166–183. [Google Scholar] [CrossRef] [Green Version]
- Pombo, S.; Da Costa, N.F. On the long-term status of treatment-seeking, heroin addicted patients: A 22-year follow-up study on mortality and drug use in Portugal. Heroin Addict. Relat. Clin. Probl. 2018, 20, 21–39. [Google Scholar]
- Rafaiee, R.; Olyaee, S.; Sargolzaiee, A. The relationship between the type of crime and drugs in addicted prisoners in Zahedan Central Prison. Int. J. High Risk Behav. Addict. 2013, 2, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belenko, S.; Hiller, M.; Hamilton, L. Treating substance use disorders in the criminal justice system. Curr. Psychiatry Rep. 2013, 15, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, M.; Hayhurst, K.; Bird, S.M.; Hickman, M.; Seddon, T.; Dunn, G.; Millar, T. Insights into the link between drug use and criminality: Lifetime offending of criminally-active opiate users. Drug Alcohol Depend. 2017, 179, 309–316. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, A.; Hardwick, R. Behind bars: The truth about drugs in prisons. Forensic Res. Criminol. Int. J. 2017, 5, 00158. [Google Scholar] [CrossRef] [Green Version]
- Montanari, L.; Royuela, L.; Pasinetti, M.; Giraudon, I.; Wiessing, L.; Vicente, J. Drug use and related consequences among prison populations in European countries. In Prisons and Health; WHO Regional Office for Europe: Copenhagen, Denmark, 2014; p. 107. [Google Scholar]
- Reekie, J.M.; Levy, M.H.; Richards, A.H.; Wake, C.J.; Siddall, D.A.; Beasley, H.M.; Kumar, S.; Butler, T.G. Trends in HIV, hepatitis B and hepatitis C prevalence among Australian prisoners-2004, 2007, 2010. Med. J. Aust. 2014, 200, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Khademi, N.; Skakiba, E.; Khoramdad, M. Seroprevalence and related risk behaviors of hepatitis C, hepatitis B and HIV infections among male prisoners in Kermanshah, Iran. Arch. Iran. Med. 2019, 22, 588. [Google Scholar]
- Sabol, W.J.; Couture, H. Prison Inmates at Midyear 2007; Bureau of Justice Statistics: Washington, DC, USA, 2008.
- Semaille, C.; Le Strat, Y.; Chiron, E.; Chemlal, K.; Valantin, M.A.; Serre, P.; Caté, L.; Barbier, C.; Jauffret-Roustide, M. Prevalence of human immunodeficiency virus and hepatitis C virus among French prison inmates in 2010: A challenge for public health policy. Eurosurveillance 2013, 18, 20524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rich, J.D.; Beckwith, C.; Macmadu, A.; Marshall, B.D.; Brinkley-Rubinstein, L.; Amon, J.J.; Milloy, M.J.; King, M.R.; Sanchez, J.; Atwoli, L.; et al. Clinical care of incarcerated people with HIV, viral hepatitis, or tuberculosis. Lancet 2016, 388, 1103–1114. [Google Scholar] [CrossRef] [Green Version]
- Fazel, S.; Baillargeon, J. The health of prisoners. Lancet 2011, 377, 956–965. [Google Scholar] [CrossRef]
- Macalino, G.E.; Hou, J.C.; Kumar, M.S.; Taylor, L.E.; Sumantera, I.G.; Rich, J.D. Hepatitis C infection and incarcerated populations. Int. J. Drug Policy 2004, 15, 103–114. [Google Scholar] [CrossRef]
- Substance Abuse Treatment: Addressing the Specific Needs of Women [Internet]. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2009. (Treatment Improvement Protocol (TIP) Series, No. 51.). Available online: https://www.ncbi.nlm.nih.gov/books/NBK83252/ (accessed on 1 January 2020).
- de Vogel, V.; Stam, J.; Bouman, Y.H.; Ter Horst, P.; Lancel, M. Gender Differences in Substance Abuse History and Offending Behavior: A Multicentre Study in Dutch Forensic Psychiatry. J. Forensic Psychol. Res. Pract. 2021, 22, 1–17. [Google Scholar] [CrossRef]
- El-Bassel, N.; Terlikbaeva, A.; Pinkham, S. HIV and women who use drugs: Double neglect, double risk. Lancet 2010, 376, 312–314. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Phys. Ther. 2009, 89, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, K.; Javadinia, S.A.; Saadat, S.H.; Ramezani, M.A.; Sedghijalal, H. Triangular relationship among risky sexual behavior, addiction, and aggression: A systematic review. Electron. Physician 2017, 9, 5129. [Google Scholar] [CrossRef] [Green Version]
- Hogarth, L.; Martin, L.; Seedat, S. Relationship between childhood abuse and substance misuse problems is mediated by substance use coping motives, in school attending South African adolescents. Drug Alcohol Depend. 2019, 194, 69–74. [Google Scholar] [CrossRef]
- Heyman, G.M. Is Addiction a Chronic, Relapsing Disease? Harvard University Press: Cambridge, MA, USA, 2001; Volume 13, pp. 81–117. [Google Scholar] [CrossRef]
- Kaló, Z. Women Who Use Drugs and Mental Health. In The Impact of Global Drug Policy on Women: Shifting the Needle; Buxton, J., Margo, G., Burger, L., Eds.; Emerald Publishing Limited: Bingley, UK, 2020; pp. 67–74. [Google Scholar] [CrossRef]
- Kerr-Corrêa, F.; Igami, T.Z.; Hiroce, V.; Tucci, A.M. Patterns of alcohol use between genders: A cross-cultural evaluation. J. Affect. Disord. 2007, 102, 265–275. [Google Scholar] [CrossRef]
- Karpyak, V.M.; Geske, J.R.; Hall-Flavin, D.K.; Loukianova, L.L.; Schneekloth, T.D.; Skime, M.K.; Seppala, M.D.; Dawson, G.A.; Frye, M.A.; Choi, D.; et al. Sex-specific association of depressive disorder and transient emotional states with alcohol consumption in male and female alcoholics. Drug Alcohol Depend. 2019, 196, 31–39. [Google Scholar] [CrossRef]
- Cross, D.; Crow, T.; Powers, A.; Bradley, B. Childhood trauma, PTSD, and problematic alcohol and substance use in low-income, African-American men and women. Child Abus. Negl. 2015, 44, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Brady, K.T.; Haynes, L.F.; Hartwell, K.J.; Killeen, T.K. Substance use disorders and anxiety: A treatment challenge for social workers. Soc. Work. Public Health 2013, 28, 407–423. [Google Scholar] [CrossRef] [Green Version]
- Velopulos, C.G.; Carmichael, H.; Zakrison, T.L.; Crandall, M. Comparison of male and female victims of intimate partner homicide and bidirectionality-an analysis of the national violent death reporting system. J. Trauma Acute Care Surg. 2019, 87, 331–336. [Google Scholar] [CrossRef]
- Greenfield, S.F.; Back, S.E.; Lawson, K.; Brady, K.T. Substance abuse in women. Psychiatr. Clin N. Am. 2010, 33, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Valentiner, D.P.; Mounts, N.S.; Deacon, B.J. Panic attacks, depression and anxiety symptoms, and substance use behaviors during late adolescence. J. Anxiety Disord. 2004, 18, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Uhl, G.R. Molecular genetic underpinnings of human substance abuse vulnerability: Likely contributions to understanding addiction as a mnemonic process. Neuropharmacology 2004, 47, 140–147. [Google Scholar] [CrossRef]
- Riley, A.L.; Hempel, B.J.; Clasen, M.M. Sex as a biological variable: Drug use and abuse. Physiol. Behav. 2018, 187, 79–96. [Google Scholar] [CrossRef]
- Parlier-Ahmad, A.B.; Martin, C.E.; Radic, M.; Svikis, D.S. An exploratory study of sex and gender differences in demographic, psychosocial, clinical, and substance use treatment characteristics of patients in outpatient opioid use disorder treatment with buprenorphine. Transl. Issues Psychol. Sci. 2021, 7, 141–153. [Google Scholar] [CrossRef] [PubMed]
- McGeough, B. A systematic review of substance use treatments for sexual minority women. J. Gay Lesbian Soc. Serv. 2021, 33, 180–210. [Google Scholar] [CrossRef]
- Beckwith, C.G.; Zaller, N.D.; Fu, J.J.; Montague, B.T.; Rich, J.D. Opportunities to diagnose, treat, and prevent HIV in the criminal justice system. J. Acquir. Immune Defic. Syndr. 2010, 55, S49. [Google Scholar] [CrossRef] [Green Version]
- Pinkerton, S.D.; Galletly, C.L.; Seal, D.W. Model-based estimates of HIV acquisition due to prison rape. Prison J. 2007, 87, 295–310. [Google Scholar] [CrossRef] [Green Version]
- Arain, A.; Robaeys, G.; Stöver, H. Hepatitis C in European prisons: A call for an evidence-informed response. BMC Infect. Dis. 2014, 14, S17. [Google Scholar] [CrossRef] [Green Version]
- Wells, C.D.; Cegielski, J.P.; Nelson, L.J.; Laserson, K.F.; Holtz, T.H.; Finlay, A.; Castro, K.G.; Weyer, K. HIV infection and multidrug-resistant tuberculosis—the perfect storm. J. Infect. Dis. 2007, 196, S86–S107. [Google Scholar] [CrossRef] [Green Version]
- Spaulding, A.C.; Seals, R.M.; Page, M.J.; Brzozowski, A.K.; Rhodes, W.; Hammett, T.M. HIV/AIDS among inmates of and releasees from US correctional facilities, 2006: Declining share of epidemic but persistent public health opportunity. PLoS ONE 2009, 4, e7558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drobniewski, F.A.; Balabanova, Y.M.; Ruddy, M.C.; Graham, C.; Kuznetzov, S.I.; Gusarova, G.I.; Zakharova, S.M.; Melentyev, A.S.; Fedorin, I.M. Tuberculosis, HIV seroprevalence and intravenous drug abuse in prisoners. Eur. Clin. Respir. J. 2005, 26, 298–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zampino, R.; Coppola, N.; Sagnelli, C.; Di Caprio, G.; Sagnelli, E. Hepatitis C virus infection and prisoners: Epidemiology, outcome and treatment. World J. Hepatol. 2015, 7, 2323. [Google Scholar] [CrossRef] [PubMed]
- Larney, S.; Kopinski, H.; Beckwith, C.G.; Zaller, N.D.; Jarlais, D.D.; Hagan, H.; Rich, J.D.; Van den Bergh, B.J.; Degenhardt, L. Incidence and prevalence of hepatitis C in prisons and other closed settings: Results of a systematic review and meta-analysis. J. Hepatol. 2013, 58, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Okie, S. Sex, drugs, prisons, and HIV. N. Engl. J. Med. 2007, 356, 105–108. [Google Scholar] [CrossRef]
- Calzavara, L.; Ramuscak, N.; Burchell, A.N.; Swantee, C.; Myers, T.; Ford, P.; Fearon, M.; Raymond, S. Prevalence of HIV and hepatitis C virus infections among inmates of Ontario remand facilities. CMAJ 2007, 177, 257–261. [Google Scholar] [CrossRef] [Green Version]
- Dolan, K.; Kite, B.; Black, E.; Aceijas, C.; Stimson, G.V.; Reference Group on HIV/AIDS Prevention and Care among Injecting Drug Users in Developing and Transitional Countries. HIV in prison in low-income and middle-income countries. Lancet Infect. Dis. 2007, 7, 32–41. [Google Scholar] [CrossRef]
- Gough, E.; Kempf, M.C.; Graham, L.; Manzanero, M.; Hook, E.W.; Bartolucci, A.; Chamot, E. HIV and hepatitis B and C incidence rates in US correctional populations and high risk groups: A systematic review and meta-analysis. BMC Public Health 2010, 10, 777. [Google Scholar] [CrossRef] [Green Version]
- McHugh, R.K.; Votaw, V.R.; Sugarman, D.E.; Greenfield, S.F. Sex and gender differences in substance use disorders. Clin. Psychol. Rev. 2018, 66, 12–23. [Google Scholar] [CrossRef]
- Winger, G.; Woods, J.H.; Galuska, C.M.; Wade-Galuska, T. Behavioral perspectives on the neuroscience of drug addiction. Exp. Anal. Hum. Behav. Bull. 2005, 84, 667–681. [Google Scholar] [CrossRef] [Green Version]
- Becker, J.B. Sex differences in addiction. Dialogues Clin. Neurosci. 2016, 18, 395. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.E.; Fontana, B.D.; Bertoncello, K.T.; Franscescon, F.; Mezzomo, N.J.; Canzian, J.; Stefanello, F.V.; Parker, M.O.; Gerlai, R.; Rosemberg, D.B. Understanding the neurobiological effects of drug abuse: Lessons from zebrafish models. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 100, 109873. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.B.; Koob, G.F. Sex differences in animal models: Focus on addiction. Pharm. Rev. 2016, 68, 242–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkow, N.D.; Koob, G.F.; McLellan, A.T. Neurobiologic advances from the brain disease model of addiction. N. Engl. J. Med. 2016, 374, 363–371. [Google Scholar] [CrossRef]
- Marrocu, A.; Giacobbe, J.; Pariante, C.; Borsini, A. The molecular neurobiology of addiction. Behav. Neurosci. Encycl. 2020. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Martinez, D.; Kim, J.H.; Krystal, J.; Abi-Dargham, A. Imaging the neurochemistry of alcohol and substance abuse. Neuroimaging Clin. N. Am. 2007, 17, 539–555. [Google Scholar] [CrossRef]
- US Substance Abuse and Mental Health Services Administration; US Office of the Surgeon General. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health; US Department of Health and Human Services: Washington, DC, USA, 2016.
- Jîtca, G.; Osz, B.E.; Tero-Vescan, A.; Miklos, A.P.; Rusz, C.M.; Batrînu, M.G.; Vari, C.E. Positive Aspects of Oxidative Stress at Different Levels of the Human Body: A Review. Antioxidants 2022, 11, 572. [Google Scholar] [CrossRef]
- NIDA. How does Cocaine Produce Its Effects? Available online: https://nida.nih.gov/publications/research-reports/cocaine/ (accessed on 15 June 2022).
- Duncan, J.R.; Lawrence, A.J. Addiction Neuroethics: The Ethics of Addiction Neuroscience Research and Treatment; Carter, W.A., Hall, J.I., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 27–54. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.J. The addicted human brain viewed in the light of imaging studies: Brain circuits and treatment strategies. Neuropharmacology 2004, 47, 3–13. [Google Scholar] [CrossRef]
- Jones, J.; Comer, S.D. A review of pharmacogenetic studies of substance-related disorders. Drug Alcohol Depend. 2015, 1, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sulzer, D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 2011, 69, 628–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wise, R.A.; Robble, M.A. Dopamine and addiction. Ann. Rev. Psychol. 2020, 71, 79–106. [Google Scholar] [CrossRef] [PubMed]
- Kehr, J.; Ichinose, F.; Yoshitake, S.; Goiny, M.; Sievertsson, T.; Nyberg, F.; Yoshitake, T. (Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br. J. Pharm. 2011, 164, 1949–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jîtcă, G.; Ősz, B.E.; Tero-Vescan, A.; Vari, C.E. Psychoactive Drugs—From Chemical Structure to Oxidative Stress Related to Dopaminergic Neurotransmission. A Review. Antioxidants 2021, 10, 381. [Google Scholar] [CrossRef]
- Auclair, A.; Drouin, C.; Cotecchia, S.; Glowinski, J.; Tassin, J.P. 5-HT2A and α1b-adrenergic receptors entirely mediate dopamine release, locomotor response and behavioural sensitization to opiates and psychostimulants. Eur. J. Neurosci. 2004, 20, 3073–3084. [Google Scholar] [CrossRef]
- Verdejo-Garcia, M.; Pérez-García, M.; Bechara, A. Emotion, decision-making and substance dependence: A somatic-marker model of addiction. Curr. Neuropharmacol. 2006, 4, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, N. Neurotransmitters in alcoholism: A review of neurobiological and genetic studies. Indian J. Hum. Genet. 2014, 20, 20–31. [Google Scholar] [CrossRef] [Green Version]
- Girault, J.A.; Greengard, P. The neurobiology of dopamine signaling. Arch. Neurol. 2004, 61, 641–644. [Google Scholar] [CrossRef] [Green Version]
- Navarro, G.; Moreno, E.; Bonaventura, J.; Brugarolas, M.; Farré, D.; Aguinaga, D.; Mallol, J.; Cortés, A.; Casadó, V.; Lluís, C.; et al. Cocaine inhibits dopamine D2 receptor signaling via sigma-1-D2 receptor heteromers. PLoS ONE 2013, 8, e61245. [Google Scholar] [CrossRef] [Green Version]
- Di Chiara, G.; Bassareo, V.; Fenu, S.; De Luca, M.A.; Spina, L.; Cadoni, C.; Acquas, E.; Carboni, E.; Valentini, V.; Lecca, D. Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology 2004, 47, 227–241. [Google Scholar] [CrossRef]
- Trifilieff, P.; Martinez, D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology 2014, 76, 498–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkov, N.D.; Fowler, J.S.; Wang, G.J.; Baler, R.; Telang, F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology 2009, 56, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Che, T.; Levit, A.; Shoichet, B.K.; Wacker, D.; Roth, B.L. Structure of the D2 dopamine receptor bound to the atypical antipsychotic drug risperidone. Nature 2018, 555, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Addy, N.A.; Solecki, W.B. Clinical syndromes of substance use disorder. In Genomics, Circuits, and Pathways in Clinical Neuropsychiatry; Academic Press: Cambridge, MA, USA, 2016; pp. 619–634. [Google Scholar] [CrossRef]
- Kirby, L.G.; Zeeb, F.D.; Winstanley, C.A. Contributions of serotonin in addiction vulnerability. Neuropharmacology 2011, 61, 421–432. [Google Scholar] [CrossRef] [Green Version]
- Jiao, D.; Liu, Y.; Li, X.; Liu, J.; Zhao, M. The role of the GABA system in amphetamine-type stimulant use disorders. Front. Cell. Neurosci. 2015, 9, 162. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, M.S. Glutamatergic transmission in drug reward: Implications for drug addiction. Front. Neurosci. 2015, 9, 404. [Google Scholar] [CrossRef] [Green Version]
- Nestler, E.J. Review. Transcriptional mechanisms of addiction: Role of DeltaFosB. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2008, 363, 3245–3255. [Google Scholar] [CrossRef] [Green Version]
- Nestler, E.J.; Lüscher, C. The Molecular Basis of Drug Addiction: Linking Epigenetic to Synaptic and Circuit Mechanisms. Neuron 2019, 102, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Nestler, E.J. Transcriptional mechanisms of drug addiction. Clin. Psychopharmacol. Neurosci. 2012, 10, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Hurst, T. World drug report. Encycl. Women Crime 2019, 1–2. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Docea, A.O.; Calina, D.; Tsarouhas, K.; Zamfira, L.M.; Mitrut, R.; Sharifi-Rad, J.; Kovatsi, L.; Siokas, V.; Dardiotis, E.; et al. A mechanistic and pathophysiological approach for stroke associated with drugs of abuse. J. Clin. Med. 2019, 8, 1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, J.B.; Hu, M. Sex differences in drug abuse. Front. Neuroendocrin. 2008, 29, 36–47. [Google Scholar] [CrossRef] [Green Version]
- Huhn, A.S.; Berry, M.S.; Dunn, K.E. Sex-based differences in treatment outcomes for persons with opioid use disorder. Am. J. Addict. 2019, 28, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.; Whisler, K.N.; Fuller, D.C.; Orsulak, P.J.; Self, D.W. Addiction-related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self-administration. Neuropsychopharmacology 2007, 32, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Kohtz, A.S.; Aston-Jones, G. Cocaine seeking during initial abstinence is driven by noradrenergic and serotonergic signaling in hippocampus in a sex-dependent manner. Neuropsychopharmacology 2017, 42, 408–418. [Google Scholar] [CrossRef] [Green Version]
- Drug Abuse Statistic. Available online: https://drugabusestatistics.org/ (accessed on 15 June 2022).
- World Drug Report 2021_Annex–UNODC. Available online: https://www.unodc.org/unodc/en/data-and-analysis/wdr2021.html (accessed on 1 January 2021).
- Volkow, N.D.; Morales, M. The brain on drugs: From reward to addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, A.G. Cannabis and Lung Health: Does the Bad Outweigh the Good? Pulm. Ther. 2021, 7, 395–408. [Google Scholar] [CrossRef]
- Ruisoto, P.; Contador, I. The role of stress in drug addiction. An integrative review. Physiol. Behav. 2019, 202, 62–68. [Google Scholar] [CrossRef]
- Fox, H.C.; Sinha, R. Sex differences in drug-related stress-system changes: Implications for treatment in substance-abusing women. Harv. Rev. Psychiatry 2009, 17, 103–119. [Google Scholar] [CrossRef]
- Poyatos, L.; Torres, A.; Papaseit, E.; Pérez-Mañá, C.; Hladun, O.; Núñez-Montero, M.; De la Rosa, G.; Torrens, M.; Fuster, D.; Muga, R.; et al. Abuse Potential of Cathinones in Humans: A Systematic Review. J. Clin. Med. 2022, 11, 1004. [Google Scholar] [CrossRef]
- Evans, S.M. The role of estradiol and progesterone in modulating the subjective effects of stimulants in humans. Exp. Clin. Psychopharmacol. 2007, 15, 418. [Google Scholar] [CrossRef]
- Fonseca, F.; Robles-Martínez, M.; Tirado-Muñoz, J.; Alías-Ferri, M.; Mestre-Pintó, J.I.; Coratu, A.M.; Torrens, M. A gender perspective of addictive disorders. Curr. Addict. Rep. 2021, 81, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Moran-Santa Maria, M.; Flanagan, J.; Brady, K. Ovarian hormones and drug abuse. Curr. Psychiatry Rep. 2014, 16, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, B.; Hoffman, L.; Garcia, C.C.; Nixon, S.J. Race and socioeconomic status in substance use progression and treatment entry. J. Ethn. Subst. Abus. 2018, 17, 150–166. [Google Scholar] [CrossRef] [PubMed]
- White, T.L.; Justice, A.J.; de Wit, H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol. Biochem. Behav. 2002, 73, 729–741. [Google Scholar] [CrossRef]
- Nicolas, C.; Zlebnik, N.E.; Farokhnia, M.; Leggio, L.; Ikemoto, S.; Shaham, Y. Sex differences in opioid and psychostimulant craving and relapse: A critical review. Pharm. Rev. 2022, 74, 119–140. [Google Scholar] [CrossRef]
- Feltenstein, M.W.; See, R.E. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. J. Alcohol Drug Depend. 2007, 89, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Jackson, L.R.; Robinson, T.E.; Becker, J.B. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology 2006, 31, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo-Lopez, E.; Pletzer, B. Interactive effects of dopamine baseline levels and cycle phase on executive functions: The role of progesterone. Front. Neurosci. 2017, 11, 403. [Google Scholar] [CrossRef] [Green Version]
- Fox, H.C.; Hong, K.A.; Paliwal, P.; Morgan, P.T.; Sinha, R. Altered levels of sex and stress steroid hormones assessed daily over a 28-day cycle in early abstinent cocaine-dependent females. Psychopharmacology 2008, 195, 527–536. [Google Scholar] [CrossRef] [Green Version]
- Yonkers, K.A.; Forray, A.; Nich, C.; Carroll, K.M.; Hine, C.; Merry, B.C.; Shaw, H.; Shaw, J.; Sofuoglu, M. Progesterone Reduces Cocaine Use in Postpartum Women with a Cocaine Use Disorder: A Randomized, Double-Blind Study. Lancet Psychiatry 2014, 1, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Mayo, L.M.; Paul, E.; DeArcangelis, J.; Van Hedger, K.; De Wit, H. Gender differences in the behavioral and subjective effects of methamphetamine in healthy humans. Psychopharmacology 2019, 236, 2413–2423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbett, C.M.; Dunn, E.; Loweth, J.A. Effects of Sex and Estrous Cycle on the Time Course of Incubation of Cue-Induced Craving following Extended-Access Cocaine Self-Administration. eNeuro 2021, 8, ENEURO.0054-21.2021. [Google Scholar] [CrossRef] [PubMed]
- Quigley, J.A.; Logsdon, M.K.; Turner, C.A.; Gonzalez, I.; Leonardo, N.; Becker, J.B. Sex differences in vulnerability to addiction. Neuropharmacology 2021, 187, 108491. [Google Scholar] [CrossRef] [PubMed]
| Regions | Drug Type | Trend |
|---|---|---|
| Western and Central Europe | Cannabis | Large increase/large decrease * |
| Cocaine | Large increase | |
| ATS | Large increase/large decrease * | |
| Opioids | Large increase | |
| Heroin | Large increase | |
| Hallucinogens | Large increase/large decrease * | |
| LSD | Large increase | |
| Southeastern Europe | Cannabis | No data |
| Cocaine | No data | |
| ATS | No data | |
| Opioids | No great change | |
| Heroin | No data | |
| Hallucinogens | No great change | |
| LSD | No great change | |
| Eastern Europe | Cannabis | No data |
| Cocaine | No great change | |
| ATS | No great change | |
| Opioids | No great change | |
| Heroin | No great change | |
| Hallucinogens | Large decrease ** | |
| LSD | Large decrease ** |
| Drug Abuse | Man, % | Woman, % |
|---|---|---|
| Opioids | 4 | 3.5 |
| Heroin | 0.5 | 0.2 |
| Cocaine | 2.6 | 1.5 |
| Cannabis | 18.5 | 13.5 |
| Methamphetamines | 0.8 | 0.4 |
| Misuse prescription pain killer pills | 3.9 | 3.4 |
| Misuse prescription tranquilants | 2.2 | 2.0 |
| Misuse prescription sedatives | 0.5 | 0.5 |
| Man, % | Woman, % | |
|---|---|---|
| COUNTRY | Cannabis | |
| Estonia | 9.20 | 4.81 |
| Finland | 11.20 | 5.20 |
| Germany | 8.87 | 5.25 |
| Netherlands | 13.90 | 6.30 |
| Norway | 7.00 | 3.70 |
| United Kingdom (England and Wales) | 10.28 | 5.00 |
| Cocaine | ||
| Estonia | 1.32 | 0.83 |
| Finland | 1.40 | 0.50 |
| Germany | 1.41 | 0.82 |
| Netherlands | 2.70 | 1.30 |
| Norway | 2.10 | 0.20 |
| United Kingdom (England and Wales) | 4.10 | 1.76 |
| Amphetamine and Methamphetamine | ||
| Estonia | 1.65 | 0.45 |
| Finland | 2.50 | 0.90 |
| Germany | 1.50 | 0.90 |
| Netherlands | 1.80 | 0.90 |
| Norway | 1.10 | 0.10 |
| United Kingdom (England and Wales) | 0.76 | 0.39 |
| Illicit opioids ●/Prescription opioids ●● | ||
| Estonia | 0.33● /0.22 ●● | 0.08● /0.08 ●● |
| Finland | 0.90 ●● | 0.80 ●● |
| Germany | 0.48 ● | 0.39 ●● |
| Netherlands | No data | No data |
| Norway | No data | No data |
| United Kingdom (England and Wales) | No data/0.07 ●● | No data/0.04 ●● |
| Barbiturates and Benzodiazepines | ||
| Estonia | 0.88 | 0.23 |
| Finland | No data | No data |
| Germany | No data | No data |
| Netherlands | 7.00 | 13.30 |
| Norway | No data | No data |
| United Kingdom (England and Wales) | 0.57 | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgieva, E.; Benkova, K.; Vlaeva, N.; Karamalakova, Y.; Miteva, R.; Abrashev, H.; Nikolova, G. Is Illicit Substance Use Gender-Specific? The Basic Points of Mental and Health Disorders. Toxics 2022, 10, 344. https://doi.org/10.3390/toxics10070344
Georgieva E, Benkova K, Vlaeva N, Karamalakova Y, Miteva R, Abrashev H, Nikolova G. Is Illicit Substance Use Gender-Specific? The Basic Points of Mental and Health Disorders. Toxics. 2022; 10(7):344. https://doi.org/10.3390/toxics10070344
Chicago/Turabian StyleGeorgieva, Ekaterina, Krasimira Benkova, Nadya Vlaeva, Yanka Karamalakova, Radostina Miteva, Hristo Abrashev, and Galina Nikolova. 2022. "Is Illicit Substance Use Gender-Specific? The Basic Points of Mental and Health Disorders" Toxics 10, no. 7: 344. https://doi.org/10.3390/toxics10070344
APA StyleGeorgieva, E., Benkova, K., Vlaeva, N., Karamalakova, Y., Miteva, R., Abrashev, H., & Nikolova, G. (2022). Is Illicit Substance Use Gender-Specific? The Basic Points of Mental and Health Disorders. Toxics, 10(7), 344. https://doi.org/10.3390/toxics10070344









