The Mixture of Bisphenol-A and Its Substitutes Bisphenol-S and Bisphenol-F Exerts Obesogenic Activity on Human Adipose-Derived Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Adipogenic Differentiation
2.3. Quantitative Oil Red O Staining Assay
2.4. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.5. Western Blot
2.6. Cell Viability
2.7. Statistical Analysis
3. Results
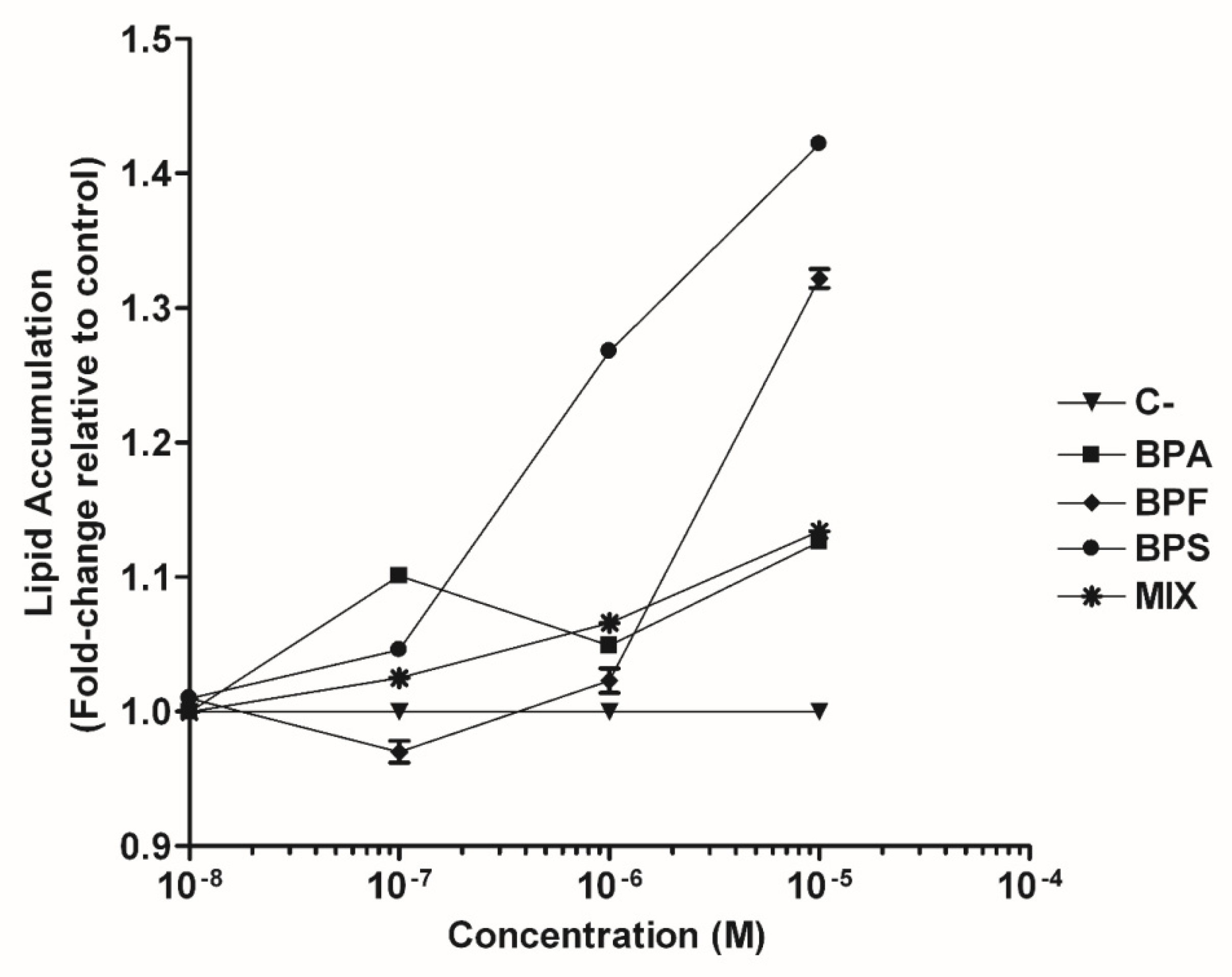
3.1. The Bisphenol Mixture Promotes Intracellular Lipid Accumulation in a Dose-Dependent Manner
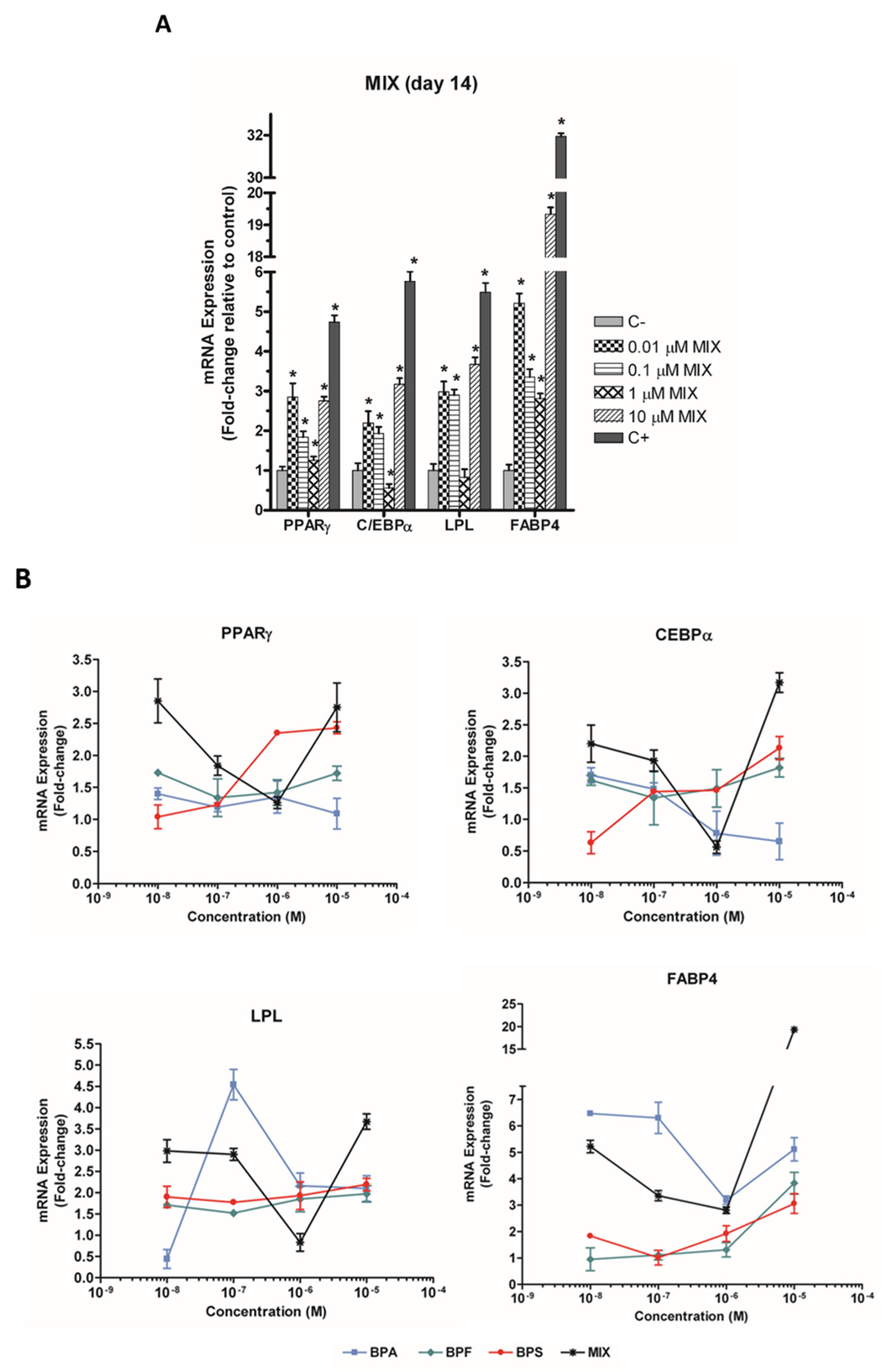
3.2. The Bisphenol Mixture Alters the Expression of Adipogenesis-Related Genes in a Non-Monotonic Manner
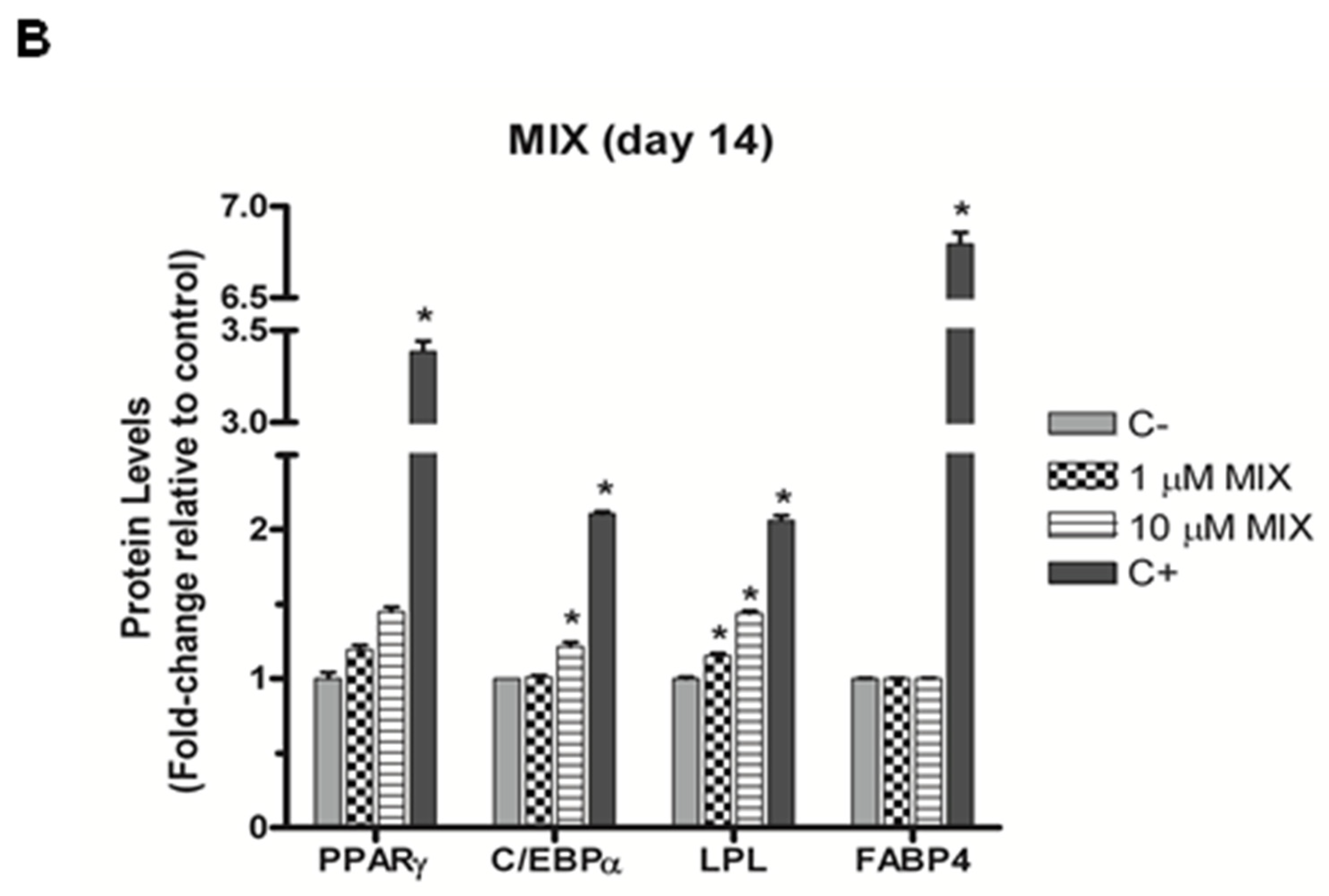
3.3. The Bisphenol Mixture Alters Protein Levels of Selected Adipogenic Markers
3.4. Effects of Bisphenols Mixture on hASC Viability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report. 2018. Available online: http://dietandcancerreport.org (accessed on 11 November 2021).
- Janesick, A.S.; Blumberg, B. Obesogens: An emerging threat to public health. Am. J. Obstet. Gynecol. 2016, 214, 559–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauretta, R.; Sansone, A.; Sansone, M.; Romanelli, F.; Appetecchia, M. Endocrine disrupting chemicals: Effects on endocrine glands. Front. Endocrinol. (Lausanne) 2019, 10, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regnier, S.M.; Sargis, R.M. Adipocytes under assault: Environmental disruption of adipose physiology. Biochim. Biophys. Acta 2014, 1842, 520–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papalou, O.; Kandaraki, E.A.; Papadakis, G.; Diamanti-Kandarakis, E. Endocrine disrupting chemicals: An occult mediator of metabolic disease. Front. Endocrinol. (Lausanne) 2019, 10, 112. [Google Scholar] [CrossRef] [Green Version]
- Tandon, P.; Wafer, R.; Minchin, J.E.N. Adipose morphology and metabolic disease. J. Exp. Biol. 2018, 221, jeb164970. [Google Scholar] [CrossRef] [Green Version]
- Artacho-Cordón, F.; Fernández, M.F.; Frederiksen, H.; Iribarne-Durán, L.M.; Jiménez-Díaz, I.; Vela-Soria, F.; Andersson, A.M.; Martin-Olmedo, P.; Peinado, F.M.; Olea, N.; et al. Environmental phenols and parabens in adipose tissue from hospitalized adults in Southern Spain. Environ. Int. 2018, 119, 203–211. [Google Scholar] [CrossRef]
- Mustieles, V.; Arrebola, J.P. How polluted is your fat? What the study of adipose tissue can contribute to environmental epidemiology. J. Epidemiol. Community Health 2020, 74, 401–407. [Google Scholar] [CrossRef]
- Fernandez, M.F.; Arrebola, J.P.; Taoufiki, J.; Navalón, A.; Ballesteros, O.; Pulgar, R.; Vilchez, J.L.; Olea, N. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod. Toxicol. 2007, 24, 259–264. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Thomas Zoeller, R.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-disrupting chemicals and public health protection: A statement of principles from The Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J. History of the Obesogen Field: Looking Back to Look Forward. Front. Endocrinol. (Lausanne) 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heindel, J.J.; Blumberg, B. Environmental Obesogens: Mechanisms and Controversies. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Mustieles, V.; D’Cruz, S.C.; Couderq, S.; Rodríguez-Carrillo, A.; Fini, J.B.; Hofer, T.; Steffensen, I.L.; Dirven, H.; Barouki, R.; Olea, N.; et al. Bisphenol A and its analogues: A comprehensive review to identify and prioritize effect biomarkers for human biomonitoring. Environ. Int. 2020, 144, 105811. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) No 321/2011 of 1 April 2011 Amending Regulation (EU) No 10/2011 as Regards the Restriction of Use of Bisphenol A in Plastic Infant Feeding Bottles. Available online: http://data.europa.eu/eli/reg_impl/2011/321/oj (accessed on 11 September 2021).
- Karrer, C.; De Boer, W.; Delmaar, C.; Cai, Y.; Crépet, A.; Hungerbühler, K.; Von Goetz, N. Linking Probabilistic Exposure and Pharmacokinetic Modeling to Assess the Cumulative Risk from the Bisphenols BPA, BPS, BPF, and BPAF for Europeans. Environ. Sci. Technol. 2019, 53, 9181–9191. [Google Scholar] [CrossRef]
- Lehmler, H.J.; Liu, B.; Gadogbe, M.; Bao, W. Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in U.S. Adults and Children: The National Health and Nutrition Examination Survey 2013—2014. ACS Omega 2018, 3, 6523–6532. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wattar, N.; Field, C.J.; Dinu, I.; Dewey, D.; Martin, J.W. Exposure and dietary sources of bisphenol A (BPA) and BPA-alternatives among mothers in the APrON cohort study. Environ. Int. 2018, 119, 319–326. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol a substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Ye, X.; Wong, L.Y.; Kramer, J.; Zhou, X.; Jia, T.; Calafat, A.M. Urinary Concentrations of Bisphenol A and Three Other Bisphenols in Convenience Samples of U.S. Adults during 2000–2014. Environ. Sci. Technol. 2015, 49, 11834–11839. [Google Scholar] [CrossRef]
- Backhaus, T.; Faust, M. Predictive environmental risk assessment of chemical mixtures: A conceptual framework. Environ. Sci. Technol. 2012, 46, 2564–2573. [Google Scholar] [CrossRef]
- Gómez, L.; Niegowska, M.; Navarro, A.; Amendola, L.; Arukwe, A.; Ait-Aissa, S.; Balzamo, S.; Barreca, S.; Belkin, S.; Bittner, M.; et al. Estrogenicity of chemical mixtures revealed by a panel of bioassays. Sci. Total Environ. 2021, 785, 147284. [Google Scholar] [CrossRef] [PubMed]
- Kortenkamp, A.; Faust, M. Regulate to reduce chemical mixture risk. Science 2018, 361, 224–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skledar, D.G.; Mašič, L.P. In vitro estrogenic activity of binary and multicomponent mixtures with bisphenol A. Sci. Total Environ. 2020, 707, 1352111. [Google Scholar] [CrossRef] [PubMed]
- Thrupp, T.J.; Runnalls, T.J.; Scholze, M.; Kugathas, S.; Kortenkamp, A.; Sumpter, J.P. The consequences of exposure to mixtures of chemicals: Something from “nothing” and “a lot from a little” when fish are exposed to steroid hormones. Sci. Total Environ. 2018, 619–620, 1482–1492. [Google Scholar] [CrossRef]
- Doan, T.Q.; Cannolly, L.; Igout, A.; Nott, K.; Muller, M.; Scippo, M.I. In vitro profiling of the potential endocrine disrupting activities affecting steroid and aryl hydrocarbon receptors of compounds and mixtures prevalent in human drinking water resources. Chemosphere 2020, 258, 127332. [Google Scholar] [CrossRef]
- Silva, E.; Rajapakse, N.; Kortenkamp, A. Something from “nothing”—Eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ. Sci. Technol. 2002, 36, 1751–1756. [Google Scholar] [CrossRef]
- Christiansen, S.; Axelstad, M.; Scholze, M.; Johansson, H.K.L.; Hass, U.; Mandrup, K.; Frandsen, H.L.; Frederiksen, H.; Isling, L.K.; Boberg, J. Grouping of endocrine disrupting chemicals for mixture risk assessment—Evidence from a rat study. Environ. Int. 2020, 142, 105870. [Google Scholar] [CrossRef]
- Hercog, K.; Maisanaba, S.; Filipič, M.; Sollner-Dolenc, M.; Kač, L.; Žegura, B. Genotoxic activity of bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF and their mixtures in human hepatocellular carcinoma (HepG2) cells. Sci. Total Environ. 2019, 687, 267–276. [Google Scholar] [CrossRef]
- Jatkowska, N.; Kudłak, B.; Lewandowska, P.; Liu, W.; Williams, M.J.; Schiöth, H.B. Identification of synergistic and antagonistic actions of environmental pollutants: Bisphenols A, S and F in the presence of DEP, DBP, BADGE and BADGE·2HCl in three component mixtures. Sci. Total Environ. 2021, 767, 144286. [Google Scholar] [CrossRef]
- Repouskou, A.; Papadopoulou, A.K.; Panagiotidou, E.; Trichas, P.; Lindh, C.; Bergman, Å.; Gennings, C.; Bornehag, C.G.; Rüegg, J.; Kitraki, E.; et al. Long term transcriptional and behavioral effects in mice developmentally exposed to a mixture of endocrine disruptors associated with delayed human neurodevelopment. Sci. Rep. 2020, 10, 9367. [Google Scholar] [CrossRef]
- Yu, H.; Caldwell, D.J.; Suri, R.P. In vitro estrogenic activity of representative endocrine disrupting chemicals mixtures at environmentally relevant concentrations. Chemosphere 2019, 215, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Kassotis, C.D.; Hoffman, K.; Phillips, A.L.; Zhang, S.; Cooper, E.M.; Webster, T.F.; Stapleton, H.M. Characterization of adipogenic, PPARγ, and TRβ activities in house dust extracts and their associations with organic contaminants. Sci. Total Environ. 2021, 758, 143707. [Google Scholar] [CrossRef] [PubMed]
- Biemann, R.; Fischer, B.; Navarrete Santos, A. Adipogenic effects of a combination of the endocrine-disrupting compounds bisphenol A, diethylhexylphthalate, and tributyltin. Obes. Facts 2014, 7, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Mentor, A.; Brunström, B.; Mattsson, A.; Jönsson, M. Developmental exposure to a human relevant mixture of endocrine disruptors alters metabolism and adipogenesis in zebrafish (Danio rerio). Chemosphere 2020, 238, 124584. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, 593–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimaldi, M.; Boulahtouf, A.; Toporova, L.; Balaguer, P. Functional profiling of bisphenols for nuclear receptors. Toxicology 2019, 420, 39–45. [Google Scholar] [CrossRef]
- Riu, A.; le Maire, A.; Grimaldi, M.; Audebert, M.; Hillenweck, A.; Bourguet, W.; Balaguer, P.; Zalko, D. Characterization of Novel Ligands of ERα, Erβ, and PPARγ: The Case of Halogenated Bisphenol A and Their Conjugated Metabolites. Toxicol. Sci. 2011, 122, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Riu, A.; Grimaldi, M.; le Maire, A.; Bey, G.; Phillips, K.; Boulahtouf, A.; Perdu, E.; Zalko, D.; Bourguet, W.; Balaguer, P. Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ. Health Perspect. 2011, 119, 1227–1232. [Google Scholar] [CrossRef]
- Molina-Molina, J.M.; Amaya, E.; Grimaldi, M.; Sáenz, J.M.; Real, M.; Fernández, M.F.; Balaguer, P.; Olea, N. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol. Appl. Pharmacol. 2013, 272, 127–136. [Google Scholar] [CrossRef]
- Delfosse, V.; Grimaldi, M.; Pons, J.L.; Boulahtouf, A.; Le Maire, A.; Cavailles, V.; Labesse, G.; Bourguet, W.; Balaguer, P. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc. Natl. Acad. Sci. USA 2012, 109, 14930–14935. [Google Scholar] [CrossRef] [Green Version]
- Reina-Pérez, I.; Olivas-Martínez, A.; Mustieles, V.; Ruiz-Ojeda, F.J.; Molina-Molina, J.M.; Olea, N.; Fernández, M.F. Bisphenol F and bisphenol S promote lipid accumulation and adipogenesis in human adipose-derived stem cells. Food Chem. Toxicol. 2021, 152, 112216. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Xia, M.; Zhang, L.; Cheng, W.; Yan, J.; Sun, Y.; Wang, Y.; Jiang, H. Individual and combined effects of BPA, BPS and BPAF on the cardiomyocyte differentiation of embryonic stem cells. Ecotoxicol. Environ. Saf. 2021, 220, 112366. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ojeda, F.J.; Rupérez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M. Cell Models and Their Application for Studying Adipogenic Differentiation in Relation to Obesity: A Review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, C.; Grimaldi, M.; Boulahtouf, A.; Pakdel, F.; Brion, F.; Aït-Aïssa, S.; Cavaillès, V.; Bourguet, W.; Gustafsson, J.A.; Bondesson, M.; et al. Selectivity of natural, synthetic and environmental estrogens for zebrafish estrogen receptors. Toxicol. Appl. Pharmacol. 2014, 280, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Park, C.; Song, H.; Choi, J.; Sim, S.; Kojima, H.; Park, J.; Iida, M.; Lee, Y.J. The mixture effects of bisphenol derivatives on estrogen receptor and androgen receptor. Environ. Pollut. 2020, 260, 114036. [Google Scholar] [CrossRef]
- Ramírez-Zacarías, J.L.; Castro-Muñozledo, F.; Kuri-Harcuch, W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with oil red O. Histochemistry 1992, 97, 493–497. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 11, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Boucher, J.G.; Ahmed, S.; Atlas, E. Bisphenol S induces adipogenesis in primary human preadipocytes from female donors. Endocrinology 2016, 157, 1397–1407. [Google Scholar] [CrossRef] [Green Version]
- Boucher, J.G.; Gagné, R.; Rowan-Carroll, A.; Boudreau, A.; Yauk, C.L.; Atlas, E. Bisphenol A and bisphenol S induce distinct transcriptional profiles in differentiating human primary preadipocytes. PLoS ONE 2016, 11, e0163318. [Google Scholar] [CrossRef] [Green Version]
- Ohlstein, J.F.; Strong, A.L.; McLachlan, J.A.; Gimble, J.M.; Burow, M.E.; Bunnell, B.A. Bisphenol a enhances adipogenic differentiation of human adipose stromal/stem cells. J. Mol. Endocrinol. 2014, 53, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Abbott, R.D.; Zieba, A.; Borowsky, F.E.; Kaplan, D.L. Development of a Three-Dimensional Adipose Tissue Model for Studying Embryonic Exposures to Obesogenic Chemicals. Ann. Biomed. Eng. 2017, 45, 1807–1818. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.G.; Boudreau, A.; Atlas, E. Bisphenol A induces differentiation of human preadipocytes in the absence of glucocorticoid and is inhibited by an estrogen-receptor antagonist. Nutr. Diabetes 2014, 4, e102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, P.; Wang, L.; Yang, N.; Wen, J.; Zhao, M.; Su, G.; Zhang, J.; Weng, D. Peroxisome proliferator-activated receptor gamma (PPARγ) activation and metabolism disturbance induced by bisphenol A and its replacement analog bisphenol S using in vitro macrophages and in vivo mouse models. Environ. Int. 2020, 134, 1005328. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Ferrini, M.G.; Jellyman, J.K.; Han, G.; Ross, M.G. In vivo and in vitro bisphenol A exposure effects on adiposity. J. Dev. Orig. Health Dis. 2018, 9, 678–687. [Google Scholar] [CrossRef]
- Ahmed, S.; Atlas, E. Bisphenol S- and bisphenol A-induced adipogenesis of murine preadipocytes occurs through direct peroxisome proliferator-activated receptor gamma activation. Int. J. Obes. (Lond) 2016, 40, 1566–1573. [Google Scholar] [CrossRef]
- Choi, S.I.; Lee, J.S.; Lee, S.; Sim, W.S.; Kim, Y.C.; Lee, O.H. Potentilla rugulosa nakai extract attenuates bisphenol A-, S-and F-induced ROS production and differentiation of 3T3-L1 preadipocytes in the absence of dexamethasone. Antioxidants (Basel). 2020, 9, 113. [Google Scholar] [CrossRef] [Green Version]
- Héliès-Toussaint, C.; Peyre, L.; Costanzo, C.; Chagnon, M.C.; Rahmani, R. Is bisphenol S a safe substitute for bisphenol A in terms of metabolic function? An in vitro study. Toxicol. Appl. Pharmacol. 2014, 280, 224–235. [Google Scholar] [CrossRef]
- Martínez, M.; Blanco, J.; Rovira, J.; Kumar, V.; Domingo, J.L.; Schuhmacher, M. Bisphenol A analogues (BPS and BPF) present a greater obesogenic capacity in 3T3-L1 cell line. Food Chem. Toxicol. 2020, 140, 111298. [Google Scholar] [CrossRef]
- Ramskov Tetzlaff, C.N.; Svingen, T.; Vinggaard, A.M.; Rosenmai, A.K.; Taxvig, C. Bisphenols B, E, F, and S and 4-cumylphenol induce lipid accumulation in mouse adipocytes similarly to bisphenol A. Environ. Toxicol. 2020, 35, 543–552. [Google Scholar] [CrossRef]
- Dong, H.; Yao, X.; Liu, S.; Yin, N.; Faiola, F. Non-cytotoxic nanomolar concentrations of bisphenol A induce human mesenchymal stem cell adipogenesis and osteogenesis. Ecotoxicol. Environ. Saf. 2018, 164, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.C.; Cohenour, E.R.; Harnett, K.G.; Schuh, S.M. BPA, BPAF and TMBPF Alter Adipogenesis and Fat Accumulation in Human Mesenchymal Stem Cells, with Implications for Obesity. Int. J. Mol. Sci. 2021, 22, 5363. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wu, G.; Xing, Y.; Wang, C.; Yuan, X.; Li, B. Evaluation of single and combined toxicity of bisphenol A and its analogues using a highly-sensitive micro-biosensor. J. Hazard. Mater. 2020, 381, 120908. [Google Scholar] [CrossRef]
- Harnett, K.G.; Chin, A.; Schuh, S.M. Cytotoxic and apoptotic data of BPA and BPA alternatives TMBPF, BPAF, and BPS in female adult rat and human stem cells. Data Brief 2021, 37, 107183. [Google Scholar] [CrossRef] [PubMed]
- Harnett, K.G.; Chin, A.; Schuh, S.M. BPA and BPA alternatives BPS, BPAF, and TMBPF, induce cytotoxicity and apoptosis in rat and human stem cells. Ecotoxicol. Environ. Saf. 2021, 216, 112210. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Hou, M.; Pan, X.; Li, X. The environmental obesogen bisphenol A promotes adipogenesis by increasing the amount of 11β-hydroxysteroid dehydrogenase type 1 in the adipose tissue of children. Int. J. Obes. (Lond) 2013, 37, 999–1005. [Google Scholar] [CrossRef]
- Kassotis, C.D.; Stapleton, H.M. Endocrine-mediated mechanisms of metabolic disruption and new approaches to examine the public health threat. Front. Endocrinol. (Lausanne) 2019, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [Green Version]
- Lefterova, M.I.; Lazar, M.A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [CrossRef]
- Le Fol, V.; Aït-Aïssa, S.; Sonavane, M.; Porcher, J.M.; Balaguer, P.; Cravedi, J.P.; Zalko, D.; Brion, F. In vitro and in vivo estrogenic activity of BPA, BPF and BPS in zebrafish-specific assays. Ecotoxicol. Environ. Saf. 2017, 142, 150–156. [Google Scholar] [CrossRef]
- Schaffert, A.; Krieg, L.; Weiner, J.; Schlichting, R.; Ueberham, E.; Karkossa, I.; Bauer, M.; Landgraf, K.; Junge, K.M.; Wabitsch, M.; et al. Alternatives for the worse: Molecular insights into adverse effects of bisphenol a and substitutes during human adipocyte differentiation. Environ. Int. 2021, 156, 106730. [Google Scholar] [CrossRef] [PubMed]
- Kudłak, B.; Wieczerzak, M.; Namieśnik, J. Bisphenols (A, S, and F) affect the basic hormonal activity determined for pharmaceuticals—Study of Saccharomyces cerevisiae. Environ. Pollut. 2019, 246, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Kassotis, C.D.; Hoffman, K.; Völker, J.; Pu, Y.; Veiga-Lopez, A.; Kim, S.M.; Schlezinger, J.J.; Bovolin, P.; Cottone, E.; Saraceni, A.; et al. Reproducibility of adipogenic responses to metabolism disrupting chemicals in the 3T3-L1 pre-adipocyte model system: An interlaboratory study. Toxicology 2021, 461, 152900. [Google Scholar] [CrossRef] [PubMed]
- Kassotis, C.D.; Vom Saal, F.S.; Babin, P.J.; Lagadic-Gossmann, D.; Le Mentec, H.; Blumberg, B.; Mohajer, N.; Legrand, A.; Munic Kos, V.; Martin-Chouly, C.; et al. Obesity III: Obesogen assays: Limitations, strengths, and new directions. Biochem. Pharmacol. 2022, 199, 115014. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reina-Pérez, I.; Olivas-Martínez, A.; Mustieles, V.; Salamanca-Fernández, E.; Molina-Molina, J.M.; Olea, N.; Fernández, M.F. The Mixture of Bisphenol-A and Its Substitutes Bisphenol-S and Bisphenol-F Exerts Obesogenic Activity on Human Adipose-Derived Stem Cells. Toxics 2022, 10, 287. https://doi.org/10.3390/toxics10060287
Reina-Pérez I, Olivas-Martínez A, Mustieles V, Salamanca-Fernández E, Molina-Molina JM, Olea N, Fernández MF. The Mixture of Bisphenol-A and Its Substitutes Bisphenol-S and Bisphenol-F Exerts Obesogenic Activity on Human Adipose-Derived Stem Cells. Toxics. 2022; 10(6):287. https://doi.org/10.3390/toxics10060287
Chicago/Turabian StyleReina-Pérez, Iris, Alicia Olivas-Martínez, Vicente Mustieles, Elena Salamanca-Fernández, José Manuel Molina-Molina, Nicolás Olea, and Mariana F. Fernández. 2022. "The Mixture of Bisphenol-A and Its Substitutes Bisphenol-S and Bisphenol-F Exerts Obesogenic Activity on Human Adipose-Derived Stem Cells" Toxics 10, no. 6: 287. https://doi.org/10.3390/toxics10060287
APA StyleReina-Pérez, I., Olivas-Martínez, A., Mustieles, V., Salamanca-Fernández, E., Molina-Molina, J. M., Olea, N., & Fernández, M. F. (2022). The Mixture of Bisphenol-A and Its Substitutes Bisphenol-S and Bisphenol-F Exerts Obesogenic Activity on Human Adipose-Derived Stem Cells. Toxics, 10(6), 287. https://doi.org/10.3390/toxics10060287







