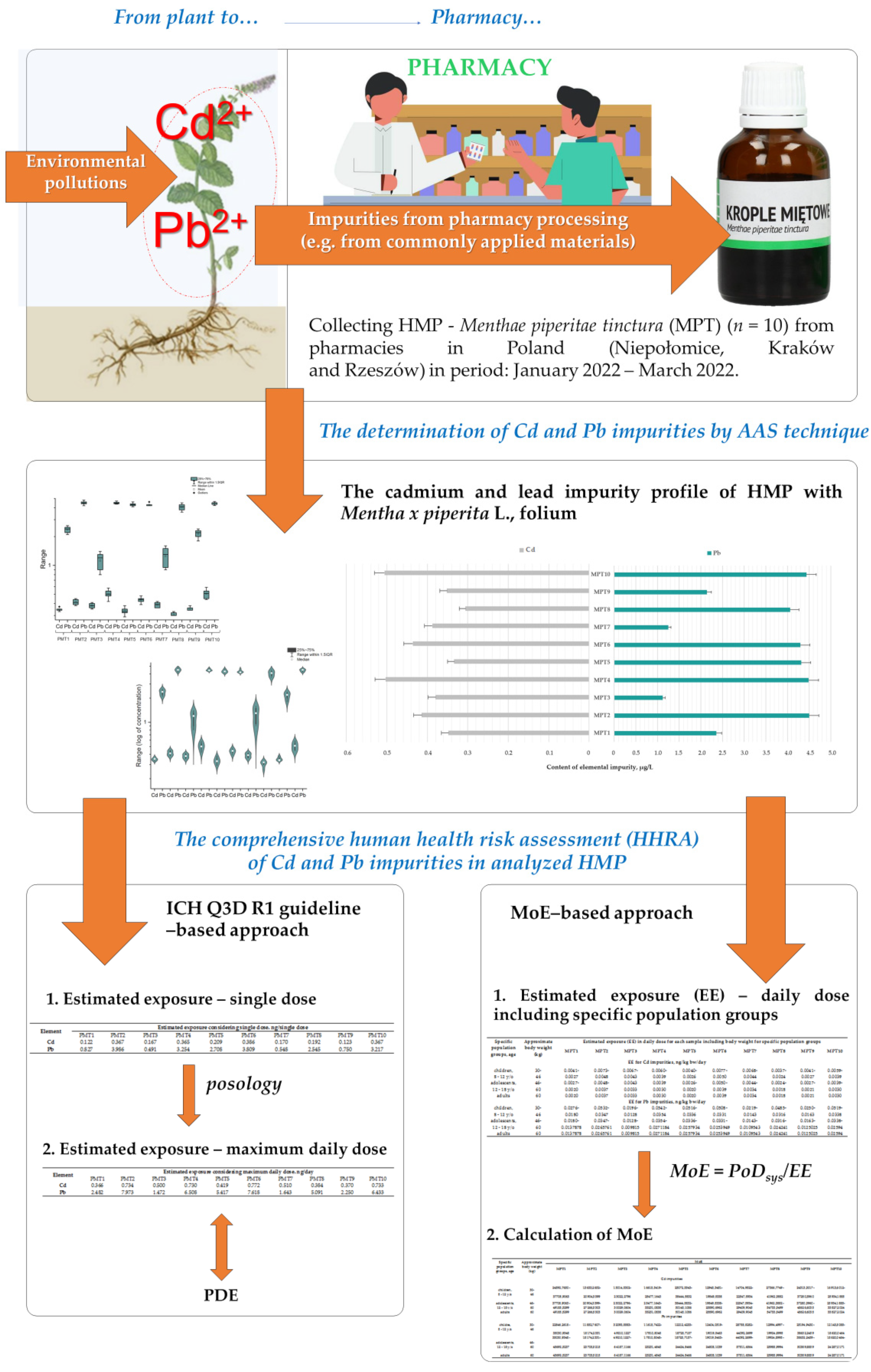
The Human Health Risk Assessment of Heavy Metals Impurities (Cd and Pb) in Herbal Medicinal Products as Menthae piperitae tinctura (Mentha × piperita L., folium) Available in Pharmacies from Poland
Abstract
:1. Introduction
- Children 8–12 years: 8–10 drops maximum 2–3 times per day;
- Adolescents 12–18 years: 10–15 drops maximum 3 times per day;
- Adults: 15–20 oral drops 3 times per day.
- (1)
- The fact that Cd and Pb impurities in final pharmaceuticals are most important from toxicological point of view (first class of elemental impurities in ICH Q3D R1 guideline [1]);
- (2)
- (3)
- (4)
- The rarity of this type of research in the field of comprehensive HHRA of elemental impurities in final pharmaceutical products;
- (5)
- The need to fill a gap in the scientific literature regarding the control of these elemental impurities in phytopharmaceutical product.
2. Materials and Methods
2.1. Samples
2.2. Samples Preparation, Determination of Cd and Pb, Analytical Calibration, Quality Control, and Data Processing
2.3. The Applied Strategies in Comprehensive Human Health Risk Assessment (HHRA) of Cd and Pb Impurities in HMP with Mentha × piperita L., folium
3. Results
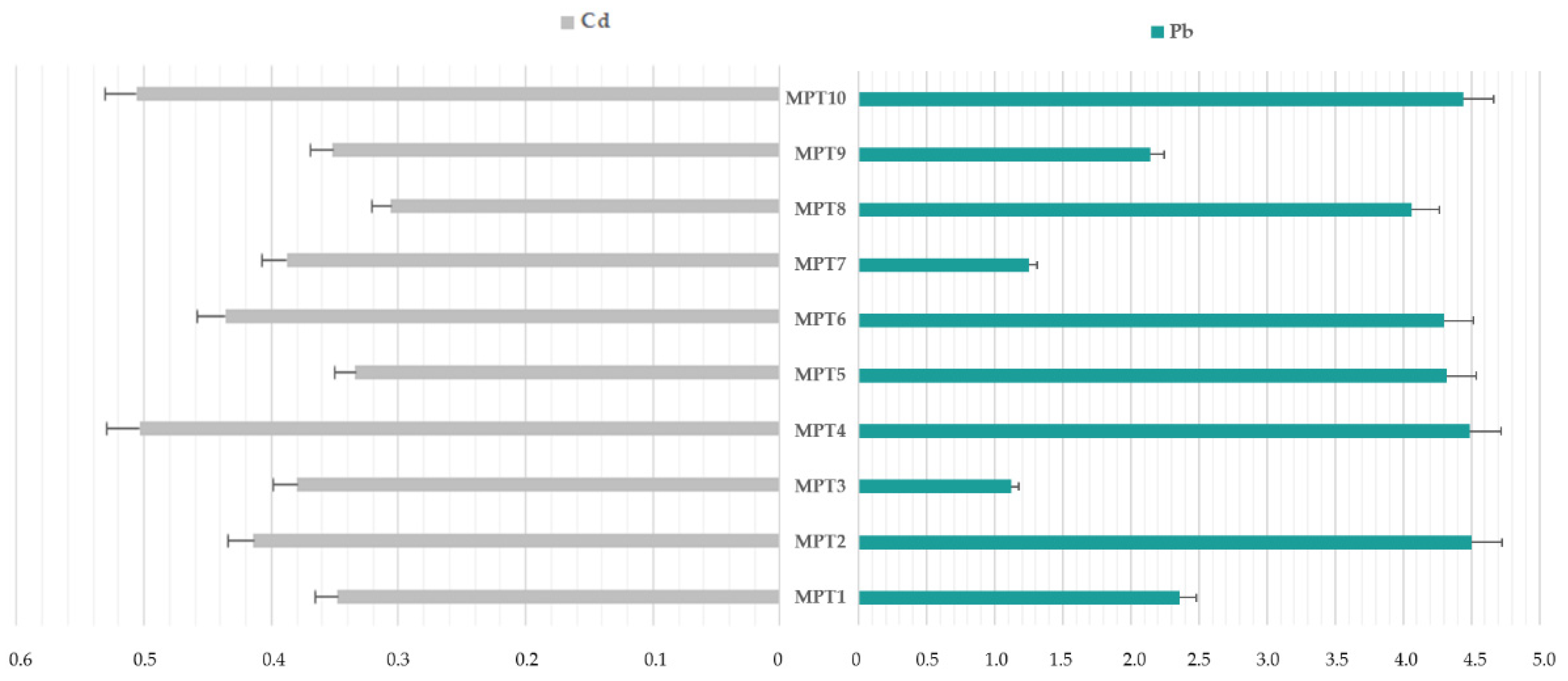
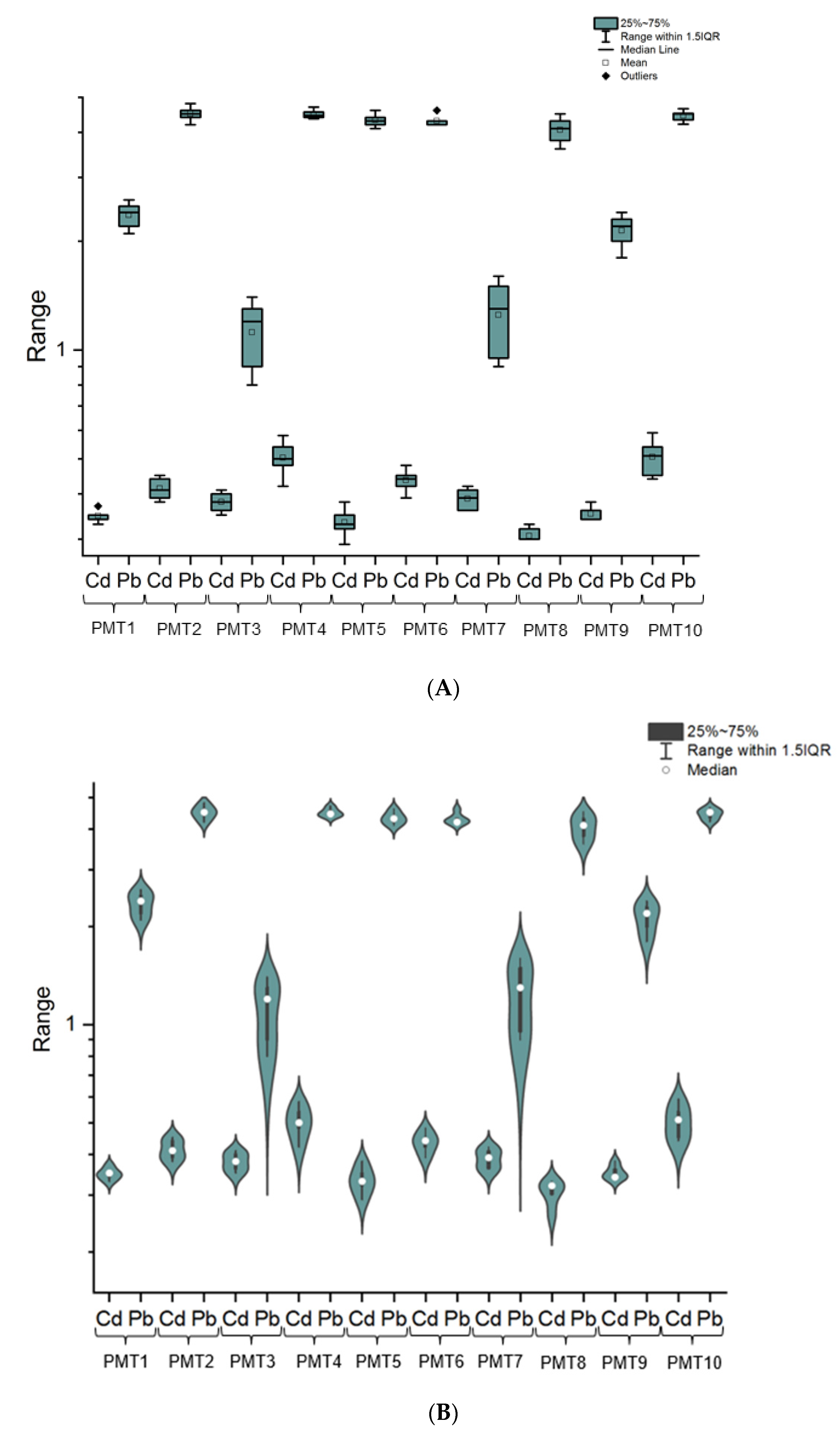
3.1. The Cadmium and Lead Impurity Profile of HMP with Mentha × piperita L., folium Available in Polish Pharmacies
3.2. The HHRA of Cd and Pb Impurities Considering ICH Q3D R1 Guideline-Based Approach
3.3. The HHRA of Cd and Pb Impurities Considering MoE-Based Approach
4. Discussion
4.1. The Cadmium and Lead Impurity Profile of HMP with Mentha × piperita L., folium Available in Polish Pharmacies
4.2. The HHRA of Cd and Pb Impurities Considering ICH Q3D R1 Guideline-Based Approach
4.3. The HHRA of Cd and Pb Impurities Considering an MoE-Based Approach
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICH Guideline Q3D (R1) on Elemental Impurities. Committee for Human Medicinal Products. 28 March 2019EMA/CHMP/ICH/353369/2013. Available online: https://www.ema.europa.eu/en/ich-q3d-elemental-impurities (accessed on 19 April 2022).
- Görög, S. Chemical and analytical characterization of related organic impurities in drugs. Anal. Bioanal. Chem. 2003, 377, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Garrett, C.E.; Prasad, K. The art of meeting palladium specifications in active pharmaceutical ingredients produced by Pd-catalyzed reactions. Adv. Synth. Cat. 2004, 346, 889–900. [Google Scholar] [CrossRef]
- Merusomayajula, K.V.; Tirukkovalluri, S.R.; Kommula, R.S.; Chakkirala, S.V.; Vundavilli, J.K.; Kottapalli, P.K.S. Development and validation of a simple and rapid ICP-OES method for quantification of elemental impurities in voriconazole drug substance. Future J. Pharm. Sci. 2021, 7, 1–12. [Google Scholar] [CrossRef]
- Sadhu, A.; Upadhyay, P.; Singh, P.K.; Agrawal, A.; Ilango, K.; Karmakar, D.; Dubey, G.P. Quantitative analysis of heavy metals in medicinal plants collected from environmentally diverse locations in India for use in a novel phytopharmaceutical product. Environ. Mon. Assess. 2015, 187, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.M.; KumarMeena, A. Detection of toxic heavy metals and pesticide residue in herbal plants which are commonly used in the herbal formulations. Environ. Monit. Assess. 2011, 181, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Directive 2004/24/EC of the European Parliament and of the Council of 31 March 2004 Amending, as Regards Traditional Herbal Medicinal Products, Directive 2001/83/EC on the Community Code Relating to Medicinal Products for Human Use. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32004L0024 (accessed on 19 April 2022).
- Chan, K. Some aspects of toxic contaminants in herbal medicines. Chemosphere 2003, 52, 1361–1371. [Google Scholar] [CrossRef] [Green Version]
- Jurowski, K.; Krośniak, M.; Fołta, M.; Tatar, B.; Cole, M.; Piekoszewski, W. The Toxicological Analysis of Ni and Cr in Prescription Food for Special Medical Purposes and Modified Milk Products for Babies in Infancy Available in Pharmacies in Poland. J. Biol. Tr. Elem. Res. 2019, 192, 129–135. [Google Scholar] [CrossRef]
- Ernst, E. Herbal medicines–They are popular, but are they also safe? Eur. J. Clin. Pharm. 2006, 62, 1–2. [Google Scholar] [CrossRef]
- Murray, M.J.; Lincoln, D.E.; Marble, P.M. Oil composition of Mentha aquatica x M. spicata F1 hybrids in relation to the origin of XM. piperita. Can. J. Genet. Cytol. 1972, 14, 13–29. [Google Scholar] [CrossRef]
- EMA/HMPC/572705/2014; Committee on Herbal Medicinal Products (HMPC). European Union Herbal Monograph on Mentha x piperita L., Folium (Final—Revision 1). 15 January 2020. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/draft-european-union-herbal-monograph-mentha-x-piperita-l-folium-revision-1_en.pdf (accessed on 19 April 2022).
- EMA/HMPC/226633/2020; EMA/HMPC/M/H/0237. Committee on Herbal Medicinal Products (HMPC), Opinion of the HMPC on a European Union Herbal Monograph on Mentha x piperita L., Folium. 15 January 2020. Available online: https://www.ema.europa.eu/en/documents/herbal-opinion/opinion-hmpc-european-union-herbal-monograph-mentha-x-piperita-l-folium-revision-1_en.pdf (accessed on 19 April 2022).
- Krekels, E.H.; Calvier, E.A.; van der Graaf, P.H.; Knibbe, C.A. Children are not small adults, but can we treat them as such? CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 34–38. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Rehm, J. Comparative risk assessment of alcohol, tobacco, cannabis and other illicit drugs using the margin of exposure approach. Sci. Rep. 2015, 5, 8126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Scientific Committee. Statement on the applicability of the Margin of Exposure approach for the safety assessment of impurities which are both genotoxic and carcinogenic in substances added to food/feed. EFSA J. 2012, 10, 2578. [Google Scholar]
- Abdullah, R.; Diaz, L.N.; Wesseling, S.; Rietjens, I.M. Risk assessment of plant food supplements and other herbal products containing aristolochic acids using the margin of exposure (MOE) approach. Food Addit. Contam. Part A 2017, 34, 135–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurowski, K.; Fołta, M.; Tatar, B.; Berkoz, M.; Krośniak, M. The Toxicological Risk Assessment of Lead and Cadmium in Valeriana officinalis L., radix (Valerian root) as Herbal Medicinal Product for the Relief of Mild Nervous Tension and Sleep Disorders Available in Polish Pharmacies. Biol. Trace Elem. Res. 2022, 200, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Jurowski, K.; Krośniak, M. The Toxicological Assessment of Content and Exposure of Heavy Metals (Pb and Cd) in Traditional Herbal Medicinal Products with Marshmallow Root (Althaea officinalis L., radix) from Polish Pharmacies. Toxics 2022, 10, 188. [Google Scholar] [CrossRef]
- Jurowski, K.; Krośniak, M.; Fołta, M.; Cole, M.; Piekoszewski, W. Toxicological analysis of Pb and Cd by ET AAS in local anaesthetics for teething (teething gels) based on herbs available in Polish pharmacies. J. Tr. Elem. Med. Biol. 2019, 52, 18–21. [Google Scholar] [CrossRef] [Green Version]
- Shahkarami, S.; Goudarzi, F.; Amiri-Ardekani, E.; Dehghani, S. Heavy metals contamination in two species of medicinal plants in the Iranian market. Int. J. Envir. Anal. Chem. 2021, 1–10. [Google Scholar] [CrossRef]
- Peyvandi, M.; Aboie Mehrizi, Z.; Ebrahimzadeh, M. The effect of cadmium on growth and composition of essential oils of Mentha piperita L. Iran. J. Plant Phys. 2016, 6, 1715–1720. [Google Scholar]
- de Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Buchet, J.; Lauwerys, R. Renal effects of cadmium body burden of the general population. Lancet 1990, 336, 699–702. [Google Scholar] [CrossRef]
- Hogan, K.; Marcus, A.; Smith, R.; White, P. Integrated exposure uptake biokineticmodel for lead in children: Empirical comparisons with epidemiologic data. Environ. Health Perspect. 1998, 106, 1557–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues. World Health Organization, Geneva, Switzerland. 2006. Available online: https://apps.who.int/iris/handle/10665/43510 (accessed on 19 April 2022).
| Sample | Posology | Density, G/mL | Composition | OTC | ||
|---|---|---|---|---|---|---|
| Children (8–12 y/o) | Adolescents (8–12 y/o) | Adults | ||||
| MPT1 | Orally: 8–10 drops/ 2 times daily | Orally: 10–15 drops/ 3 times daily | Orally: 15–20 drops/ 3 times daily | 0.93 | Tincture 1:20 Mentha × piperita L. folium, extraction solvent ethanol 80–85% v/v | Yes |
| MPT2 | Orally: 8–10 drops/ 3 times daily | Orally: 10–20 drops/ 2 times daily | Orally: 20–50 drops/ 2 times daily | 0.92 | Tincture 1:20 Mentha × piperita L. folium, extraction solvent ethanol 90% v/v | Yes |
| MPT3 | Orally: 8–10 drops/ 3 times daily | Orally: 10–15 drops/ 3 times daily | Orally: 15–25 drops/ 3 times daily | 0.93 | Tincture 1:20 Mentha × piperita L. folium, extraction solvent ethanol 90% v/v | Yes |
| MPT4 | Orally: 8–10 drops/ 2 times daily | Orally: 15–20 drops/ 2 times daily | Orally: 20–40 drops/ 2 times daily | 0.90 | Tincture 1:20 Mentha × piperita L. folium, extraction solvent ethanol 90% v/v | Yes |
| MPT5 | Orally: 10 drops/ 2 times daily | Orally: 10–20 drops/ 2 times daily | Orally: 20–35 drops/ 2 times daily | 0.91 | Tincture 1:20 Mentha × piperita L. folium, extraction solvent ethanol 80–85% v/v | Yes |
| MPT6 | Orally: 8–10 drops/ 3 times daily | Orally: 10–20 drops/ 2 times daily | Orally: 20–50 drops/ 2 times daily | 0.92 | Tincture 1:20 Mentha × piperita L. folium, extraction solvent ethanol 90% v/v | Yes |
| MPT7 | Orally: 8–10 drops/ 3 times daily | Orally: 10–15 drops/ 3 times daily | Orally: 15–25 drops/ 3 times daily | 0.93 | Tincture 1:20 Mentha × piperita L. folium, extraction solvent ethanol 90% v/v | Yes |
| MPT8 | Orally: 10 drops/ 2 times daily | Orally: 10–20 drops/ 2 times daily | Orally: 20–35 drops/ 2 times daily | 0.91 | Tincture 1:20 Mentha × piperita L. folium, extraction solvent ethanol 80–85% v/v | Yes |
| MPT9 | Orally: 8–10 drops/ 2 times daily | Orally: 10–15 drops/ 3 times daily | Orally: 15–20 drops/ 3 times daily | 0.93 | Tincture 1:20 Mentha × piperita L. folium, extraction solvent ethanol 80–85% v/v | Yes |
| MPT10 | Orally: 8–10 drops/ 2 times daily | Orally: 15–20 drops/ 2 times daily | Orally: 20–40 drops/ 2 times daily | 0.90 | Tincture 1:20 Mentha × piperita L. folium, extraction solvent ethanol 90% v/v | Yes |
| Parameter(s) | Element | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cd | Pb | ||||||||
| Wavelength, nm | 228.8 | 283.3 | |||||||
| Slit width, nm | 0.7 | 0.7 | |||||||
| Lamp current, mA | 5 | 8 | |||||||
| Optimum working range, µg/kg | 0.02–0.20 | 1.0–10.0 | |||||||
| Time– Temperature Program | Step | Temperature, °C | Ramp, s | Hold, s | Gas Flow, mL/Min | Temperature,°C | Ramp, s | Hold, s | Gas Flow, mL/Min |
| 1 | 120 | 10 | 25 | 250 | 120 | 1 | 30 | 250 | |
| 2 | 300 | 5 | 15 | 250 | 950 | 10 | 20 | 250 | |
| 3 | 1600 | 0 | 3 | 0 | 1450 | 0 | 5 | 0 | |
| 5 | 2400 | 1 | 2 | 250 | 2400 | 1 | 2 | 250 | |
| Elemental Impurity | Minimum, µg/L | Maximum, µg/L | Range, µg/L | Mean, µg/L | Skewness | Kurtosis | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cd | 0.305 | 0.506 | 0.201 | 0.397 | 1.23 | 2.52 | ||||
| Pb | 1.122 | 4.492 | 3.370 | 3.407 | 0.67 | −2.42 | ||||
| Ratio Pb/Cd | MPT1 | MPT2 | MPT3 | MPT4 | MPT5 | MPT6 | MPT7 | MPT8 | MPT9 | MPT10 |
| 6.78 | 10.87 | 2.95 | 8.91 | 12.93 | 9.86 | 3.22 | 13.27 | 6.08 | 8.77 | |
| Element | Estimated Exposure Considering Single Dose. Ng/Single Dose | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PMT1 | PMT2 | PMT3 | PMT4 | PMT5 | PMT6 | PMT7 | PMT8 | PMT9 | PMT10 | |
| Cd | 0.122 | 0.367 | 0.167 | 0.365 | 0.209 | 0.386 | 0.170 | 0.192 | 0.123 | 0.367 |
| Pb | 0.827 | 3.986 | 0.491 | 3.254 | 2.708 | 3.809 | 0.548 | 2.545 | 0.750 | 3.217 |
| Element | Estimated Exposure Considering Maximum Daily Dose. Ng/Day | |||||||||
| PMT1 | PMT2 | PMT3 | PMT4 | PMT5 | PMT6 | PMT7 | PMT8 | PMT9 | PMT10 | |
| Cd | 0.366 | 0.734 | 0.500 | 0.730 | 0.419 | 0.772 | 0.510 | 0.384 | 0.370 | 0.733 |
| Pb | 2.482 | 7.973 | 1.472 | 6.508 | 5.417 | 7.618 | 1.643 | 5.091 | 2.250 | 6.433 |
| Specific Population Groups, Age | Approximate Body Weight (Kg) | Estimated Exposure (EE) in Daily Dose for Each Sample, including Body Weight for Specific Population Groups | |||||||||
| MPT1 | MPT2 | MPT3 | MPT4 | MPT5 | MPT6 | MPT7 | MPT8 | MPT9 | MPT10 | ||
| EE for Cd Impurities, ng/kg bw/day | |||||||||||
| Children, 8–12 y/o | 30– 46 | 0.0041– 0.0027 | 0.007– 0.0048 | 0.0067– 0.0043 | 0.0060– 0.0039 | 0.0040– 0.0026 | 0.0077– 0.0050 | 0.0068– 0.0044 | 0.0037– 0.0024 | 0.0041– 0.0027 | 0.0059– 0.0039 |
| Adolescents, 12–18 y/o | 46– 60 | 0.0027– 0.0020 | 0.0048– 0.0037 | 0.0043– 0.0033 | 0.0039– 0.0030 | 0.0026– 0.0020 | 0.0050– 0.0039 | 0.0044– 0.0034 | 0.0024– 0.0018 | 0.0027– 0.0021 | 0.0039– 0.0030 |
| Adults | 60 | 0.0020 | 0.0037 | 0.0033 | 0.0030 | 0.0020 | 0.0039 | 0.0034 | 0.0018 | 0.0021 | 0.0030 |
| EE for Pb Impurities, ng/kg bw/day | |||||||||||
| Children, 8–12 y/o | 30– 46 | 0.0276– 0.0180 | 0.0532– 0.0347 | 0.0196– 0.0128 | 0.0542– 0.0354 | 0.0516– 0.0336 | 0.0508– 0.0331 | 0.0219– 0.0143 | 0.0485– 0.0316 | 0.0250– 0.0163 | 0.0519– 0.0338 |
| Adolescents, 12–18 y/o | 46– 60 | 0.0180– 0.0137878 | 0.0347– 0.0265761 | 0.0128– 0.009815 | 0.0354– 0.0271184 | 0.0336– 0.0257934 | 0.0331– 0.0253949 | 0.0143– 0.0109543 | 0.0316– 0.024241 | 0.0163– 0.0125025 | 0.0338– 0.02594 |
| Adults | 60 | 0.0137878 | 0.0265761 | 0.009815 | 0.0271184 | 0.0257934 | 0.0253949 | 0.0109543 | 0.024241 | 0.0125025 | 0.02594 |
| Specific Population Groups, Age | Approximate Body Weight (Kg) | MoE | |||||||||
| MPT1 | MPT2 | MPT3 | MPT4 | MPT5 | MPT6 | MPT7 | MPT8 | MPT9 | MPT10 | ||
| Cd Impurities | |||||||||||
| Children, 8–12 y/o | 30– 46 | 245,927,650– 377,089,063 | 136,332,652– 209,043,399 | 150,145,302– 230,222,796 | 166,155,419– 254,771,643 | 250,725,543– 384,445,832 | 129,453,481– 198,495,338 | 147,049,522– 225,475,934 | 273,667,749– 419,623,882 | 243,133,017– 372,803,960 | 169,136,012– 259,341,885 |
| Adolescents, 12–18 y/o | 46– 60 | 377,089,063– 491,855,299 | 209,043,399– 272,665,303 | 230,222,796– 300,290,604 | 254,771,643– 332,310,838 | 384,445,832– 501,451,086 | 198,495,338– 258,906,962 | 225,475,934– 294,099,045 | 419,623,882– 547,335,499 | 372,803,960– 486,266,035 | 259,341,885– 338,272,024 |
| Adults | 60 | 491,855,299 | 272,665,303 | 300,290,604 | 332,310,838 | 501,451,086 | 258,906,962 | 294,099,045 | 547,335,499 | 486,266,035 | 338,272,024 |
| Pb Impurities | |||||||||||
| Children, 8–12 y/o | 30– 46 | 228,462,618– 350,309,348 | 118,527,607– 181,742,331 | 320,935,583– 492,101,227 | 116,157,422– 178,108,048 | 122,124,233– 187,257,157 | 124,040,519– 190,195,463 | 287,558,282– 440,922,699 | 129,944,997– 199,248,995 | 251,949,430– 386,322,459 | 121,435,085– 186,200,464 |
| Adolescents, 12–18 y/o | 46– 60 | 350,309,348– 456,925,237 | 181,742,331– 237,055,215 | 492,101,227– 641,871,166 | 178,108,048– 232,314,845 | 187,257,157– 244,248,466 | 190,195,463– 248,081,039 | 440,922,699– 575,116,564 | 199,248,995– 259,889,994 | 386,322,459– 503,898,859 | 186,200,464– 242,870,171 |
| Adults | 60 | 456,925,237 | 237,055,215 | 641,871,166 | 232,314,845 | 244,248,466 | 248,081,039 | 575,116,564 | 259,889,994 | 503,898,859 | 242,870,171 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurowski, K.; Krośniak, M. The Human Health Risk Assessment of Heavy Metals Impurities (Cd and Pb) in Herbal Medicinal Products as Menthae piperitae tinctura (Mentha × piperita L., folium) Available in Pharmacies from Poland. Toxics 2022, 10, 273. https://doi.org/10.3390/toxics10050273
Jurowski K, Krośniak M. The Human Health Risk Assessment of Heavy Metals Impurities (Cd and Pb) in Herbal Medicinal Products as Menthae piperitae tinctura (Mentha × piperita L., folium) Available in Pharmacies from Poland. Toxics. 2022; 10(5):273. https://doi.org/10.3390/toxics10050273
Chicago/Turabian StyleJurowski, Kamil, and Mirosław Krośniak. 2022. "The Human Health Risk Assessment of Heavy Metals Impurities (Cd and Pb) in Herbal Medicinal Products as Menthae piperitae tinctura (Mentha × piperita L., folium) Available in Pharmacies from Poland" Toxics 10, no. 5: 273. https://doi.org/10.3390/toxics10050273
APA StyleJurowski, K., & Krośniak, M. (2022). The Human Health Risk Assessment of Heavy Metals Impurities (Cd and Pb) in Herbal Medicinal Products as Menthae piperitae tinctura (Mentha × piperita L., folium) Available in Pharmacies from Poland. Toxics, 10(5), 273. https://doi.org/10.3390/toxics10050273





