Environmental Toxicity Assessment of Sodium Fluoride and Platinum-Derived Drugs Co-Exposure on Aquatic Organisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Concentrations
2.2. Solutions Preparation
2.3. Zebrafish Maintenance and Breeding
2.4. Zebrafish Embryo Toxicity (ZFET) Assay
- embryo coagulation—can also occur within a few hours of the start of exposure and indicates a generic acute toxic effect;
- lack of somite formation— if a somite is not visible 12 h after fertilization, the embryo will not develop further, resulting in death;
- non-detachment of the tail— the dissociation of the tail from the yolk sac can be seen 24 h after fertilization, indicating that the embryo is growing normally;
- absence of heartbeat— the absence of a heartbeat 30 h after fertilization implies that the embryo has died; embryo coagulation and the absence of a heartbeat were utilized as endpoints of mortality.
2.5. Total RNA Extraction and RT-PCR
2.6. MDA, Antioxidant Enzyme and Acetylcholinesterase (AChE) Activity Measurement
2.7. Data Analysis
3. Results
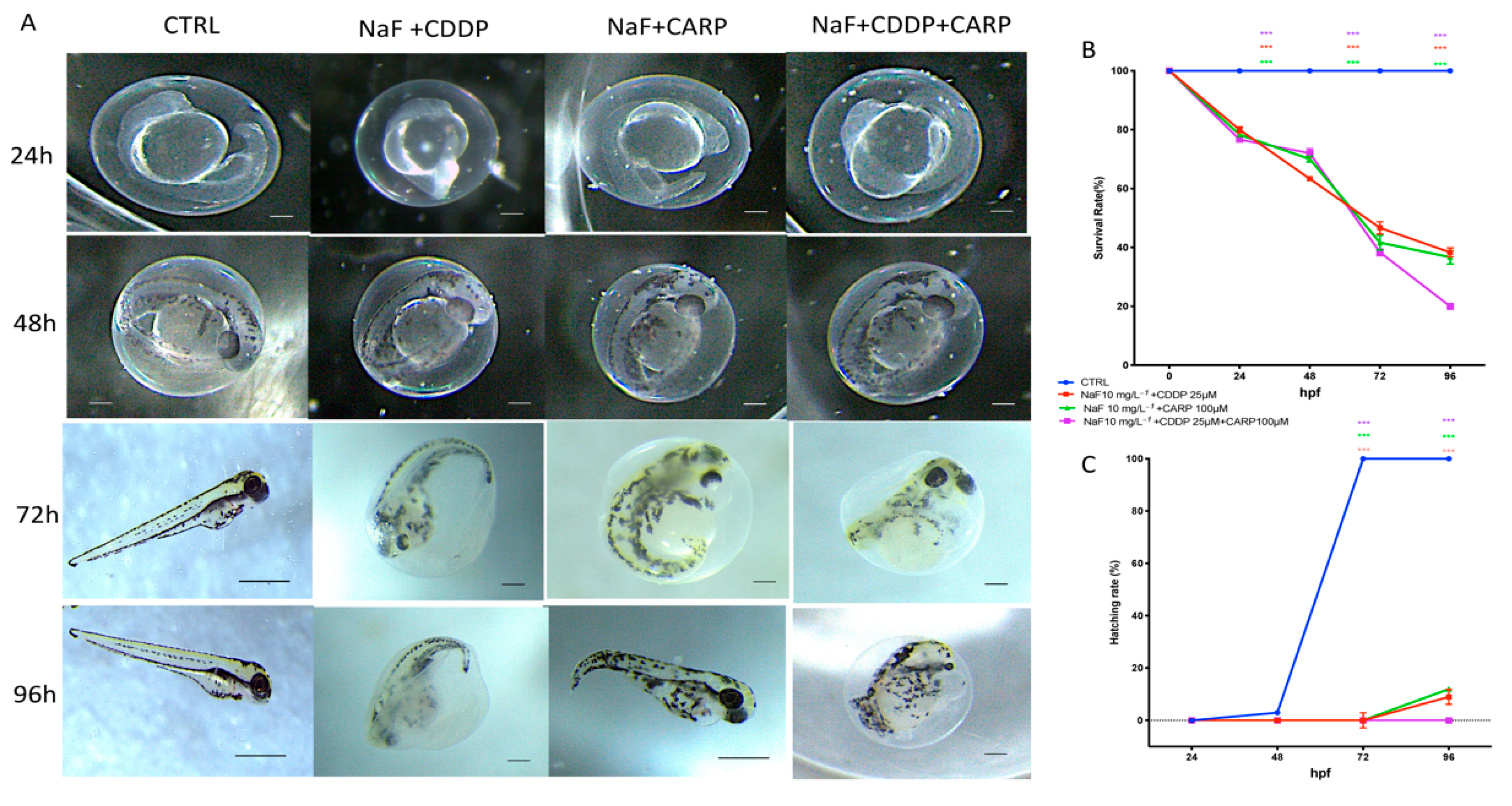
3.1. Viability and Morphology of Zebrafish Embryos after CDDP, CARP, and NaF Exposure
3.2. Survival and Hatching Rate
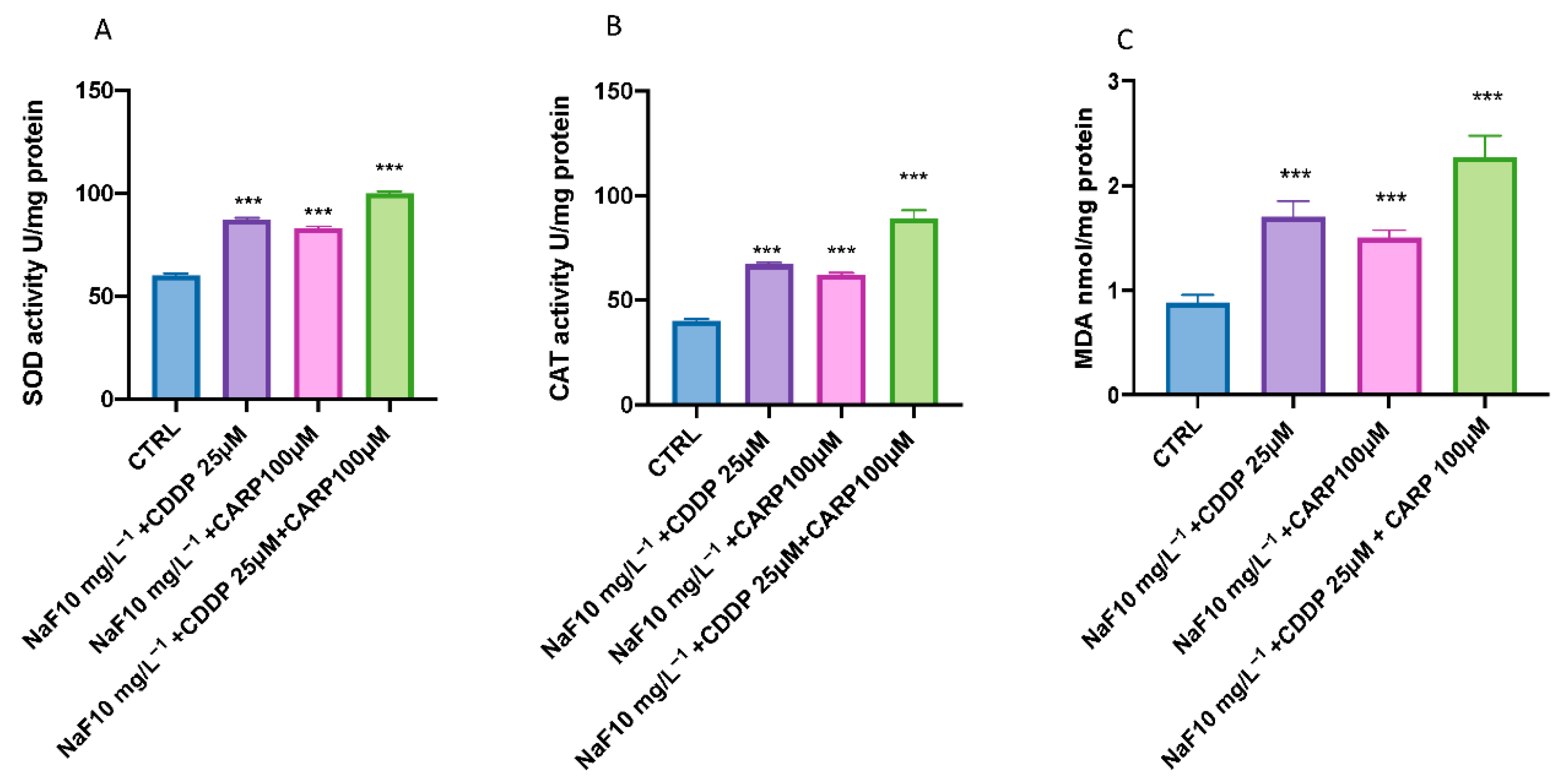
3.3. Effect of NaF, CDDP, and CARP on Lipid Peroxidation and Stress Oxidative Pathway
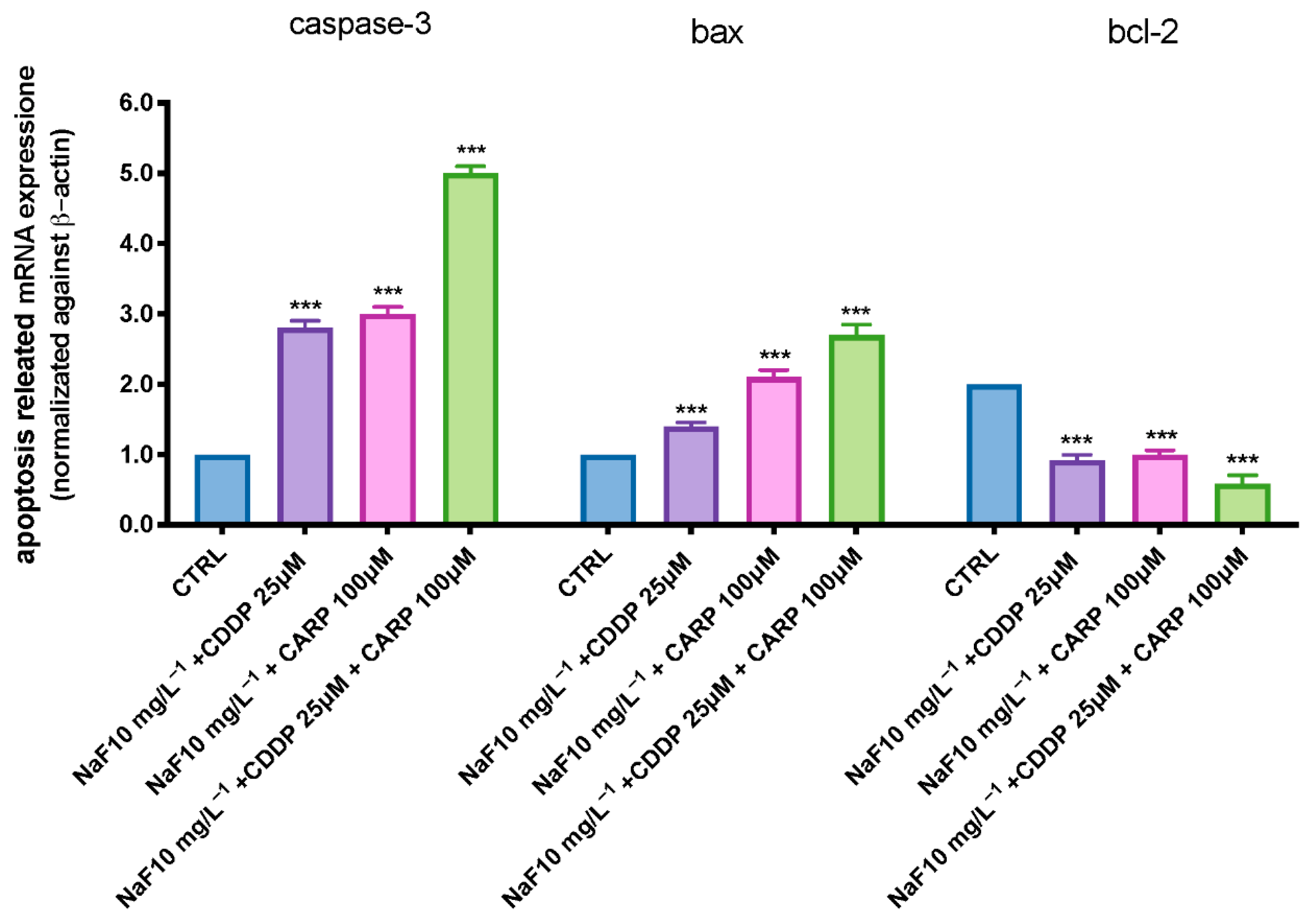
3.4. Apoptosis Process
3.5. AChE Activity after NaF, CDDP, and CARP Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kauffman, J.M. Water fluoridation: A review of recent research and actions. J. Am. Physicians Surg. 2005, 10, 38. [Google Scholar]
- Adedara, I.A.; Olabiyi, B.F.; Ojuade, T.D.; Idris, U.F.; Onibiyo, E.M.; Farombi, E.O. Taurine reverses sodium fluoride-mediated increase in inflammation, caspase-3 activity, and oxidative damage along the brain–pituitary–gonadal axis in male rats. Can. J. Physiol. Pharmacol. 2017, 95, 1019–1029. [Google Scholar] [CrossRef]
- WHO. Staff, Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004; Volume 1. [Google Scholar]
- Alarcón-Herrera, M.T.; Bundschuh, J.; Nath, B.; Nicolli, H.B.; Gutierrez, M.; Reyes-Gomez, V.M.; Nuñez, D.; Martin-Dominguez, I.R.; Sracek, O. Co-occurrence of arsenic and fluoride in groundwater of semi-arid regions in Latin America: Genesis, mobility and remediation. J. Hazard. Mater. 2013, 262, 960–969. [Google Scholar] [CrossRef]
- Mondal, P.; Shaw, P.; Bhowmik, A.D.; Bandyopadhyay, A.; Sudarshan, M.; Chakraborty, A.; Chattopadhyay, A. Combined effect of arsenic and fluoride at environmentally relevant concentrations in zebrafish (Danio rerio) brain: Alterations in stress marker and apoptotic gene expression. Chemosphere 2020, 269, 128678. [Google Scholar] [CrossRef]
- Duan, Q.; Jiao, J.; Chen, X.; Wang, X. Association between water fluoride and the level of children’s intelligence: A dose–response meta-analysis. Public Health 2018, 154, 87–97. [Google Scholar] [CrossRef]
- Yu, X.; Chen, J.; Li, Y.; Liu, H.; Hou, C.; Zeng, Q.; Cui, Y.; Zhao, L.; Li, P.; Zhou, Z.; et al. Threshold effects of moderately excessive fluoride exposure on children’s health: A potential association between dental fluorosis and loss of excellent intelligence. Environ. Int. 2018, 118, 116–124. [Google Scholar] [CrossRef]
- Aguirre-Sierra, A.; Alonso, A.; Camargo, J.A. Fluoride Bioaccumulation and Toxic Effects on the Survival and Behavior of the Endangered White-Clawed Crayfish Austropotamobius pallipes (Lereboullet). Arch. Environ. Contam. Toxicol. 2013, 65, 244–250. [Google Scholar] [CrossRef]
- Guth, S.; Hüser, S.; Roth, A.; Degen, G.; Diel, P.; Edlund, K.; Eisenbrand, G.; Engel, K.-H.; Epe, B.; Grune, T.; et al. Toxicity of fluoride: Critical evaluation of evidence for human developmental neurotoxicity in epidemiological studies, animal experiments and in vitro analyses. Arch. Toxicol. 2020, 94, 1375–1415. [Google Scholar] [CrossRef]
- Paul, V.; Ekambaram, P.; Jayakumar, A. Effects of sodium fluoride on locomotor behavior and a few biochemical parameters in rats. Environ. Toxicol. Pharmacol. 1998, 6, 187–191. [Google Scholar] [CrossRef]
- Wang, A.-G.; Xia, T.; Chu, Q.L.; Zhang, M.; Liu, F.; Chen, X.M.; Yang, K.D. Effects of flueoride on lipid peroxidation, DNA damige and apoptosis in human embryo hepatocytes. Biomed. Environ. Sci. 2004, 17, 217–222. [Google Scholar]
- Chioca, L.R.; Raupp, I.M.; Da Cunha, C.; Losso, E.M.; Andreatini, R. Subchronic fluoride intake induces impairment in habituation and active avoidance tasks in rats. Eur. J. Pharmacol. 2008, 579, 196–201. [Google Scholar] [CrossRef]
- Mullenix, P.J.; Denbesten, P.K.; Schunior, A.; Kernan, W.J. Neurotoxicity of sodium fluoride in rats. Neurotoxicol. Teratol. 1995, 17, 169–177. [Google Scholar] [CrossRef]
- Qian, W.; Miao, K.; Li, T.; Zhang, Z. Effect of Selenium on Fluoride-Induced Changes in Synaptic Plasticity in Rat Hippocampus. Biol. Trace Element Res. 2013, 155, 253–260. [Google Scholar] [CrossRef]
- Wei, Y.; Zeng, B.; Zhang, H.; Chen, C.; Wu, Y.; Wang, N.; Wu, Y.; Zhao, D.; Zhao, Y.; Iqbal, J.; et al. Comparative proteomic analysis of fluoride treated rat bone provides new insights into the molecular mechanisms of fluoride toxicity. Toxicol. Lett. 2018, 291, 39–50. [Google Scholar] [CrossRef]
- Wei, Q.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. A mini review of fluoride-induced apoptotic pathways. Environ. Sci. Pollut. Res. 2018, 25, 33926–33935. [Google Scholar] [CrossRef]
- Chirumari, K.; Reddy, P.K. Dose-dependent effects of fluoride on neurochemical milieu in the hippocampus and neocortex of rat brain. Fluoride 2007, 40, 101–110. [Google Scholar]
- Shan, K.-R.; Qi, X.-L.; Long, Y.-G.; Nordberg, A.; Guan, Z.-Z. Decreased nicotinic receptors in PC12 cells and rat brains influenced by fluoride toxicity—a mechanism relating to a damage at the level in post-transcription of the receptor genes. Toxicology 2004, 200, 169–177. [Google Scholar] [CrossRef]
- Rico, E.P.; Rosemberg, D.; Dias, R.D.; Bogo, M.R.; Bonan, C.D. Ethanol alters acetylcholinesterase activity and gene expression in zebrafish brain. Toxicol. Lett. 2007, 174, 25–30. [Google Scholar] [CrossRef]
- Goschorska, M.; Baranowska-Bosiacka, I.; Gutowska, I.; Metryka, E.; Skórka-Majewicz, M.; Chlubek, D. Potential Role of Fluoride in the Etiopathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2018, 19, 3965. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Singh, A.; Verma, K.; Paliwal, S.; Sharma, S.; Dwivedi, J. Fluoride: A review of pre-clinical and clinical studies. Environ. Toxicol. Pharmacol. 2017, 56, 297–313. [Google Scholar] [CrossRef]
- Dondossola, E.R.; Pacheco, S.D.; Visentin, S.C.; Mendes, N.V.; Baldin, S.L.; Bernardo, H.T.; Scussel, R.; Rico, E.P. Prolonged fluoride exposure alters neurotransmission and oxidative stress in the zebrafish brain. NeuroToxicology 2022, 89, 92–98. [Google Scholar] [CrossRef]
- Poklar, N.; Pilch, D.S.; Lippard, S.J.; Redding, E.A.; Dunham, S.U.; Breslauer, K.J. Influence of cisplatin intrastrand crosslinking on the conformation, thermal stability, and energetics of a 20-mer DNA duplex. Proc. Natl. Acad. Sci. USA 1996, 93, 7606–7611. [Google Scholar] [CrossRef] [Green Version]
- Rudd, G.; Hartley, J.; Souhami, R. Persistence of cisplatin-induced DNA interstrand crosslinking in peripheral blood mononuclear cells from elderly and young individuals. Cancer Chemother. Pharmacol. 1995, 35, 323–326. [Google Scholar] [CrossRef]
- Osterauer, R.; Faßbender, C.; Braunbeck, T.; Köhler, H.-R. Genotoxicity of platinum in embryos of zebrafish (Danio rerio) and ramshorn snail (Marisa cornuarietis). Sci. Total Environ. 2011, 409, 2114–2119. [Google Scholar] [CrossRef]
- Karas, B.F.; Hotz, J.M.; Buckley, B.T.; Cooper, K.R. Cisplatin alkylating activity in zebrafish causes resistance to chorionic degradation and inhibition of osteogenesis. Aquat. Toxicol. 2020, 229, 105656. [Google Scholar] [CrossRef]
- Alt, F.; Eschnauer, H.R.; Mergler, B.; Messerschmidt, J.; Tölg, G. A contribution to the ecology and enology of platinum. Anal. Bioanal. Chem. 1997, 357, 1013–1019. [Google Scholar] [CrossRef]
- Lenz, K.; Hann, S.; Koellensperger, G.; Stefanka, Z.; Stingeder, G.; Weissenbacher, N.; Mahnik, S.N.; Fuerhacker, M. Presence of cancerostatic platinum compounds in hospital wastewater and possible elimination by adsorption to activated sludge. Sci. Total Environ. 2005, 345, 141–152. [Google Scholar] [CrossRef]
- Li, D.; Chen, H.; Liu, H.; Schlenk, D.; Mu, J.; Lacorte, S.; Ying, G.-G.; Xie, L. Anticancer drugs in the aquatic ecosystem: Environmental occurrence, ecotoxicological effect and risk assessment. Environ. Int. 2021, 153, 106543. [Google Scholar] [CrossRef]
- Zhang, C.; Willett, C.; Fremgen, T. Zebrafish: An Animal Model for Toxicological Studies. Curr. Protoc. Toxicol. 2003, 17, 1.7.1–1.7.18. [Google Scholar] [CrossRef]
- McGrath, P.; Li, C.-Q. Zebrafish: A predictive model for assessing drug-induced toxicity. Drug Discov. Today 2008, 13, 394–401. [Google Scholar] [CrossRef]
- Madhavan, N.; Subramanian, V. Environmental Impact Assessment, Remediation and Evolution of Fluoride and Arsenic Contamination Process in Groundwater. In Groundwater; Springer: Dordrecht, The Netherlands, 2007; pp. 128–155. [Google Scholar] [CrossRef]
- Cronin, S.J.; Neall, V.; Lecointre, J.; Hedley, M.; Loganathan, P. Environmental hazards of fluoride in volcanic ash: A case study from Ruapehu volcano, New Zealand. J. Volcanol. Geotherm. Res. 2003, 121, 271–291. [Google Scholar] [CrossRef]
- Queirós, V.; Azeiteiro, U.M.; Soares, A.M.; Freitas, R. The antineoplastic drugs cyclophosphamide and cisplatin in the aquatic environment—Review. J. Hazard. Mater. 2021, 412, 125028. [Google Scholar] [CrossRef]
- Di Paola, D.; Iaria, C.; Capparucci, F.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Aflatoxin B1 Toxicity in Zebrafish Larva (Danio rerio): Protective Role of Hericium erinaceus. Toxins 2021, 13, 710. [Google Scholar] [CrossRef]
- Di Paola, D.; Capparucci, F.; Lanteri, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Combined Toxicity of Xenobiotics Bisphenol A and Heavy Metals on Zebrafish Embryos (Danio rerio). Toxics 2021, 9, 344. [Google Scholar] [CrossRef]
- Di Paola, D.; Capparucci, F.; Abbate, J.M.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Genovese, T.; Impellizzeri, D.; et al. Environmental Risk Assessment of Oxaliplatin Exposure on Early Life Stages of Zebrafish (Danio rerio). Toxics 2022, 10, 81. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wei, Y.; Zhang, H.; Xu, M.; Dai, J. Induction of time-dependent oxidative stress and related transcriptional effects of perfluorododecanoic acid in zebrafish liver. Aquat. Toxicol. 2008, 89, 242–250. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, X.; Shu, L.; Chen, L.; Sun, L.; Qian, H.; Liu, W.; Fu, Z. Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere 2009, 78, 846–852. [Google Scholar] [CrossRef]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017, 584-585, 1022–1031. [Google Scholar] [CrossRef]
- Vani, M.L.; Reddy, K.P. Effects of fluoride accumulation on some enzymes of brain and gastrocnemius muscle of mice. Fluoride 2000, 33, 17–26. [Google Scholar]
- Williams, R.T. Human Pharmaceuticals: Assessing the Impacts on Aquatic Ecosystems; Allen Press/ACG Publishing: Pensacola, FL, USA; SETAC Press: Pensacola, FL, USA, 2008. [Google Scholar]
- Rosi-Marshall, E.J.; Royer, T.V. Pharmaceutical Compounds and Ecosystem Function: An Emerging Research Challenge for Aquatic Ecologists. Ecosystems 2012, 15, 867–880. [Google Scholar] [CrossRef]
- Samaee, S.-M.; Rabbani, S.; Jovanović, B.; Mohajeri-Tehrani, M.R.; Haghpanah, V. Efficacy of the hatching event in assessing the embryo toxicity of the nano-sized TiO2 particles in zebrafish: A comparison between two different classes of hatching-derived variables. Ecotoxicol. Environ. Saf. 2015, 116, 121–128. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Luo, G.-Z.; Wang, X.; Yue, Y.; Wang, X.; Zong, X.; Chen, K.; Yin, H.; Fu, Y.; et al. Abundant DNA 6mA methylation during early embryogenesis of zebrafish and pig. Nat. Commun. 2016, 7, 13052. [Google Scholar] [CrossRef]
- Papiya, S.; Kanamadi, R. Effect of mercurial fungicide Emisan®-6 on the embryonic developmental stages of zebrafish, Brachydanio (Danio) rerio. J. Adv. Zool. 2000, 21, 12–18. [Google Scholar]
- Ismail, A.; Yusof, S. Effect of mercury and cadmium on early life stages of Java medaka (Oryzias javanicus): A potential tropical test fish. Mar. Pollut. Bull. 2011, 63, 347–349. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Chattopadhyay, A. Induction of Oxidative Stress and Related Transcriptional Effects of Sodium Fluoride in Female Zebrafish Liver. Bull. Environ. Contam. Toxicol. 2014, 93, 64–70. [Google Scholar] [CrossRef]
- Domarecka, E.; Skarzynska, M.; Szczepek, A.J.; Hatzopoulos, S. Use of zebrafish larvae lateral line to study protection against cisplatin-induced ototoxicity: A scoping review. Int. J. Immunopathol. Pharmacol. 2020, 34. [Google Scholar] [CrossRef]
- Husain, K.; Whitworth, C.; Somani, S.; Rybak, L. Carboplatin-induced oxidative stress in rat cochlea. Hear. Res. 2001, 159, 14–22. [Google Scholar] [CrossRef]
- Steiling, H.; Munz, B.; Werner, S.; Brauchle, M. Different types of ROS-scavenging enzymes are expressed during cutaneous wound repair. Exp. Cell Res. 1999, 247, 484–494. [Google Scholar] [CrossRef]
- Kanzaki, H.; Wada, S.; Narimiya, T.; Yamaguchi, Y.; Katsumata, Y.; Itohiya, K.; Fukaya, S.; Miyamoto, Y.; Nakamura, Y. Pathways that Regulate ROS Scavenging Enzymes, and Their Role in Defense Against Tissue Destruction in Periodontitis. Front. Physiol. 2017, 8, 351. [Google Scholar] [CrossRef] [Green Version]
- Lubrano, V.; Balzan, S. Enzymatic antioxidant system in vascular inflammation and coronary artery disease. World J. Exp. Med. 2015, 5, 218. [Google Scholar] [CrossRef]
- Yasui, K.; Baba, A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm. Res. 2006, 55, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Negre-Salvayre, A.; Coatrieux, C.; Ingueneau, C.; Salvayre, R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. J. Cereb. Blood Flow Metab. 2008, 153, 6–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niki, E. Lipid peroxidation products as oxidative stress biomarkers. BioFactors 2008, 34, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, T.; Houari, S.; Mauro, N.; MacDougall, M.; Peters, H.; Berdal, A. Fluoride at non-toxic dose affects odontoblast gene expression in vitro. Toxicology 2008, 249, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Mišík, M.; Filipic, M.; Nersesyan, A.; Kundi, M.; Isidori, M.; Knasmueller, S. Environmental risk assessment of widely used anticancer drugs (5-fluorouracil, cisplatin, etoposide, imatinib mesylate). Water Res. 2019, 164, 114953. [Google Scholar] [CrossRef]
- Ghafuria, Y.; Yunesian, M.; Nabizadeh, R.; Mesdaghinia, A.; Dehghani, M.H.; Alimohammadi, M. Environmental risk assessment of platinum cytotoxic drugs: A focus on toxicity characterization of hospital effluents. Int. J. Environ. Sci. Technol. 2017, 15, 1983–1990. [Google Scholar] [CrossRef]
- Lee, S.K.; Oh, K.H.; Chung, A.Y.; Park, H.C.; Lee, S.H.; Kwon, S.Y.; Choi, J. Protective role of quercetin against cisplatin-induced hair cell damage in zebrafish embryos. Hum. Exp. Toxicol. 2015, 34, 1043–1052. [Google Scholar] [CrossRef]
- Vidot, S.; Witham, J.; Agarwal, R.; Greenhough, S.; Bamrah, H.S.; Tigyi, G.J.; Kaye, S.B.; Richardson, A. Autotaxin delays apoptosis induced by carboplatin in ovarian cancer cells. Cell. Signal. 2010, 22, 926–935. [Google Scholar] [CrossRef]
- Franco, R.; Sánchez-Olea, R.; Reyes-Reyes, E.M.; Panayiotidis, M.I. Environmental toxicity, oxidative stress and apoptosis: Menage a trois. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2009, 674, 3–22. [Google Scholar] [CrossRef]
- Ozben, T. Oxidative stress and apoptosis: Impact on cancer therapy. J. Pharm. Sci. 2007, 96, 2181–2196. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Kim, S.; Ahn, J.H.; Youn, P.; Kang, J.S.; Park, K.; Yi, J.; Ryu, D.-Y. Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat. Toxicol. 2010, 100, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Wei, L.; Zhang, Y.; Kong, H.; Shi, Y.; Wang, X.; Chen, X.; Han, L.; Liu, K. Psoralen Induces Developmental Toxicity in Zebrafish Embryos/Larvae Through Oxidative Stress, Apoptosis, and Energy Metabolism Disorder. Front. Pharmacol. 2018, 9, 1457. [Google Scholar] [CrossRef] [PubMed]
- Deidda, I.; Russo, R.; Bonaventura, R.; Costa, C.; Zito, F.; Lampiasi, N. Neurotoxicity in Marine Invertebrates: An Update. Biology 2021, 10, 161. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Orientation | Nucleotide Sequence |
|---|---|---|
| b-actin | forward | 5′-AGAGCTATGAGCTGCCTGACG-3′ |
| reverse | 5′-CCGCAAGATTCCATACCCA-3′ | |
| casp-3 | forward | 5′-CCGCTGCCCATCACTA-3′ |
| reverse | 5′-ATCCTTTCACGACCATCT-3′ | |
| Bax | forward | 5′-GGCTATTTCAACCAGGGTTCC-3′ |
| reverse | 5′-TGCGAATCACCAATGCTGT-3′ | |
| bcl-2 | forward | 5′-TCACTCGTTCAGACCCTCAT-3′ |
| reverse | 5′-ACGCTTTCCACGCACAT-3′ |
| Malformations | Mortality | Hatching | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 96 h | 24 h | 48 h | 72 h | 96 h | 24 h | 48 h | 72 h | 96 h | |
| CTRL | 0 | 0 | 0 | 0 | 0 | 0 | 8% | 96% | 100% |
| NaF | |||||||||
| 50 mg/L−1 | SC, PE and YE | 0 | 0 | 82% | 100% | 0 | 0 | 100% | 100% |
| 25 mg/L−1 | SC | 0 | 0 | 11% | 15% | 0 | 0 | 15% | 85% |
| 10 mg/L−1 | 0 | 0 | 0 | 4% | 5% | 0 | 0 | 90% | 95% |
| 5 mg/L−1 | 0 | 0 | 0 | 0 | 0 | 0 | 3% | 95% | 100% |
| CDDP | |||||||||
| 50μM | SC PE and YE | 0 | 0 | 30% | 60% | 0 | 0 | 0 | 0 |
| 25 μM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100% |
| 10μM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100% |
| 5 μM | 0 | 0 | 0 | 0 | 0 | 0 | 6% | 94% | 100% |
| CARP | |||||||||
| 150μM | SC, PE and YE | 0 | 0 | 40% | 75% | 0 | 0 | 75% | 0 |
| 100μM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100% |
| 50μM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100% |
| 25 μM | 0 | 0 | 0 | 0 | 0 | 0 | 5% | 20% | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Paola, D.; Capparucci, F.; Lanteri, G.; Crupi, R.; Marino, Y.; Franco, G.A.; Cuzzocrea, S.; Spanò, N.; Gugliandolo, E.; Peritore, A.F. Environmental Toxicity Assessment of Sodium Fluoride and Platinum-Derived Drugs Co-Exposure on Aquatic Organisms. Toxics 2022, 10, 272. https://doi.org/10.3390/toxics10050272
Di Paola D, Capparucci F, Lanteri G, Crupi R, Marino Y, Franco GA, Cuzzocrea S, Spanò N, Gugliandolo E, Peritore AF. Environmental Toxicity Assessment of Sodium Fluoride and Platinum-Derived Drugs Co-Exposure on Aquatic Organisms. Toxics. 2022; 10(5):272. https://doi.org/10.3390/toxics10050272
Chicago/Turabian StyleDi Paola, Davide, Fabiano Capparucci, Giovanni Lanteri, Rosalia Crupi, Ylenia Marino, Gianluca Antonio Franco, Salvatore Cuzzocrea, Nunziacarla Spanò, Enrico Gugliandolo, and Alessio Filippo Peritore. 2022. "Environmental Toxicity Assessment of Sodium Fluoride and Platinum-Derived Drugs Co-Exposure on Aquatic Organisms" Toxics 10, no. 5: 272. https://doi.org/10.3390/toxics10050272
APA StyleDi Paola, D., Capparucci, F., Lanteri, G., Crupi, R., Marino, Y., Franco, G. A., Cuzzocrea, S., Spanò, N., Gugliandolo, E., & Peritore, A. F. (2022). Environmental Toxicity Assessment of Sodium Fluoride and Platinum-Derived Drugs Co-Exposure on Aquatic Organisms. Toxics, 10(5), 272. https://doi.org/10.3390/toxics10050272











