Experimental Study on the Detection of Hazardous Chemicals Using Alternative Sensors in the Water Environment
Abstract
:1. Introduction
2. Experimental Set-Up and Procedure
2.1. Chemical Reagents and Measurement Sensors
2.2. Selectd Solvents
2.3. Experimental Method
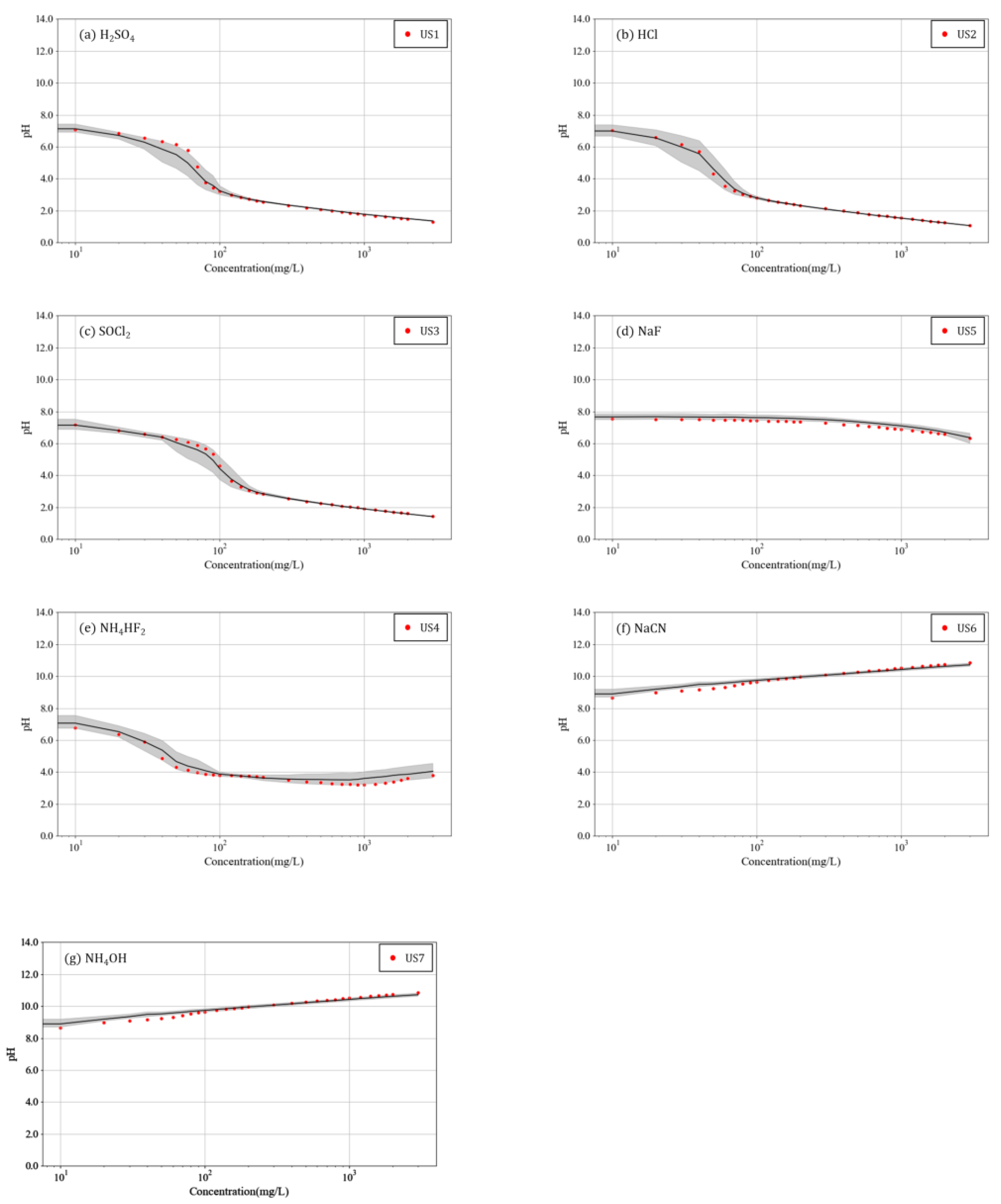
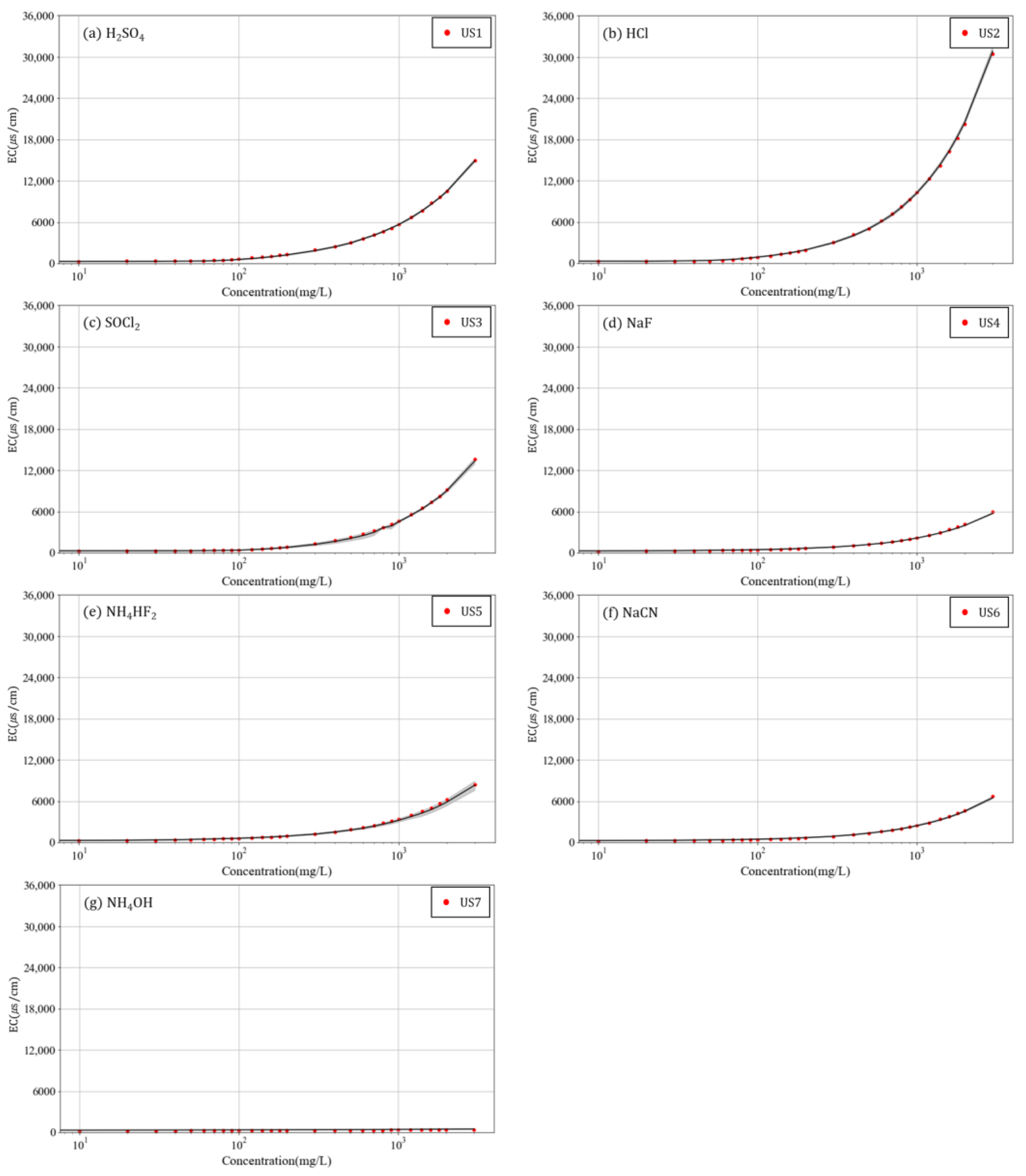
3. Results and Discussion
4. Conclusions
- (1)
- The measurement results for pH and EC according to the concentration of the seven chemicals showed very similar patterns, even when solvents with different base concentrations were used. This confirmed that the variations in pH and EC according to the concentrations of chemicals had low sensitivity to the base concentration of the solvent and that each chemical has a distinct effect on these parameters.
- (2)
- For each chemical, the average variations in pH and EC in six solvents according to the chemical concentrations were numerically compared with the variations in pH and EC according to the concentrations of unknown substances. The unknown substances were identified by assuming that the MAPEs of pH and EC would be the lowest when comparing the unknown substance with the correct chemical. Consequently, all seven unknown substances could be identified through the MAPEs, demonstrating the possibility of using pH and EC to identify chemicals.
- (3)
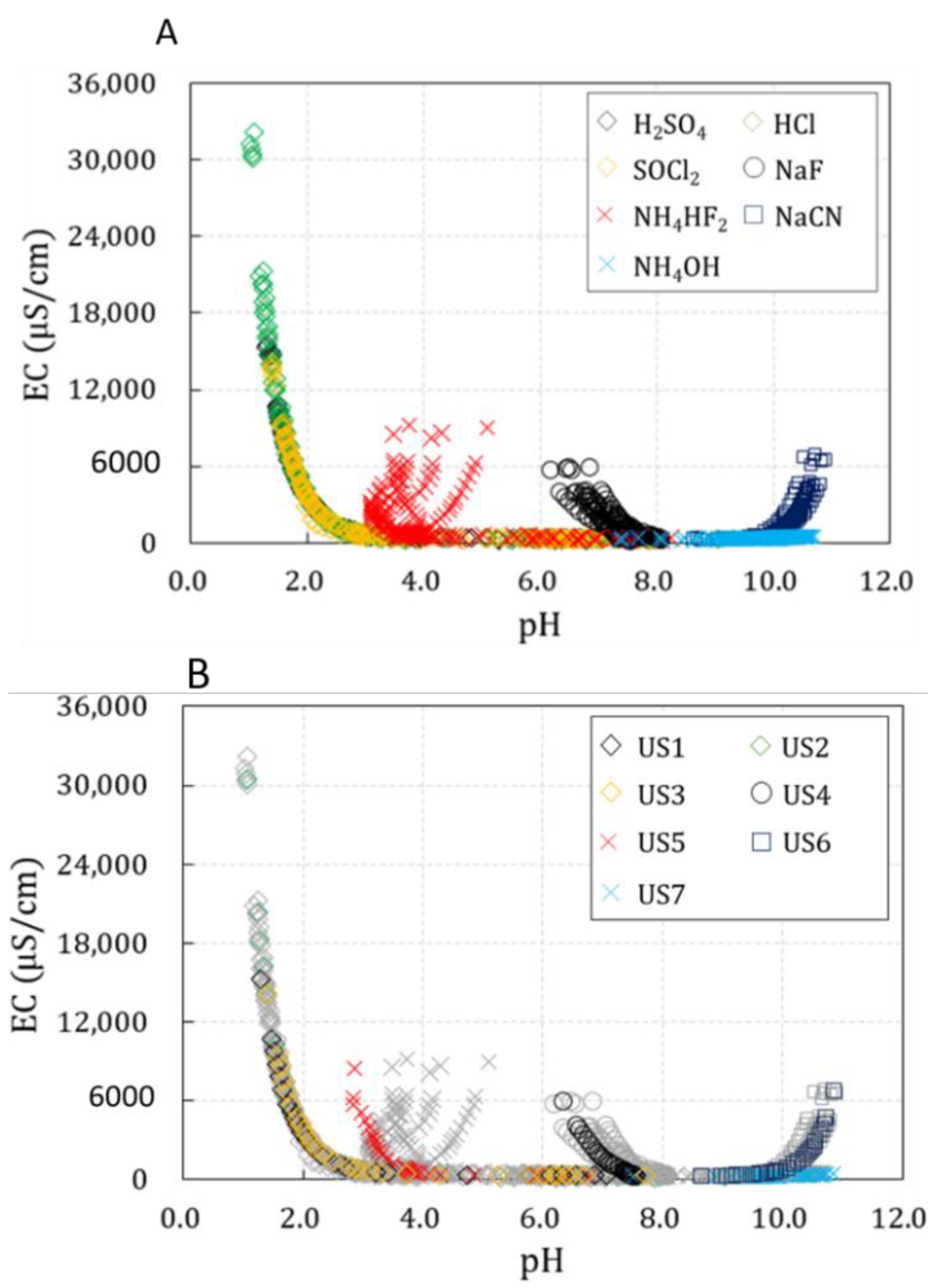
- The actual concentrations of chemicals cannot be obtained by measuring pH and EC in the event of chemical accidents in rivers. Therefore, the measurement results according to the concentrations of the seven chemicals were combined and represented as pH-EC relation curves. NaF, NH4HF2, NaCN, and NH4OH could be clearly classified by comparing their distributions in a pH-EC relation curve. However, H2SO4, HCl, and SOCl2 showed similar distributions in the pH-EC relation curve. Therefore, there are limitations in classifying these three substances using pH and EC in natural rivers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.K.; Kim, T.O.; Jung, Y.J. Analysis of Domestic Water Pollution Accident and Response Management. J. Wetl. Res. 2013, 15, 529–534. [Google Scholar]
- Gangopadhyay, R.K.; Das, S.K. Lessons learned from a fuming sulfuric acid tank overflow incident. J. Chem. Health Safe. 2008, 15, 13–15. [Google Scholar]
- Cordos, E.; Rautiu, R.; Roman, C.; Ponta, M.; Frentiu, T.; Sarkany, A.; Fodorpataki, L.; Macalik, L.; McCormick, C.; Weiss, D. Characterization of the rivers in the mining and industrial area of Baia Mare, Romania. Eur. J. Min. Process. Environ. Protect. 2003, 3, 324–335. [Google Scholar]
- Zunkel, A.; Tiebe, C.; Schlischka, J. “Stolt Rotterdam”—The sinking of an acid freigher. Engin. Fail. Anal. 2014, 43, 221–231. [Google Scholar]
- Carbon, J.Y.; Giamarchi, P.; Floch, L.S. A study of marine pollution caused by the release of metals into seawater following acid spills. Mar. Poll. Bull. 2010, 60, 998–1004. [Google Scholar]
- Hou, J.; Gai, W.M.; Cheng, W.Y.; Deng, Y.F. Hazardous chemical leakage accidents and emergency evacuation response from 2009 to 2018 in China: A review. Safe. Sci. 2021, 135, 105101. [Google Scholar]
- Korea Institute of Civil Engineering and Building Technology (KICT). Report of the Comprehensive Plan Review for Clear Water Supply in Gyeongsangbuk-do and Daegu-si Region; Ministry of Land, Infrastructure and Transport and K-Water: Sejong City, Korea, 2014; p. 7. [Google Scholar]
- Korea Institute of Civil Engineering and Building Technology (KICT). The Feasibility Study of the Relocation of Drinking Water Intake Towers from the Nakdong River; KICT: Daegu-si, Korea, 2008; p. 665. [Google Scholar]
- Lee, S.H.; Jung, H.W.; Jung, J.Y.; Min, H.J.; Kim, B.R.; Park, C.G.; Oh, J.E.; Yuu, O.; Nobuyuki, S. Characteristics of Occurrence of Pharmaceuticals in the Nakdong River. J. Kor. Soc. Environ. Eng. 2013, 35, 45–56. [Google Scholar]
- Kwon, H.K.; Park, I.H.; Ha, S.R. Wastewater Flowrate Analysis of Drainage Basin for Application of Total Water Pollution Load Management System. J. Wetl. Res. 2009, 11, 75–82. [Google Scholar]
- Lee, J.; Bae, S.; Lee, D.R.; Seo, D. Transportation Modeling of Conservative Pollutant in a River with Weirs—The Nakdong River Case. J. Kor. Soc. Environ. Eng. 2014, 36, 821–827. [Google Scholar]
- Choi, M.O. A Case Study of Environmental Policy Formation: A Focus on the Phenol Spills in Nakdong River of 1991 and 2008. GRI Rev. 2013, 15, 91–112. [Google Scholar]
- Lee, K.S. Drinking Water Resource Projects in Gyeongbuk and Daegu; Korea Development Institute (KDI): Yeongi-gun, Korea, 2011; p. 474.
- Ministry of Environment (ME). 2018 Annual Report of Water Pollution Accident and Response Management; Ministry of Environment (ME): Sejong City, Korea, 2019; pp. 3–7.
- Jang, J.; Jong, J.; Mun, H.; Kim, K.; Seo, I. Mixing Analysis of Oil Spilled into River by GPS-equipped Drifter Experiment and Numerical Modeling. J. Korean Soc. Water Environ. KSWE 2016, 32, 243–252. [Google Scholar]
- Dunsbergen, D.W.; Stalling, G.S. The Combination of Random Walk Method and a Hydrodynamic Model for the Simulation of Dispersion of Dissolved Matter in Water. Transac. Ecol. Environ. 1993, 2, 235–242. [Google Scholar]
- Craig, P.M. Implementation of a Lagrangian Particle Tracking Sub-Model for the Environmental Fluid Dynamics Code; Dynamic Solutions-International, LLC.: Knoxville, TN, USA, 2009; pp. 475–482. [Google Scholar]
- Wang, S.D.; Shen, Y.M.; Guo, Y.K.; Tang, J. Three-dimensional Numerical Simulation for Transport of Oil Spills in Seas. Ocean Engin. 2008, 35, 503–510. [Google Scholar]
- Korea Environment Corporation (K-eco). The Self-Prevention Guidebook for Efficient Response of Water Pollution Accident; Korea Environment Corporation: Seoul, Korea, 2017. [Google Scholar]
- Neely, W.B.; Blau, G.E.; Alfrey, T. Mathematical models predict concentration-time profiles resulting from chemical spill in a river. Environ. Sci. Tech. 1976, 10, 72–76. [Google Scholar]
- Fu, W.; Fu, H.; Skøtt, K.; Yang, M. Modeling the spill in the Songhua River after the explosion in the petrochemical plant in Jilin. Environ. Sci. Poll. Res. 2008, 15, 178–181. [Google Scholar]
- Yeom, J.; Kim, I.; Kim, M.; Cho, K.; Kim, S.D. Coupling of the AQUATOX and EFDC Models for Ecological Impact Assessment of Chemical Spill Scenarios in the Jeonju River, Korea. Biology 2020, 9, 340. [Google Scholar]
- EPA ORD NHSRC(a). Rapid Screening and Preliminary Identification Techniques and Method; EPA/600/R-10/090. 2010. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=227244&Lab=NHSRC (accessed on 26 January 2022).
- EPA ORD NHSRC(b). Sample Collection Information Document for Pathogens and Biotoxins; EPA/600/R-09/074. 2010. Available online: https://19january2017snapshot.epa.gov/homeland-security-research/sample-collection-information-document-pathogens-and-biotoxins-companion_.html (accessed on 26 January 2022).
- National Institute for Occupational Safty and Health. NIOSH Manual of Analytical Methods, 4th ed.; CDC: Atlanta, GA, USA, 1994.
- OSHA Analytical Methods. Available online: https://www.osha.gov/chemicaldata/sampling-analytical-methods (accessed on 18 January 2022).
- Kim, K.; Lee, S.; Hwang, S.; Kim, Y.; Seok, G. Study on the analytical method using GC-MS for the accident preparedness substances. Anal. Sci. Technol. 2013, 26, 80–85. [Google Scholar]
- Sartorius, A.G. Operation Manual Sartorius Professional Meter. 2005, p. WPP6003-e05011. Available online: http://www.scaleservice.net/manuals/sartorius/MAN-PP-15-e.pdf (accessed on 14 April 2022).
- YSI Incorporated. Pro2030 User Manual. 2010. Available online: https://www.ysi.com/pro2030 (accessed on 20 January 2022).

| Name | CAS No. | Molecular Formula | Molecular Weight (g/mol) | Number of Factories Using Substance | Chemical Family |
|---|---|---|---|---|---|
| Sulfuric acid | 7664-93-9 | H2SO4 | 98.09 | 2588 | Inorganic oxidizing acids |
| Hydrochloric acid | 7647-01-0 | HCl | 36.46 | 3058 | Inorganic non oxidizing acids |
| Thionyl chloride | 7719-09-7 | SOCl2 | 118.97 | 40 | Acid halides |
| Sodium fluoride | 7681-49-4 | NaF | 41.99 | 162 | Inorganic compounds |
| Ammonium bifluoride | 1341-49-7 | NH4HF2 | 57.04 | 385 | Inorganic compounds |
| Sodium cyanide | 143-33-9 | NaCN | 49.01 | 673 | Inorganic cyanides |
| Ammonia hydroxide | 1336-21-6 | NH4OH | 35.05 | 963 | Bases |
| River | pH | EC (µS/cm) |
|---|---|---|
| Joman River (JM) (Dry Season/Wet Season) | 7.5/7.4 | 335.6/308.2 |
| Sineo Stream (SS) (Dry Season/Wet Season) | 8.6/7.6 | 234.1/158.5 |
| West Nakdong River (WNR) (Dry Season/Wet Season) | 8.7/8.0 | 350.2/297.0 |
| Gam Stream (GC) | 9.9 | 212.4 |
| Range (mg/L) | 0–100 | 100–200 | 200–1000 | 1000–2000 | 2000–3000 |
|---|---|---|---|---|---|
| Concentration Interval (mg/L) | 10 | 20 | 100 | 200 | 1000 |
| US1 | US2 | US3 | US4 | US5 | US6 | US7 | |
|---|---|---|---|---|---|---|---|
| Unknown Substance | H2SO4 | HCl | SOCl2 | NaF | NH4HF2 | NaCN | NH4OH |
| US1 | H2SO4 | HCl | SOCl2 | NaF | NH4HF2 | NaCN | NH4OH |
| pH | 2.81% | 16.43% | 9.95% | 57.40% | 34.23% | 67.23% | 66.51% |
| EC | 6.59% | 31.34% | 38.16% | 99.22% | 45.69% | 82.88% | 698.12% |
| US2 | H2SO4 | HCl | SOCl2 | NaF | NH4HF2 | NaCN | NH4OH |
| pH | 12.77% | 1.85% | 21.61% | 63.24% | 37.89% | 71.70% | 71.08% |
| EC | 56.46% | 3.74% | 103.43% | 216.29% | 125.18% | 187.16% | 1320.96% |
| US3 | H2SO4 | HCl | SOCl2 | NaF | NH4HF2 | NaCN | NH4OH |
| pH | 14.12% | 32.38% | 1.94% | 52.17% | 33.29% | 63.25% | 62.44% |
| EC | 19.23% | 45.55% | 5.11% | 59.92% | 31.26% | 48.28% | 550.95% |
| US4 | H2SO4 | HCl | SOCl2 | NaF | NH4HF2 | NaCN | NH4OH |
| pH | 187.13% | 234.66% | 159.90% | 2.43% | 79.34% | 27.94% | 26.48% |
| EC | 41.18% | 58.29% | 28.48% | 4.92% | 29.75% | 9.71% | 239.25% |
| US5 | H2SO4 | HCl | SOCl2 | NaF | NH4HF2 | NaCN | NH4OH |
| pH | 54.46% | 75.09% | 48.51% | 46.80% | 5.41% | 60.32% | 59.50% |
| EC | 26.34% | 43.99% | 26.53% | 40.64% | 5.67% | 30.24% | 401.27% |
| US6 | H2SO4 | HCl | SOCl2 | NaF | NH4HF2 | NaCN | NH4OH |
| pH | 316.95% | 387.18% | 278.91% | 36.15% | 151.80% | 1.20% | 1.70% |
| EC | 39.43% | 57.58% | 25.38% | 11.25% | 26.14% | 6.49% | 274.75% |
| US7 | H2SO4 | HCl | SOCl2 | NaF | NH4HF2 | NaCN | NH4OH |
| pH | 315.87% | 385.83% | 277.79% | 36.45% | 152.09% | 0.19% | 1.90% |
| EC | 69.15% | 75.80% | 62.12% | 59.70% | 69.06% | 61.32% | 2.34% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, S.-H.; Ku, T.-G.; Park, Y.-L.; Kwon, J.-H.; Huh, D.-S.; Kim, Y.-D. Experimental Study on the Detection of Hazardous Chemicals Using Alternative Sensors in the Water Environment. Toxics 2022, 10, 200. https://doi.org/10.3390/toxics10050200
Nam S-H, Ku T-G, Park Y-L, Kwon J-H, Huh D-S, Kim Y-D. Experimental Study on the Detection of Hazardous Chemicals Using Alternative Sensors in the Water Environment. Toxics. 2022; 10(5):200. https://doi.org/10.3390/toxics10050200
Chicago/Turabian StyleNam, Su-Han, Tae-Geom Ku, Ye-Lim Park, Jae-Hyun Kwon, Do-Sung Huh, and Young-Do Kim. 2022. "Experimental Study on the Detection of Hazardous Chemicals Using Alternative Sensors in the Water Environment" Toxics 10, no. 5: 200. https://doi.org/10.3390/toxics10050200
APA StyleNam, S.-H., Ku, T.-G., Park, Y.-L., Kwon, J.-H., Huh, D.-S., & Kim, Y.-D. (2022). Experimental Study on the Detection of Hazardous Chemicals Using Alternative Sensors in the Water Environment. Toxics, 10(5), 200. https://doi.org/10.3390/toxics10050200






