The Impact of the Storage Conditions and Type of Clearomizers on the Increase of Heavy Metal Levels in Electronic Cigarette Liquids Retailed in Romania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
- -
- I: The initial phase: the EC liquids were directly taken from EC liquid bottles as purchased from retail;
- -
- II: EC liquid analyzed for storage period and clearomizer effect: the samples were stored for 1, 3, and 5 days in 2 different types of EC clearomizers purchased from specialized shops (VapePoint and Etigareta shops, Iasi, Romania). The clearomizers were selected based on their popularity. According to the employees from the vape shops, at the time of the purchase, these models were requested most frequently by the customers. Both clearomizers were “tank-style” electronic cigarettes and belonged to the second generation of electronic cigarettes [36,37]; clearomizer 1 was a CE4 type, while clearomizer 2 was a T3S type. The clearomizers (Figure 1) presented different tank capacities (1.6 mL and, respectively, 3.0 mL) and were made from dark plastic material (clearomizer 1) and clear, resistant plastic (clearomizer 2). Inside the tank, an atomizing unit with metallic coil and wick material were visible. The prices for the two clearomizers were also different (rating as ‘’low’’—clearomizer 1 and ‘’high’’—clearomizer 2);
- -
- III: EC liquid analyzed for storage period and temperature effect: EC liquids were stored in the two clearomizers mentioned above at two different temperatures: 22 °C (room temperature) and 40 °C. In this step, the samples were maintained in room with controlled temperature (22 °C), in the absence of direct sunlight, and in a programmable furnace (Model Nobertherm, Germany) at 40 °C for 1, 3, and 5 days in order to investigate the concentration of heavy metals that can be released through their storage under improper/inadequate conditions. For each clearomizer and both the variables (storage period and temperature), three replicates of each sample were performed. The clearomizers were filled and sealed with the e-liquid, from which an aliquot of 1 mL was separated and analyzed.
2.2. Reagents and Standards
2.3. Instrument
2.4. Data Analysis
3. Results
3.1. Calibration and Detection Limit
3.2. Heavy Metals Concentration in E-Cigarettes
3.2.1. Lead Concentration
3.2.2. Nickel Concentration
3.2.3. Zinc Concentration
3.2.4. Cobalt Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, D.; Navas-Acien, A.; Ilievski, V.; Slavkovich, V.; Olmedo, P.; Adria-Mora, B.; Domingo-Relloso, A.; Aherrera, A.; Kleiman, N.; Rule, A.M.; et al. Metal concentrations in electronic cigarette aerosol: Effect of open-system and closed-system devices and power settings. Environ. Res. 2019, 174, 125–134. [Google Scholar] [CrossRef]
- Short, M.; Cole, A.G. Factors Associated with E-Cigarette Escalation among High School Students: A Review of the Literature. Int. J. Environ. Res. Public Health 2021, 18, 10067. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhuang, Y.-L.; Zhu, S.-H. E-Cigarette Design Preference and Smoking Cessation: A U.S. Population Study. Am. J. Prev. Med. 2016, 51, 356–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margham, J.; McAdam, K.; Forster, M.; Liu, C.; Wright, C.; Mariner, D.; Proctor, C. Chemical Composition of Aerosol from an E-Cigarette: A Quantitative Comparison with Cigarette Smoke. Chem. Res. Toxicol. 2016, 29, 1662–1678. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T. Chemical evaluation of electronic cigarettes. Tob. Control 2014, 23 (Suppl. 2), ii11–ii17. [Google Scholar] [CrossRef]
- Callahan-Lyon, P. Electronic cigarettes: Human health effects. Tob. Control 2014, 23 (Suppl. 2), ii36–ii40. [Google Scholar] [CrossRef]
- Do, E.K.; O’Connor, K.; Perks, S.N.; Soule, E.K.; Eissenberg, T.; Amato, M.S.; Graham, A.L.; Martin, C.K.; Höchsmann, C.; Fuemmeler, B.F. E-cigarette device and liquid characteristics and E-cigarette dependence: A pilot study of pod-based and disposable E-cigarette users. Addict. Behav. 2021, 124, 107117. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, D.; Ma, Y.; Ma, X.; Wang, S.; Li, F.; Li, M.; Zhang, T. Toxicity of electronic cigarettes: A general review of the origins, health hazards, and toxicity mechanisms. Sci. Total Environ. 2021, 772, 145475. [Google Scholar] [CrossRef]
- Hess, C.A.; Olmedo, P.; Navas-Acien, A.; Goessler, W.; Cohen, J.E.; Rule, A.M. E-cigarettes as a source of toxic and potentially carcinogenic metals. Environ. Res. 2017, 152, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Gray, N.; Halstead, M.; Gonzalez-Jimenez, N.; Valentin-Blasini, L.; Watson, C.; Pappas, R.S. Analysis of Toxic Metals in Liquid from Electronic Cigarettes. Int. J. Environ. Res. Public Health 2019, 16, 4450. [Google Scholar] [CrossRef] [Green Version]
- Na, C.-J.; Jo, S.-H.; Kim, K.-H.; Sohn, J.-R.; Son, Y.-S. The transfer characteristics of heavy metals in electronic cigarette liquid. Environ. Res. 2019, 174, 152–159. [Google Scholar] [CrossRef] [PubMed]
- McAlinden, K.D.; Lu, W.; Eapen, M.S.; Sohal, S.S. Electronic cigarettes: Modern instruments for toxic lung delivery and posing risk for the development of chronic disease. Int. J. Biochem. Cell Biol. 2021, 137, 106039. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Kim, K.-H. Review on quantitation methods for hazardous pollutants released by e-cigarette (EC) smoking. TrAC Trends Anal. Chem. 2016, 78, 120–133. [Google Scholar] [CrossRef]
- Palazzolo, D.L.; Crow, A.P.; Nelson, J.M.; Johnson, R.A. Trace Metals Derived from Electronic Cigarette (ECIG) Generated Aerosol: Potential Problem of ECIG Devices That Contain Nickel. Front. Physiol. 2017, 7, 663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamilari, E.; Farsalinos, K.; Poulas, K.; Kontoyannis, C.G.; Orkoula, M.G. Detection and quantitative determination of heavy metals in electronic cigarette refill liquids using Total Reflection X-ray Fluorescence Spectrometry. Food Chem. Toxicol. 2018, 116 Pt B, 233–237. [Google Scholar] [CrossRef]
- Zhao, D.; Aravindakshan, A.; Hilpert, M.; Olmedo, P.; Rule, A.M.; Navas-Acien, A.; Aherrera, A. Metal/Metalloid Levels in Electronic Cigarette Liquids, Aerosols, and Human Biosamples: A Systematic Review. Environ. Health Perspect. 2020, 128, 36001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omaiye, E.E.; Williams, M.; Bozhilov, K.N.; Talbot, P. Design features and elemental/metal analysis of the atomizers in pod-style electronic cigarettes. PLoS ONE 2021, 16, e0248127. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Zielinska-Blizniewska, H.; Malinowska, K.; Zajdel, K.; Zakonnik, L.; Zajdel, R. A Summary of In Vitro and In Vivo Studies Evaluating the Impact of E-Cigarette Exposure on Living Organisms and the Environment. Int. J. Mol. Sci. 2020, 21, 652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, D.B.; Hilpert, M.; Saglimbeni, B.; Strait, M.; Ilievski, V.; Coady, M.; Talayero, M.; Wilmsen, K.; Chesnais, H.; Balac, O.; et al. Exposure to e-cigarette aerosol over two months induces accumulation of neurotoxic metals and alteration of essential metals in mouse brain. Environ. Res. 2021, 202, 111557. [Google Scholar] [CrossRef]
- Mikheev, V.B.; Brinkman, M.C.; Granville, C.A.; Gordon, S.M.; Clark, P.I. Real-Time Measurement of Electronic Cigarette Aerosol Size Distribution and Metals Content Analysis. Nicotine Tob. Res. 2016, 18, 1895–1902. [Google Scholar] [CrossRef] [Green Version]
- Pappas, R.S.; Gray, N.; Halstead, M.; Valentin-Blasini, L.; Watson, C. Toxic Metal-Containing Particles in Aerosols from Pod-Type Electronic Cigarettes. J. Anal. Toxicol. 2021, 45, 337–347. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Voudris, V.; Poulas, K. Are Metals Emitted from Electronic Cigarettes a Reason for Health Concern? A Risk-Assessment Analysis of Currently Available Literature. Int. J. Environ. Res. Public Health 2015, 12, 5215–5232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heldt, N.A.; Seliga, A.; Winfield, M.; Gajghate, S.; Reichenbach, N.; Yu, X.; Rom, S.; Tenneti, A.; May, D.; Gregory, B.D.; et al. Electronic cigarette exposure disrupts blood-brain barrier integrity and promotes neuroinflammation. Brain Behav. Immun. 2020, 88, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Fowles, J.; Barreau, T.; Wu, N. Cancer and Non-Cancer Risk Concerns from Metals in Electronic Cigarette Liquids and Aerosols. Int. J. Environ. Res. Public Health 2020, 17, 2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiener, R.C.; Bhandari, R. Association of electronic cigarette use with lead, cadmium, barium, and antimony body burden: NHANES 2015-2016. J. Trace Elements Med. Biol. 2020, 62, 126602. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Smith, D.M.; Edwards, K.C.; Blount, B.C.; Caldwell, K.L.; Feng, J.; Wang, L.; Christensen, C.; Ambrose, B.; Borek, N.; et al. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Netw. Open 2018, 1, e185937. [Google Scholar] [CrossRef] [Green Version]
- Olmedo, P.; Rodrigo, L.; Grau-Pérez, M.; Hilpert, M.; Navas-Acién, A.; Téllez-Plaza, M.; Pla, A.; Gil, F. Metal exposure and biomarker levels among e-cigarette users in Spain. Environ. Res. 2021, 202, 111667. [Google Scholar] [CrossRef]
- Gaur, S.; Agnihotri, R. Health Effects of Trace Metals in Electronic Cigarette Aerosols—A Systematic Review. Biol. Trace Element Res. 2019, 188, 295–315. [Google Scholar] [CrossRef]
- Williams, M.; Bozhilov, K.; Ghai, S.; Talbot, P. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS ONE 2017, 12, e0175430. [Google Scholar] [CrossRef]
- Agoroaei, L.; Bibire, N.; Apostu, M.; Strugaru, M.; Grigoriu, I.; Butnaru, E. Content of Heavy Metals in Tobacco of Commonly Smoked Cigarettes in Romania. Rev. Chim. 2014, 65, 1026–1028. [Google Scholar]
- Bouktif, M.; Maatouk, I.; Leblanc, J.C.; Gharbi, N.; Landoulsi, A. Dietary Intake of Tunisian Adult Population Aged from 19 To 65 in Cobalt. J. Food Nutr. 2021, 7, 1–7. [Google Scholar]
- Elliott, D.R.F.; Shah, R.; Hess, C.A.; Elicker, B.; Henry, T.S.; Rule, A.M.; Chen, R.; Golozar, M.; Jones, K.D. Giant cell interstitial pneumonia secondary to cobalt exposure from e-cigarette use. Eur. Respir. J. 2019, 54, 1901922. [Google Scholar] [CrossRef]
- Gonzalez-Jimenez, N.; Gray, N.; Pappas, R.S.; Halstead, M.; Lewis, E.; Valentin-Blasini, L.; Watson, C.; Blount, B. Analysis of Toxic Metals in Aerosols from Devices Associated with Electronic Cigarette, or Vaping, Product Use Associated Lung Injury. Toxics 2021, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Monsé, C.; Raulf, M.; Hagemeyer, O.; Van Kampen, V.; Kendzia, B.; Gering, V.; Marek, E.-M.; Jettkant, B.; Bünger, J.; Merget, R.; et al. Airway inflammation after inhalation of nano-sized zinc oxide particles in human volunteers. BMC Pulm. Med. 2019, 19, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalokalin, B.; Velimirovic, D. Potentiometric stripping analysis of zinc, cadmium and lead in tabacco leaves (Nicotiana tabacum L.) and soil samples. Int. J. Electrochem. Sci. 2012, 7, 313–323. [Google Scholar]
- Williams, M.; Li, J.; Talbot, P. Effects of Model, Method of Collection, and Topography on Chemical Elements and Metals in the Aerosol of Tank-Style Electronic Cigarettes. Sci. Rep. 2019, 9, 13969. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Talbot, P. Design Features in Multiple Generations of Electronic Cigarette Atomizers. Int. J. Environ. Res. Public Health 2019, 16, 2904. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.; Ni, Z.; Liu, M.; Shen, X.; Jia, Y. Determination of trace Cr, Ni, Hg, As, and Pb in the tipping paper and filters of cigarettes by monochromatic wavelength X-ray fluorescence spectrometry. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2021, 502, 59–65. [Google Scholar] [CrossRef]
- Olmedo, P.; Goessler, W.; Tanda, S.; Grau-Perez, M.; Jarmul, S.; Aherrera, A.; Chen, R.; Hilpert, M.; Cohen, J.E.; Navas-Acien, A.; et al. Metal Concentrations in e-Cigarette Liquid and Aerosol Samples: The Contribution of Metallic Coils. Environ. Health Perspect. 2018, 126, 27010. [Google Scholar] [CrossRef]
- Zhao, D.; Ilievski, V.; Slavkovich, V.; Olmedo, P.; Domingo-Relloso, A.; Rule, A.M.; Kleiman, N.J.; Navas-Acien, A.; Hilpert, M. Effects of e-liquid flavor, nicotine content, and puff duration on metal emissions from electronic cigarettes. Environ. Res. 2021, 204 Pt C, 112270. [Google Scholar] [CrossRef]
- Williams, M.; Bozhilov, K.N.; Talbot, P. Analysis of the elements and metals in multiple generations of electronic cigarette atomizers. Environ. Res. 2019, 175, 156–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, C. Between the Tank and the Coil: Assessing How Metals End Up in E-Cigarette Liquid and Vapor. Environ. Health Perspect. 2018, 126, 64002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, M.D.; Prasad, K.A.; Kim, J.S.; Park, J.H. Characteristics of metallic nanoparticles emitted from heated Kanthal e-cigarette coils. J. Nanopart. Res. 2019, 21, 156. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.; To, A.; Bozhilov, K.; Talbot, P. Strategies to Reduce Tin and Other Metals in Electronic Cigarette Aerosol. PLoS ONE 2015, 10, e0138933. [Google Scholar] [CrossRef] [PubMed]
- Aszyk, J.; Kubica, P.; Namieśnik, J.; Kot-Wasik, A.; Wasik, A. New approach for e-cigarette aerosol collection by an original automatic aerosol generator utilizing melt-blown non-woven fabric. Anal. Chim. Acta 2018, 1038, 67–78. [Google Scholar] [CrossRef]
- Olmedo, P.; Navas-Acien, A.; Hess, C.; Jarmul, S.; Rule, A. A direct method for e-cigarette aerosol sample collection. Environ. Res. 2016, 149, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Limit Test for Heavy Metals in Food Additive Specifications—Explanatory Note; Joint FAO/WHO Expert Committee on Food Additives (JECFA): Rome, Italy, 2002; Available online: https://www.fao.org/documents/card/en/c/f9d29932-9975-479c-8528-b8d3cc8ed34e/ (accessed on 18 February 2022).
- Dunbar, Z.R.; Das, A.; O’Connor, R.J.; Goniewicz, M.L.; Wei, B.; Travers, M.J. Brief Report: Lead Levels in Selected Electronic Cigarettes from Canada and the United States. Int. J. Environ. Res. Public Health 2018, 15, 154. [Google Scholar] [CrossRef] [Green Version]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific Opinion on the Risks to Public Health Related to the Presence of Nickel in Food and Drinking Water. EFSA J. 2015, 13, 4002. [Google Scholar] [CrossRef]
- Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31998L0083 (accessed on 18 February 2022).
- Nickel in Drinking-Water. Available online: https://www.who.int/water_sanitation_health/gdwqrevision/nickel2005.pdf (accessed on 18 February 2022).
- Summary of Evaluations Performed by the Joint FAO/WHO Expert Committee on Food Additives—Zinc. Available online: https://inchem.org/documents/jecfa/jeceval/jec_2411.htm (accessed on 18 February 2022).
- Cobalt and Its Compounds Chemical Substances Control Law. Available online: https://www.env.go.jp/en/chemi/chemicals/profile_erac/profile11/pf1-09.pdf (accessed on 15 November 2021).
- Provisional Peer Reviewed Toxicity Values for Cobalt. Available online: https://cfpub.epa.gov/ncea/pprtv/documents/Cobalt.pdf (accessed on 15 November 2021).
- Zervas, E.; Matsouki, N.; Kyriakopoulos, G.; Poulopoulos, S.; Ioannides, T.; Katsaounou, P. Transfer of metals in the liquids of electronic cigarettes. Inhal. Toxicol. 2020, 32, 240–248. [Google Scholar] [CrossRef]
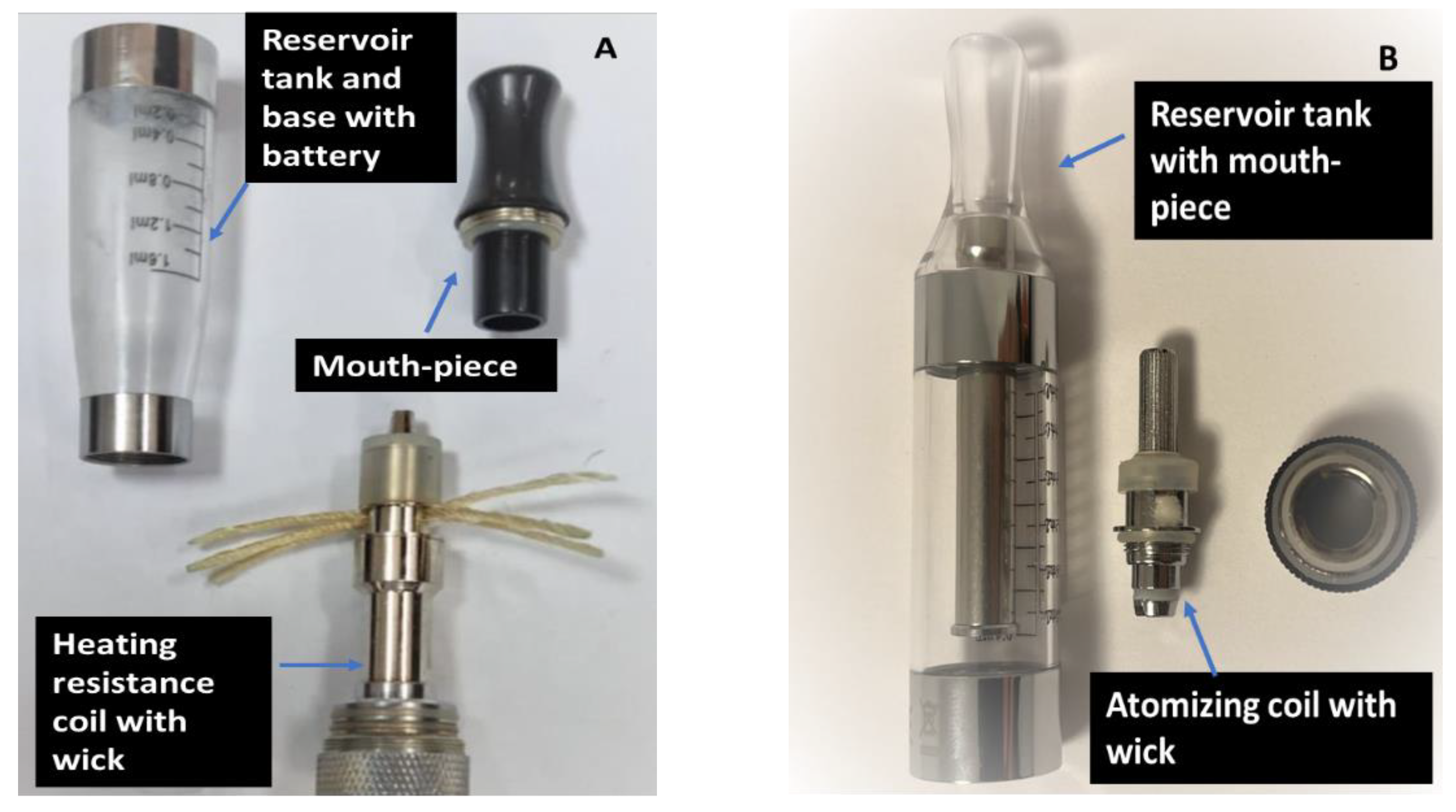

| Sample | Nicotine (mg/mL) | PG:VG Ratio (w/w) | Flavor |
|---|---|---|---|
| A | 0 | 50:50 | Dark tobacco |
| B | 6 | 70:30 | Cherry |
| C | 12 | 50:50 | Apple |
| D | 18 | 70:30 | Tobacco |
| E | 18 | 50:50 | Cuban cigar |
| Metal | Wavelength (nm) | Linear Range (mg L−1) | Detection Limit (mg L−1) | Correlation Coefficient (R2) | RSD (%) |
|---|---|---|---|---|---|
| Pb | 217.00 | 0.1–0.5 | 0.04 | 0.998 | 1.9 |
| Zn | 213.85 | 0.05–0.5 | 0.001 | 0.999 | 2.1 |
| Ni | 232.00 | 0.05–0.5 | 0.01 | 0.999 | 1.7 |
| Co | 240.72 | 0.05–0.5 | 0.005 | 0.997 | 3.4 |
| Metal | E-Liquid Sample | PG/VG Mixture | ||||||
|---|---|---|---|---|---|---|---|---|
| 1.0 mg L−1 | 5.0 mg L−1 | 1.0 mg L−1 | 5.0 mg L−1 | |||||
| Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | |
| Pb | 94.1 | 2.5 | 96.2 | 1.9 | 95.7 | 7.8 | 95.9 | 4.1 |
| Zn | 98.3 | 4.5 | 107.3 | 2.1 | 94.8 | 4.6 | 95.5 | 6.9 |
| Ni | 95.4 | 1.7 | 95.7 | 2.3 | 98.1 | 3.9 | 101.3 | 4.8 |
| Co | 96.4 | 5.5 | 104.2 | 3.4 | 95.7 | 4.2 | 98.1 | 2.4 |
| Sample | Initial Conc. (mg L−1) | Clearomizer 1 | Clearomizer 2 | ||
|---|---|---|---|---|---|
| 22 °C (mg L−1) | 40 °C (mg L−1) | 22 °C (mg L−1) | 40 °C (mg L−1) | ||
| Pb | |||||
| A(1) | 0.17 ± 0.02 | 0.28 ± 0.01 | 0.53 ± 0.01 | 0.37 ± 0.02 | 0.81 ± 0.01 |
| A(3) | 0.58 ± 0.03 | 0.84 ± 0.02 | 0.74 ± 0.05 | 0.88 ± 0.09 | |
| A(5) | 0.98 ± 0.04 | 1.84 ± 0.04 | 1.84 ± 0.07 | 1.94 ± 0.07 | |
| B(1) | 0.15 ± 0.03 | 0.47 ± 0.01 | 0.67 ± 0.03 | 0.50 ± 0.06 | 0.86 ± 0.02 |
| B(3) | 0.78 ± 0.02 | 1.15 ± 0.11 | 0.78 ± 0.03 | 1.30 ± 0.06 | |
| B(5) | 2.99 ± 0.15 | 3.22 ± 0.21 | 1.97 ± 0.7 | 1.98 ± 0.8 | |
| C(1) | 0.26 ± 0.06 | 0.58 ± 0.11 | 1.02 ± 0.16 | 0.49 ± 0.08 | 1.12 ± 0.21 |
| C(3) | 0.78 ± 0.16 | 1.45 ± 0.18 | 0.85 ± 0.10 | 1.73 ± 0.36 | |
| C(5) | 0.98 ± 0.20 | 1.86 ± 0.11 | 1.89 ± 0.08 | 2.38 ± 0.31 | |
| D(1) | 0.13 ± 0.01 | 0.20 ± 0.01 | 0.29 ± 0.04 | 0.76 ± 0.14 | 6.63 ± 1.32 |
| D(3) | 0.42 ± 0.03 | 0.90 ± 0.07 | 0.94 ± 0.19 | 8.56 ± 1.78 | |
| D(5) | 0.74 ± 0.04 | 1.77 ± 0.11 | 1.85 ± 0.21 | 10.48 ± 1.91 | |
| E(1) | 0.19 ± 0.01 | 1.20 ± 0.19 | 4.72 ± 0.83 | 1.98 ± 0.73 | 4.48 ± 1.03 |
| E(3) | 2.56 ± 0.53 | 5.36 ± 0.38 | 3.69 ± 1.03 | 7.88 ± 2.11 | |
| E(5) | 2.95 ± 0.74 | 7.27 ± 0.95 | 5.16 ± 1.38 | 9.23 ± 2.18 | |
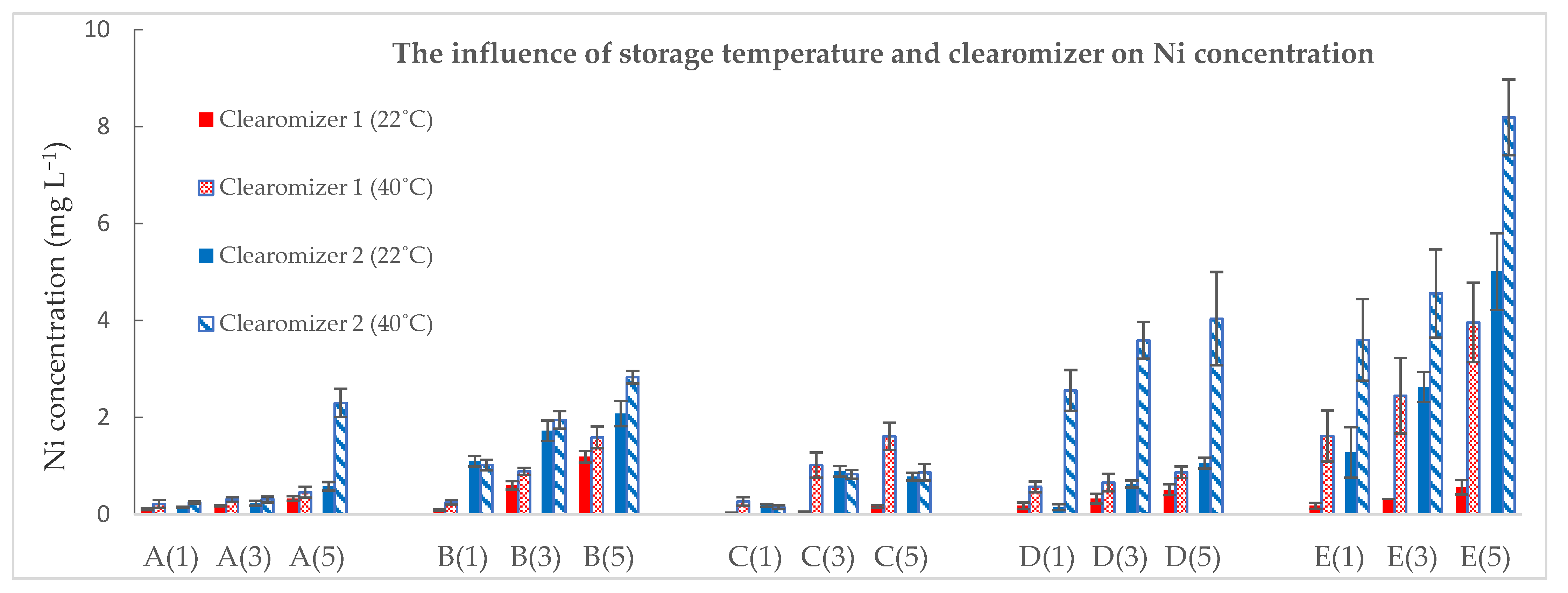
| Ni | |||||
| A(1) | 0.01 ± 0.01 | 0.11 ± 0.02 | 0.22 ± 0.08 | 0.15 ± 0.01 | 0.25 ± 0.02 |
| A(3) | 0.18 ± 0.01 | 0.31 ± 0.05 | 0.23 ± 0.05 | 0.31 ± 0.06 | |
| A(5) | 0.33 ± 0.05 | 0.46 ± 0.11 | 0.58 ± 0.09 | 2.30 ± 0.29 | |
| B(1) | 0.02 ± 0.01 | 0.09 ± 0.01 | 0.25 ± 0.05 | 1.10 ± 0.11 | 1.02 ± 0.11 |
| B(3) | 0.60 ± 0.09 | 0.89 ± 0.07 | 1.73 ± 0.21 | 1.95 ± 0.18 | |
| B(5) | 1.19 ± 0.12 | 1.59 ± 0.22 | 2.08 ± 0.26 | 2.83 ± 0.13 | |
| C(1) | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.27 ± 0.09 | 0.19 ± 0.03 | 0.15 ± 0.04 |
| C(3) | 0.05 ± 0.01 | 1.02 ± 0.26 | 0.89 ± 0.11 | 0.83 ± 0.09 | |
| C(5) | 0.16 ± 0.03 | 1.61 ± 0.28 | 0.78 ± 0.08 | 0.87 ± 0.17 | |
| D(1) | 0.01 ± 0.01 | 0.18 ± 0.07 | 0.57 ± 0.11 | 0.15 ± 0.06 | 2.56 ± 0.42 |
| D(3) | 0.33 ± 0.1 | 0.66 ± 0.18 | 0.63 ± 0.07 | 3.59 ± 0.38 | |
| D(5) | 0.51 ± 0.11 | 0.87 ± 0.12 | 1.06 ± 0.11 | 4.04 ± 0.96 | |
| E(1) | 0.02 ± 0.01 | 0.18 ± 0.06 | 1.62 ± 0.53 | 1.28 ± 0.52 | 3.60 ± 0.84 |
| E(3) | 0.32 ± 0.09 | 2.45 ± 0.78 | 2.63 ± 0.31 | 4.56 ± 0.91 | |
| E(5) | 0.56 ± 0.15 | 3.96 ± 0.82 | 5.01 ± 0.79 | 8.19 ± 0.78 | |
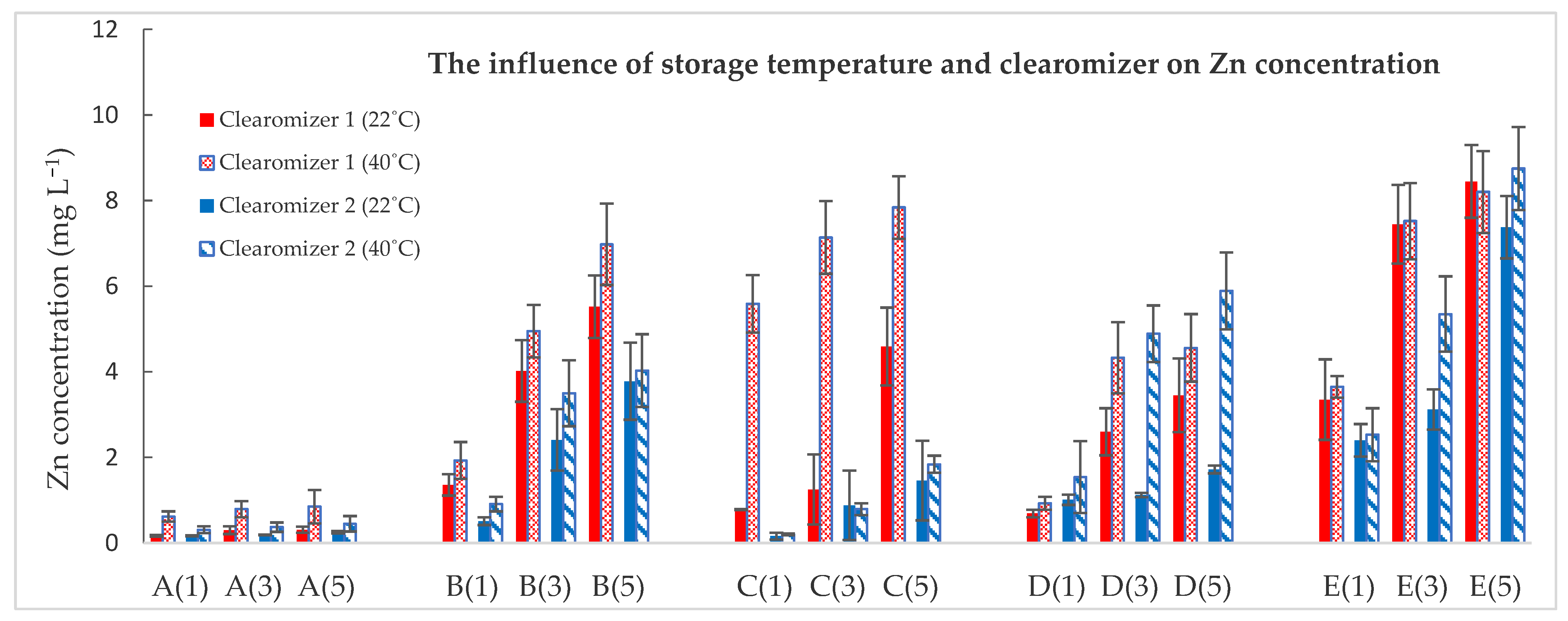
| Zn | |||||
| A(1) | 0.04 ± 0.01 | 0.17 ± 0.02 | 0.62 ± 0.12 | 0.17 ± 0.01 | 0.31 ± 0.08 |
| A(3) | 0.30 ± 0.09 | 0.79 ± 0.19 | 0.19 ± 0.01 | 0.37 ± 0.11 | |
| A(5) | 0.31 ± 0.07 | 0.85 ± 0.39 | 0.25 ± 0.03 | 0.45 ± 0.18 | |
| B(1) | 0.07 ± 0.01 | 1.36 ± 0.25 | 1.93 ± 0.43 | 0.91 ± 0.09 | 0.51 ± 0.17 |
| B(3) | 4.02 ± 0.72 | 4.95 ± 0.61 | 3.50 ± 0.72 | 2.41 ± 0.77 | |
| B(5) | 5.52 ± 0.73 | 6.98 ± 0.95 | 4.03 ± 0.90 | 3.78 ± 0.85 | |
| C(1) | 0.05 ± 0.01 | 0.78 ± 0.01 | 5.59 ± 0.67 | 0.16 ± 0.08 | 0.20 ± 0.02 |
| C(3) | 1.25 ± 0.82 | 7.14 ± 0.85 | 0.88 ± 0.81 | 0.79 ± 0.14 | |
| C(5) | 4.59 ± 0.91 | 7.84 ± 0.73 | 1.46 ± 0.93 | 1.84 ± 0.20 | |
| D(1) | 0.07 ± 0.01 | 0.69 ± 0.09 | 0.93 ± 0.15 | 1.01 ± 0.12 | 1.54 ± 0.84 |
| D(3) | 2.60 ± 0.55 | 4.33 ± 0.83 | 1.12 ± 0.05 | 4.89 ± 0.66 | |
| D(5) | 3.45 ± 0.86 | 4.56 ± 0.79 | 1.72 ± 0.09 | 5.89 ± 0.90 | |
| E(1) | 0.06 ± 0.01 | 3.35 ± 0.94 | 3.65 ± 0.25 | 2.40 ± 0.38 | 2.53 ± 0.62 |
| E(3) | 7.45 ± 0.92 | 7.52 ± 0.89 | 3.12 ± 0.47 | 5.35 ± 0.88 | |
| E(5) | 8.45 ± 0.85 | 8.20 ± 0.96 | 7.38 ± 0.73 | 8.75 ± 0.97 | |
| Storage Conditions | I vs. 1 D | I vs. 3 D | I vs. 5 D | 1 D vs. 3 D | 1 D vs. 5 D | 3 D vs. 5 D |
| Clearomizer 1 (22 °C) | 0.048 | 0.048 | 0.019 | 0.048 | 0.022 | 0.068 |
| Clearomizer 1 (40 °C) | 0.099 | 0.054 | 0.023 | 0.0006 | 0.003 | 0.008 |
| Clearomizer 2 (22 °C) | 0.048 | 0.049 | 0.011 | 0.055 | 0.004 | 0.0001 |
| Clearomizer 2 (40 °C) | 0.045 | 0.042 | 0.029 | 0.049 | 0.018 | 0.0043 |
| Storage Conditions | I vs. 1 D | I vs. 3 D | I vs. 5 D | 1 D vs. 3 D | 1 D vs. 5 D | 3 D vs. 5 D |
|---|---|---|---|---|---|---|
| Clearomizer 1 (22 °C) | 0.013 | 0.018 | 0.018 | 0.054 | 0.033 | 0.021 |
| Clearomizer 1 (40 °C) | 0.049 | 0.022 | 0.024 | 0.020 | 0.023 | 0.030 |
| Clearomizer 2 (22 °C) | 0.045 | 0.023 | 0.041 | 0.017 | 0.047 | 0.096 |
| Clearomizer 2 (40 °C) | 0.045 | 0.025 | 0.021 | 0.007 | 0.015 | 0.048 |
| Storage Conditions | I vs. 1 D | I vs. 3 D | I vs. 5 D | 1 D vs. 3 D | 1 D vs. 5 D | 3 D vs. 5 D |
|---|---|---|---|---|---|---|
| Clearomizer 1 (22 °C) | 0.046 | 0.041 | 0.017 | 0.041 | 0.012 | 0.036 |
| Clearomizer 1 (40 °C) | 0.027 | 0.009 | 0.008 | 0.015 | 0.013 | 0.049 |
| Clearomizer 2 (22 °C) | 0.064 | 0.024 | 0.041 | 0.053 | 0.041 | 0.070 |
| Clearomizer 2 (40 °C) | 0.035 | 0.023 | 0.023 | 0.025 | 0.021 | 0.051 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jităreanu, A.; Cara, I.G.; Sava, A.; Mârțu, I.; Caba, I.-C.; Agoroaei, L. The Impact of the Storage Conditions and Type of Clearomizers on the Increase of Heavy Metal Levels in Electronic Cigarette Liquids Retailed in Romania. Toxics 2022, 10, 126. https://doi.org/10.3390/toxics10030126
Jităreanu A, Cara IG, Sava A, Mârțu I, Caba I-C, Agoroaei L. The Impact of the Storage Conditions and Type of Clearomizers on the Increase of Heavy Metal Levels in Electronic Cigarette Liquids Retailed in Romania. Toxics. 2022; 10(3):126. https://doi.org/10.3390/toxics10030126
Chicago/Turabian StyleJităreanu, Alexandra, Irina Gabriela Cara, Alexandru Sava, Ioana Mârțu, Ioana-Cezara Caba, and Luminița Agoroaei. 2022. "The Impact of the Storage Conditions and Type of Clearomizers on the Increase of Heavy Metal Levels in Electronic Cigarette Liquids Retailed in Romania" Toxics 10, no. 3: 126. https://doi.org/10.3390/toxics10030126
APA StyleJităreanu, A., Cara, I. G., Sava, A., Mârțu, I., Caba, I.-C., & Agoroaei, L. (2022). The Impact of the Storage Conditions and Type of Clearomizers on the Increase of Heavy Metal Levels in Electronic Cigarette Liquids Retailed in Romania. Toxics, 10(3), 126. https://doi.org/10.3390/toxics10030126






