PBDEs Found in House Dust Impact Human Lung Epithelial Cell Homeostasis
Abstract
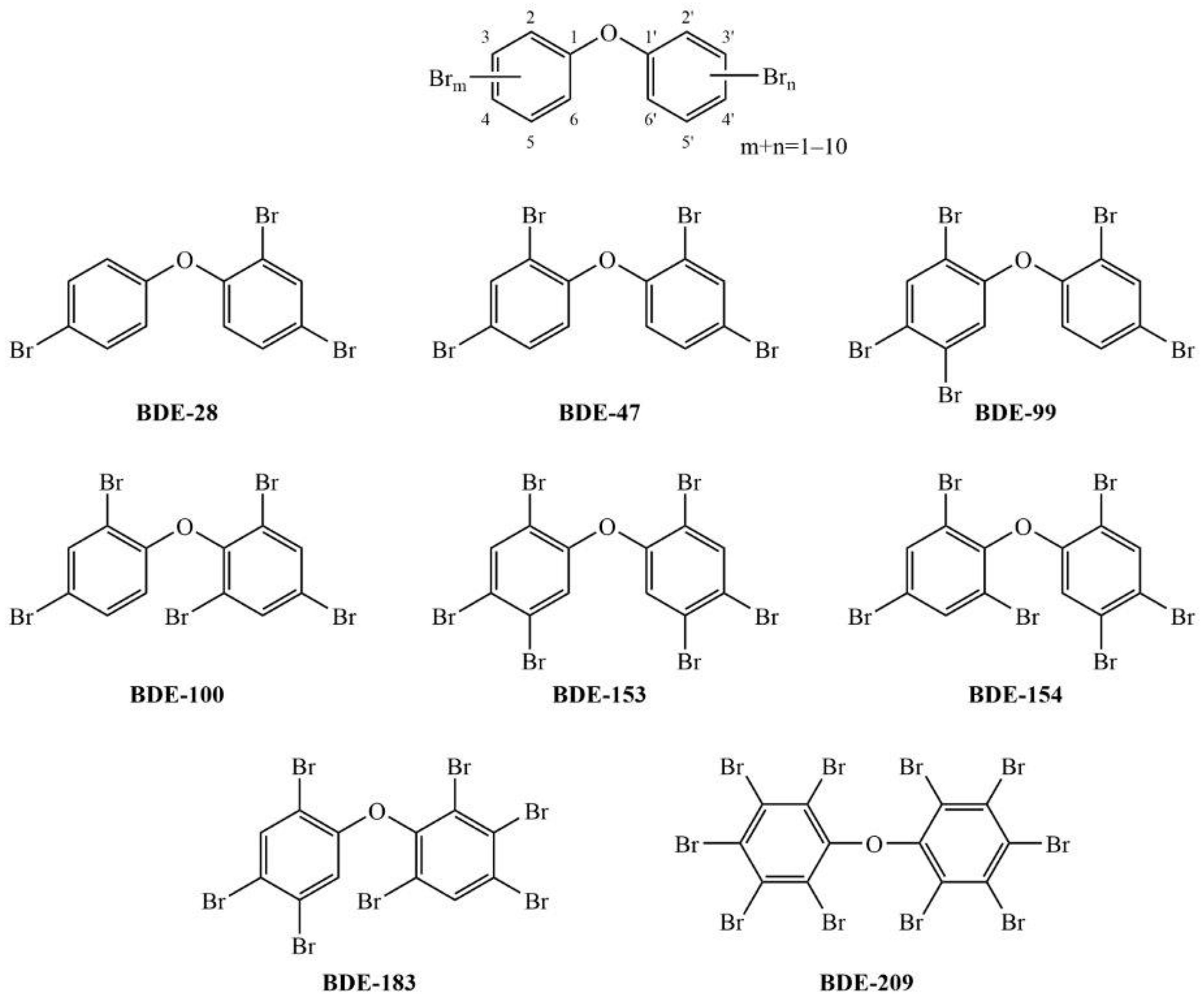
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Measurement of Cell Viability (MTS)
2.4. Cell Membrane Integrity
2.5. Determination of Reactive Oxygen Species
2.6. Determination of Glutathione
2.7. Determination of Mitochondrial Membrane Potential
2.8. Apoptosis
2.9. In Silico Predictions of CYP Inhibition
2.10. Statistics
3. Results
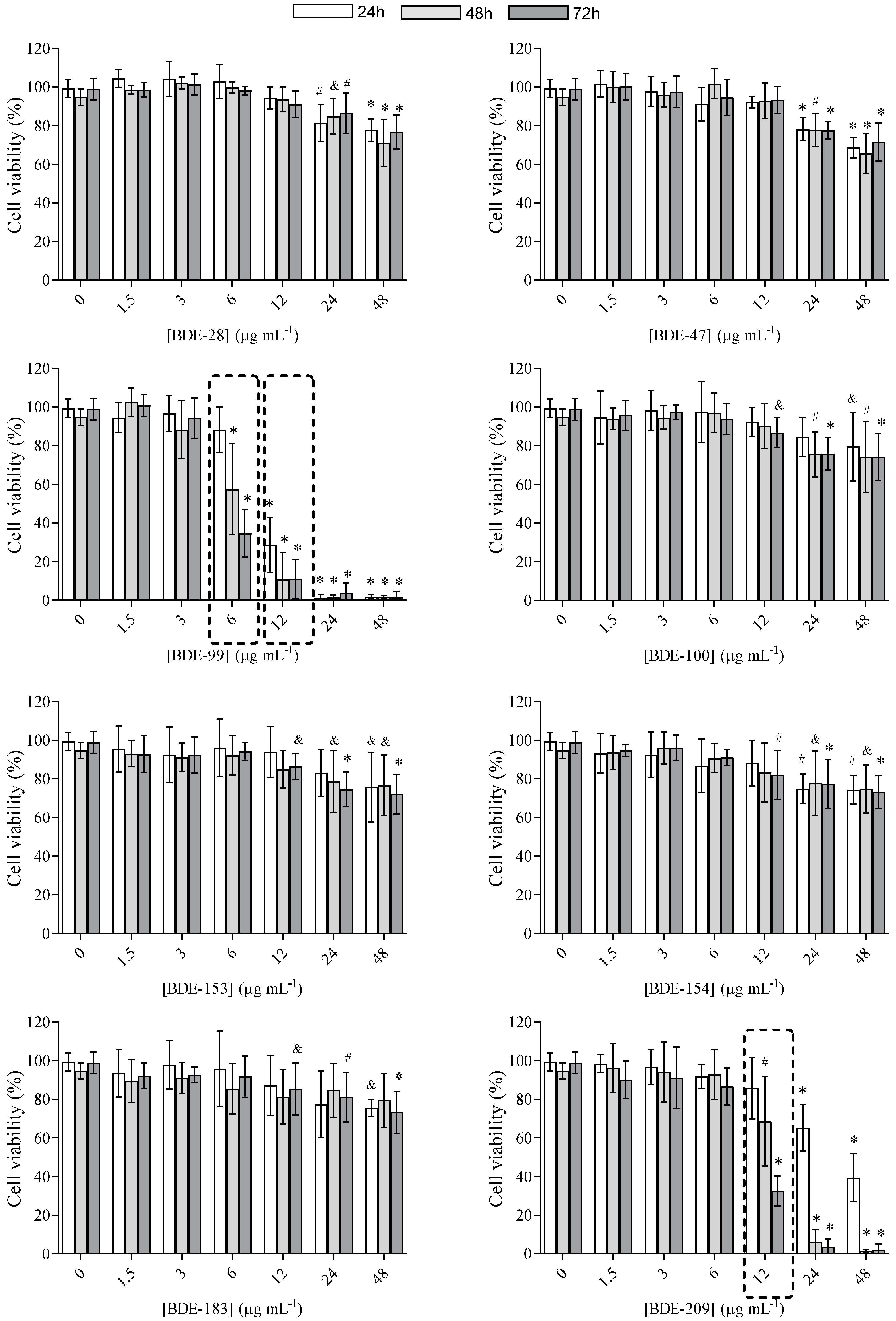
3.1. Cell Viability
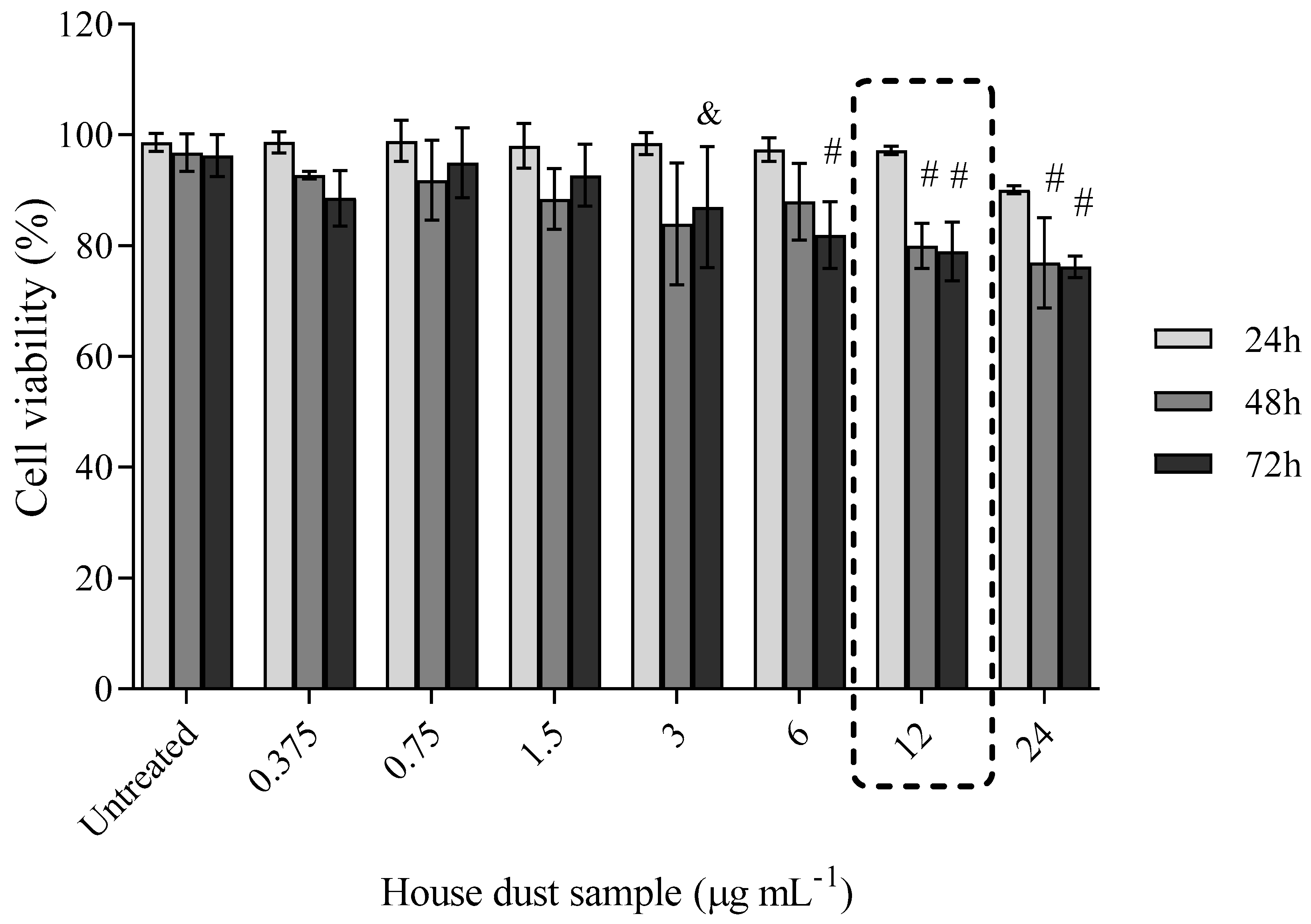
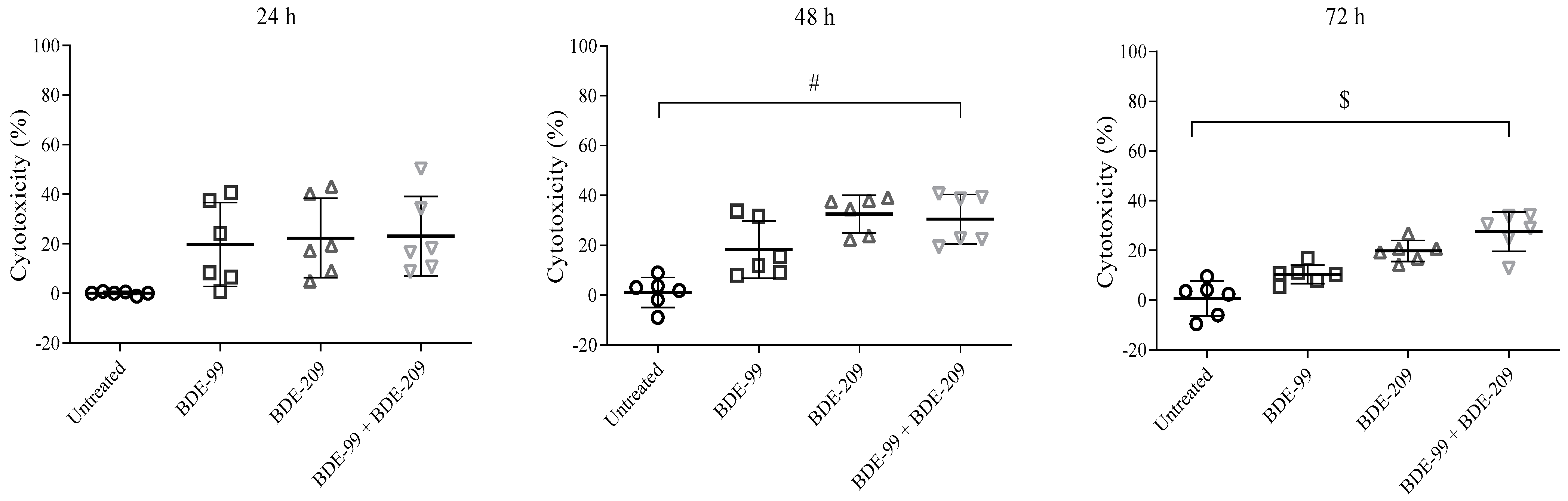
Cell Viability after Exposure to BDE-99 and -209 Mixture
3.2. Cell Status in the Presence of PBDEs
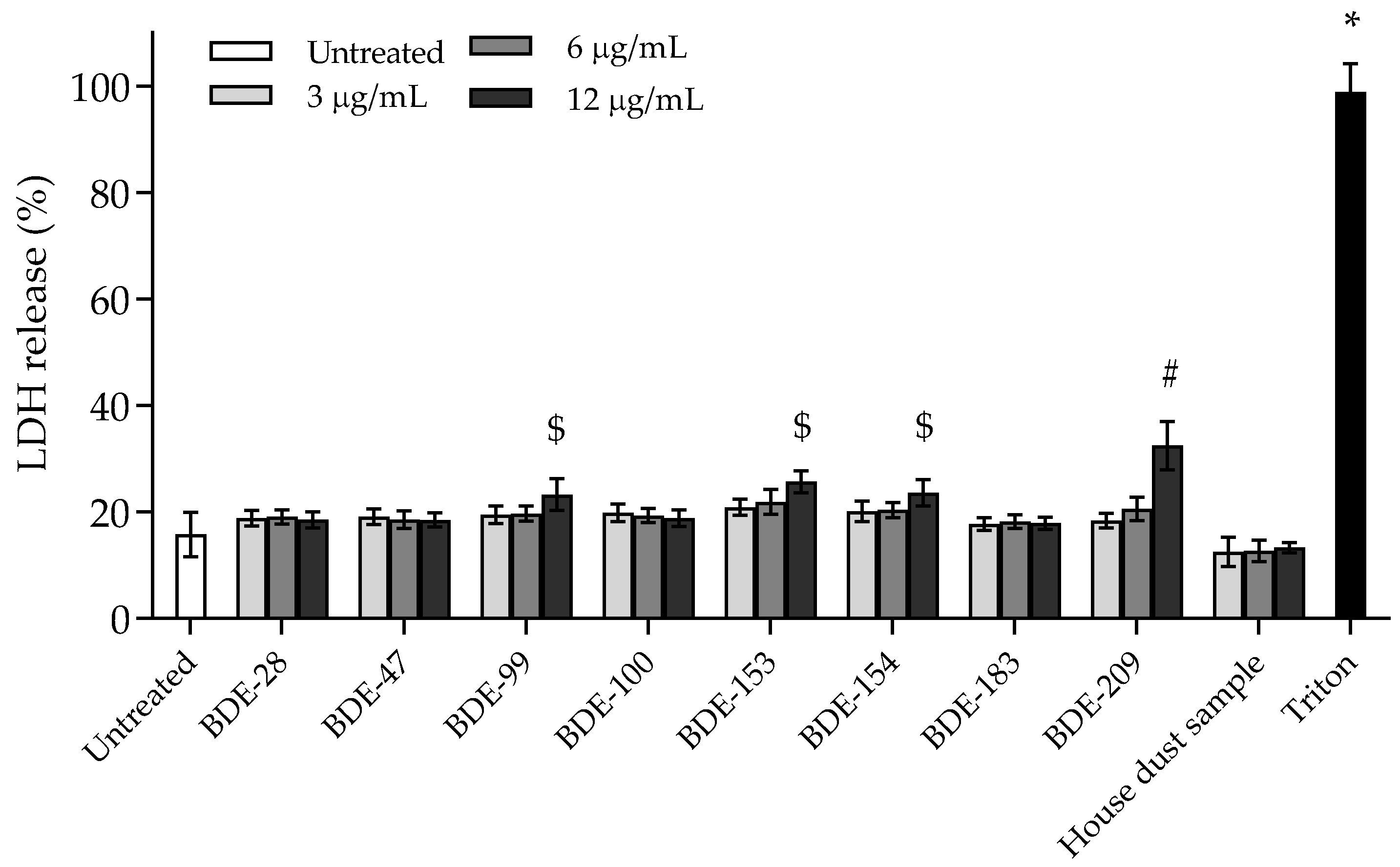
3.2.1. BDE-99, -153, -154 and -209 Affect Cell Membrane Integrity
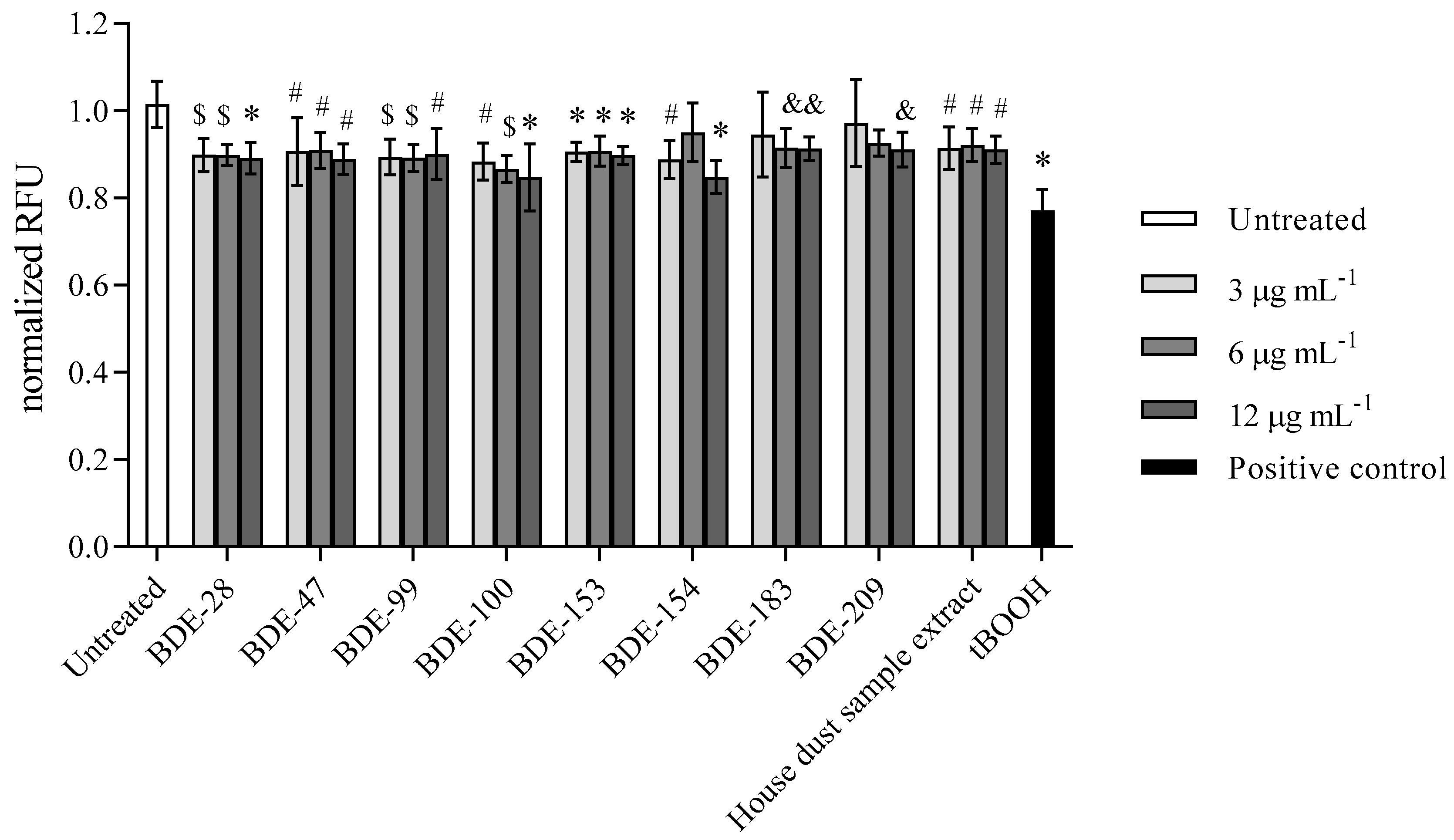
3.2.2. PBDEs Induce ROS
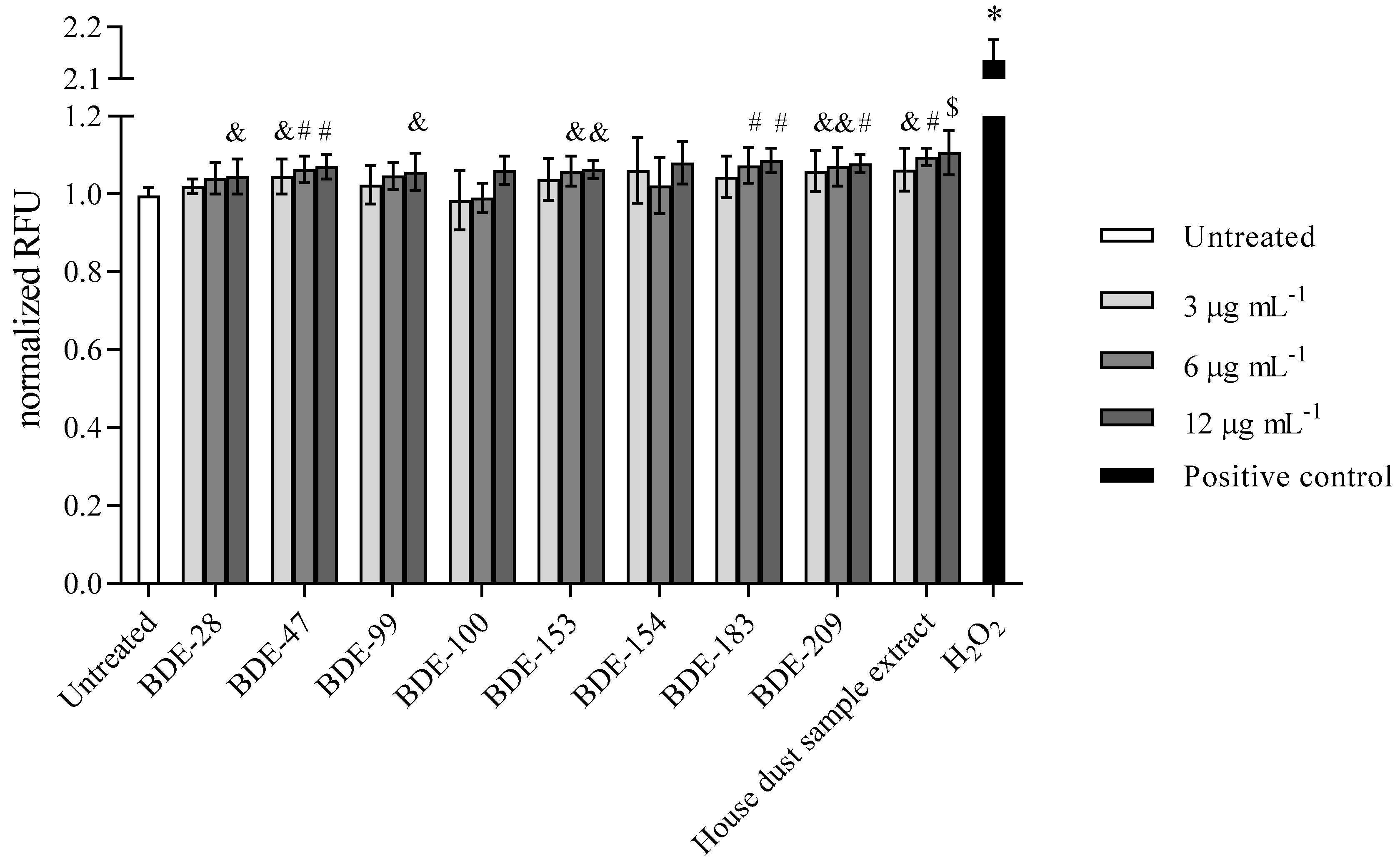
3.2.3. PBDEs Decrease GSH Levels
3.2.4. BDE-100, -154 and -209 Reduce Mitochondrial Membrane Potential
3.2.5. BDE-28 Activates Apoptosis
3.3. Predicted CYP Enzyme Inhibition by PBDEs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNEP. Guidance for the inventory of polybrominated diphenyl ethers (PBDEs) listed under the Stockholm Convention on Persistent Organic Pollutants. U. N. Environ. Progr. 2017, 127, 66–75. [Google Scholar]
- Johnson-Restrepo, B.; Kannan, K. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere 2009, 76, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Lorber, M. Exposure of Americans to polybrominated diphenyl ethers. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 2–19. [Google Scholar] [CrossRef]
- Stapleton, H.M.; Kelly, S.M.; Allen, J.G.; McClean, M.D.; Webster, T.F. Measurement of polybrominated diphenyl ethers on hand wipes: Estimating exposure from hand-to-mouth contact. Environ. Sci. Technol. 2008, 42, 3329–3334. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.; Siegel, E.; Eniola, F.; Herbstman, J.; Factor-Litvak, P. Effects of polybrominated siphenyl ethers on child cognitive, behavioral, and motor development. Int. J. Environ. Res. Public Health 2018, 15, 1636. [Google Scholar] [CrossRef] [PubMed]
- Alm, H.; Scholz, B.; Kultima, K.; Nilsson, A.; Andrén, P.E.; Savitski, M.M.; Bergman, Å.; Stigson, M.; Fex-Svenningsen, Å.; Dencker, L. In vitro neurotoxicity of PBDE-99: Immediate and concentration-dependent effects on protein expression in cerebral cortex cells. J. Proteome Res. 2010, 9, 1226–1235. [Google Scholar] [CrossRef]
- He, H.; Shi, X.; Lawrence, A.; Hrovat, J.; Turner, C.; Cui, J.Y.; Gu, H. 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) induces wide metabolic changes including attenuated mitochondrial function and enhanced glycolysis in PC12 cells. Ecotoxicol. Environ. Saf. 2020, 201, 110849. [Google Scholar] [CrossRef]
- Madia, F.; Giordano, G.; Fattori, V.; Vitalone, A.; Branchi, I.; Capone, F.; Costa, L.G. Differential in vitro neurotoxicity of the flame retardant PBDE-99 and of the PCB Aroclor 1254 in human astrocytoma cells. Toxicol. Lett. 2004, 154, 11–21. [Google Scholar] [CrossRef]
- Albano, G.D.; Moscato, M.; Montalbano, A.M.; Anzalone, G.; Gagliardo, R.; Bonanno, A.; Giacomazza, D.; Barone, R.; Drago, G.; Cibella, F.; et al. Can PBDEs affect the pathophysiologic complex of epithelium in lung diseases? Chemosphere 2020, 241, 125087. [Google Scholar] [CrossRef]
- Pereira, L.C.; Cabral Miranda, L.F.; Franco-Bernardes, M.F.; Tasso, M.J.; Duarte, F.V.; Inácio Varela, A.T.; Rolo, A.P.; Marques Palmeira, C.M.; Dorta, D.J. Mitochondrial damage and apoptosis: Key features in BDE-153-induced hepatotoxicity. Chem. Biol. Interact. 2018, 291, 192–201. [Google Scholar] [CrossRef]
- Pereira, L.C.; de Souza, A.O.; Meireles, G.; Franco-Bernardes, M.F.; Tasso, M.J.; Bruno, V.; Dorta, D.J.; de Oliveira, D.P. Comparative study of genotoxicity induced by six different PBDEs. Basic Clin. Pharmacol. Toxicol. 2016, 119, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.C.; Souza, A.O.; Dorta, D.J. Polybrominated diphenyl ether congener (BDE-100) induces mitochondrial impairment. Basic Clin. Pharmacol. Toxicol. 2013, 112, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.O.; Pereira, L.C.; Oliveira, D.P.; Dorta, D.J. BDE-99 congener induces cell death by apoptosis of human hepatoblastoma cell line—HepG2. Toxicol. Vitr. 2013, 27, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, S.; Caglieri, A.; Goldoni, M.; Pinelli, S.; Alinovi, R.; Poli, D.; Pellacani, C.; Giordano, G.; Mutti, A.; Costa, L.G. Low concentrations of the brominated flame retardants BDE-47 and BDE-99 induce synergistic oxidative stress-mediated neurotoxicity in human neuroblastoma cells. Toxicol. Vitr. 2010, 24, 116–122. [Google Scholar] [CrossRef]
- He, W.; He, P.; Wang, A.; Xia, T.; Xu, B.; Chen, X. Effects of PBDE-47 on cytotoxicity and genotoxicity in human neuroblastoma cells in vitro. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2008, 649, 62–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, P.; Li, G.; Hu, J.; Yu, Y.; An, T. Delineation of 3D dose-time-toxicity in human pulmonary epithelial Beas-2B cells induced by decabromodiphenyl ether (BDE209). Environ. Pollut. 2018, 243, 661–669. [Google Scholar] [CrossRef]
- Tang, Z.; Li, Y.; Jiang, Y.; Cheng, J.; Xu, S.; Zhang, J. Cellular metabolomics reveals glutamate and pyrimidine metabolism pathway alterations induced by BDE-47 in human neuroblastoma SK-N-SH cells. Ecotoxicol. Environ. Saf. 2019, 182, 109427. [Google Scholar] [CrossRef]
- Montalbano, A.M.; Albano, G.D.; Anzalone, G.; Moscato, M.; Gagliardo, R.; Di Sano, C.; Bonanno, A.; Ruggieri, S.; Cibella, F.; Profita, M. Cytotoxic and genotoxic effects of the flame retardants (PBDE-47, PBDE-99 and PBDE-209) in human bronchial epithelial cells. Chemosphere 2020, 245, 125600. [Google Scholar] [CrossRef]
- Jagić, K.; Dvoršćak, M.; Jurič, A.; Safner, T.; Klinčić, D. Preliminary results on polybrominated diphenyl ether contamination status in Croatian households and insights into children’s exposure. Environ. Toxicol. Pharmacol. 2021, 84, 103603. [Google Scholar] [CrossRef]
- Dulbecco, R.; Vogt, M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J. Exp. Med. 1954, 99, 167–182. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Zandona, A.; Katalinić, M.; Šinko, G.; Radman Kastelic, A.; Primožič, I.; Kovarik, Z. Targeting organophosphorus compounds poisoning by novel quinuclidine-3 oximes: Development of butyrylcholinesterase-based bioscavengers. Arch. Toxicol. 2020, 94, 3157–3171. [Google Scholar] [CrossRef] [PubMed]
- Zandona, A.; Maraković, N.; Mišetić, P.; Madunić, J.; Miš, K.; Padovan, J.; Pirkmajer, S.; Katalinić, M. Activation of (un)regulated cell death as a new perspective for bispyridinium and imidazolium oximes. Arch. Toxicol. 2021, 95, 2737–2754. [Google Scholar] [CrossRef]
- Zorbaz, T.; Malinak, D.; Maraković, N.; Maček Hrvat, N.; Zandona, A.; Novotny, M.; Skarka, A.; Andrys, R.; Benkova, M.; Soukup, O.; et al. Pyridinium oximes with ortho-positioned chlorine moiety exhibit improved physicochemical properties and efficient reactivation of human acetylcholinesterase inhibited by several nerve agents. J. Med. Chem. 2018, 61, 10753–10766. [Google Scholar] [CrossRef] [PubMed]
- Belmokhtar, C.A.; Hillion, J.; Ségal-Bendirdjian, E. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene 2001, 20, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Promega. CytoTox-ONE TM Homogeneous Membrane Integrity Assay; Promega: Madison, WI, USA, 2009; pp. 1–14. [Google Scholar]
- Simon, H.-U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Strzyz, P. Cell death: Pulling the apoptotic trigger for necrosis. Nat. Rev. Mol. Cell Biol. 2017, 18, 72. [Google Scholar] [CrossRef]
- Fresno Vara, J.Á.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; González-Barón, M. P13K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef]
- Chang, F.; Lee, J.T.; Navolanic, P.M.; Steelman, L.S.; Shelton, J.G.; Blalock, W.L.; Franklin, R.A.; McCubrey, J.A. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leukemia 2003, 17, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Ibhazehiebo, K.; Iwasaki, T.; Kimura-Kuroda, J.; Miyazaki, W.; Shimokawa, N.; Koibuchi, N. Disruption of thyroid hormone receptor–mediated transcription and thyroid hormone–induced purkinje cell dendrite arborization by polybrominated diphenyl ethers. Environ. Health Perspect. 2011, 119, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Luthe, G.; Jacobus, J.A.; Robertson, L.W. Receptor interactions by polybrominated diphenyl ethers versus polychlorinated biphenyls: A theoretical structure–activity assessment. Environ. Toxicol. Pharmacol. 2008, 25, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Kang, J.; Qiu, J.; Yang, W.; Wu, J.; Ji, D.; Yu, Y.; Ke, W.; Shi, X.; Wei, Y. Developmental exposure to BDE-99 hinders cerebrovascular growth and disturbs vascular barrier formation in zebrafish larvae. Aquat. Toxicol. 2019, 214, 105224. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-R.; Kamau, P.W.; Loch-Caruso, R. Involvement of reactive oxygen species in brominated diphenyl ether-47-induced inflammatory cytokine release from human extravillous trophoblasts in vitro. Toxicol. Appl. Pharmacol. 2014, 274, 283–292. [Google Scholar] [CrossRef]
- Legradi, J.; Pomeren, M.; Dahlberg, A.-K.; Legler, J. Effects of hydroxylated polybrominated diphenyl ethers in developing zebrafish are indicative of disruption of oxidative phosphorylation. Int. J. Mol. Sci. 2017, 18, 970. [Google Scholar] [CrossRef]
- Daniels, J.L.; Pan, I.J.; Jones, R.; Anderson, S.; Patterson, D.G.; Needham, L.L.; Sjödin, A. Individual characteristics associated with PBDE levels in U.S. human milk samples. Environ. Health Perspect. 2010, 118, 155–160. [Google Scholar] [CrossRef][Green Version]
- Siddiqi, M.A.; Laessig, R.H.; Reed, K.D. Polybrominated diphenyl ethers (PBDEs): New pollutants-old diseases. Clin. Med. Res. 2003, 1, 281–290. [Google Scholar] [CrossRef]
- Dodson, R.E.; Perovich, L.J.; Covaci, A.; Van den Eede, N.; Ionas, A.C.; Dirtu, A.C.; Brody, J.G.; Rudel, R.A. After the PBDE phase-out: A broad suite of flame retardants in repeat house dust samples from California. Environ. Sci. Technol. 2012, 46, 13056–13066. [Google Scholar] [CrossRef]
- Shoeib, M.; Harner, T.; Webster, G.M.; Sverko, E.; Cheng, Y. Legacy and current-use flame retardants in house dust from Vancouver, Canada. Environ. Pollut. 2012, 169, 175–182. [Google Scholar] [CrossRef]
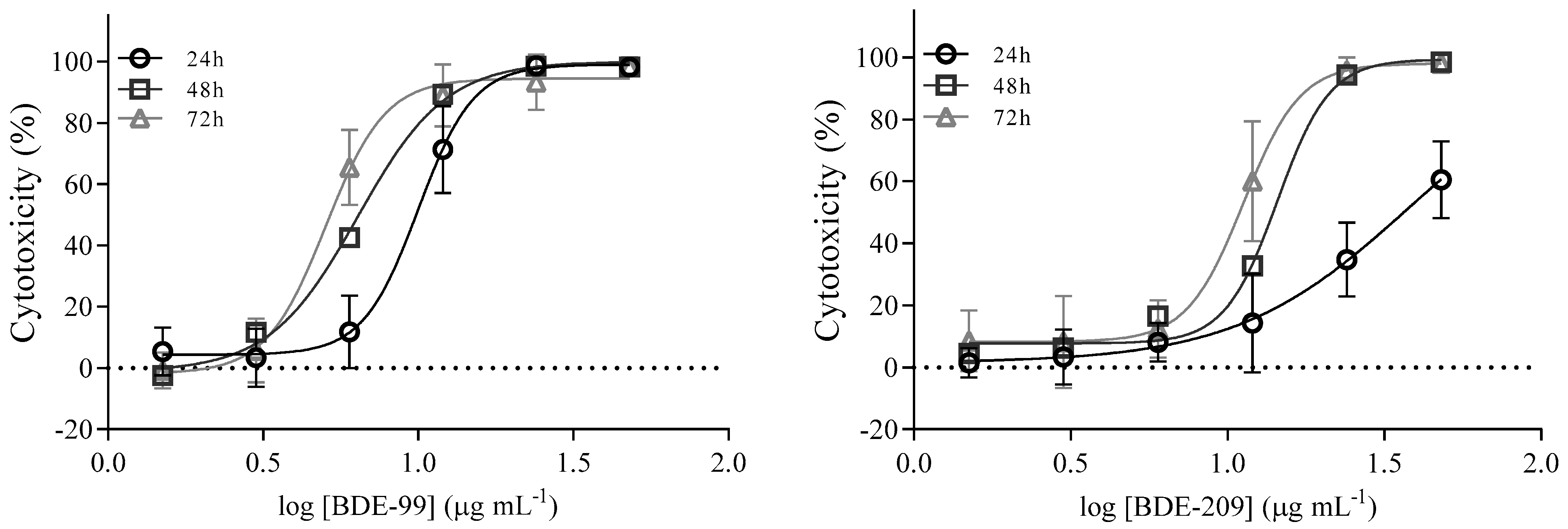
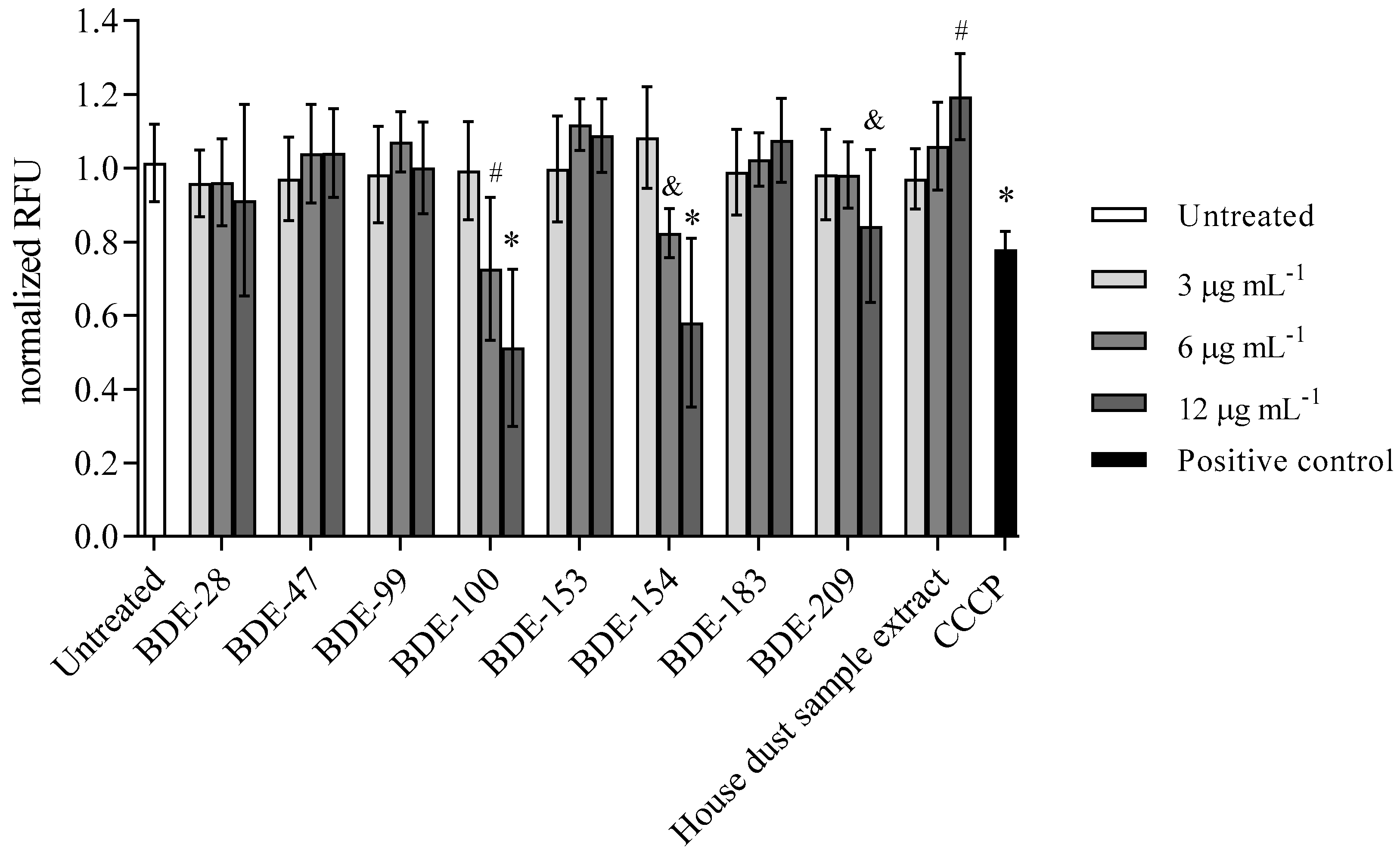
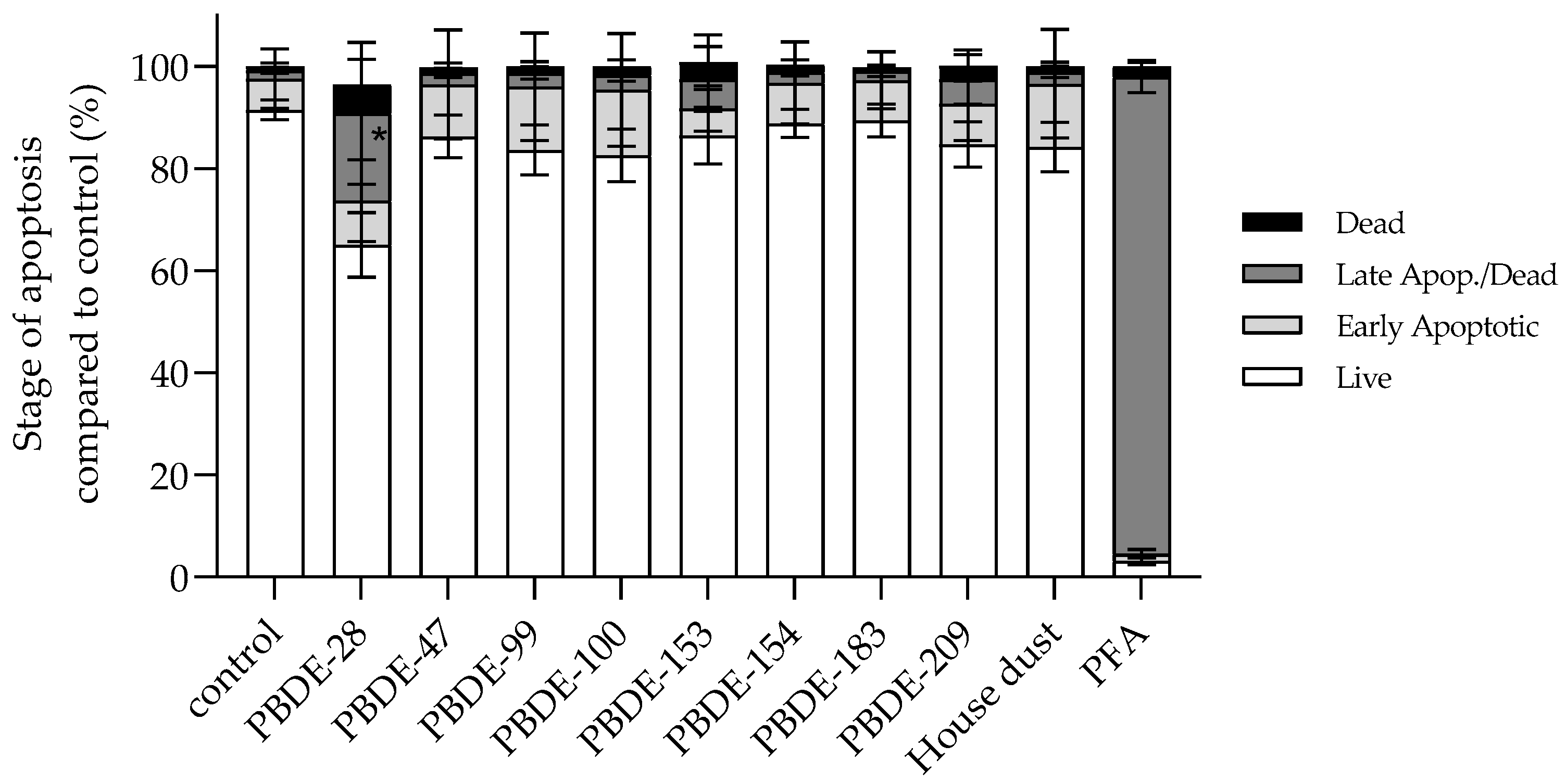
| Time (h) | IC50 ± SD (µg mL−1) BDE-99 | IC50 ± SD (µg mL−1) BDE-209 |
|---|---|---|
| 24 h | 9.8 ± 1.1 | 37.2 ± 2.9 |
| 48 h | 6.6 ± 1.1 | 13.8 ± 1.1 |
| 72 h | 5.3 ± 1.1 | 11.0 ± 1.1 |
| CYP | BDE-28 | BDE-47 | BDE-99 | BDE-100 | BDE-153 | BDE-154 | BDE-183 | BDE-209 |
|---|---|---|---|---|---|---|---|---|
| 1A2 | + | - | - | - | - | - | - | - |
| 2C19 | + | - | - | - | - | - | - | - |
| 2C9 | + | + | + | + | + | + | + | + |
| 2D6 | - | - | - | - | - | - | - | - |
| 3A4 | - | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zandona, A.; Jagić, K.; Dvoršćak, M.; Madunić, J.; Klinčić, D.; Katalinić, M. PBDEs Found in House Dust Impact Human Lung Epithelial Cell Homeostasis. Toxics 2022, 10, 97. https://doi.org/10.3390/toxics10020097
Zandona A, Jagić K, Dvoršćak M, Madunić J, Klinčić D, Katalinić M. PBDEs Found in House Dust Impact Human Lung Epithelial Cell Homeostasis. Toxics. 2022; 10(2):97. https://doi.org/10.3390/toxics10020097
Chicago/Turabian StyleZandona, Antonio, Karla Jagić, Marija Dvoršćak, Josip Madunić, Darija Klinčić, and Maja Katalinić. 2022. "PBDEs Found in House Dust Impact Human Lung Epithelial Cell Homeostasis" Toxics 10, no. 2: 97. https://doi.org/10.3390/toxics10020097
APA StyleZandona, A., Jagić, K., Dvoršćak, M., Madunić, J., Klinčić, D., & Katalinić, M. (2022). PBDEs Found in House Dust Impact Human Lung Epithelial Cell Homeostasis. Toxics, 10(2), 97. https://doi.org/10.3390/toxics10020097








