Effect of Combined Soil Amendment on Immobilization of Bioavailable As and Pb in Paddy Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Amendments
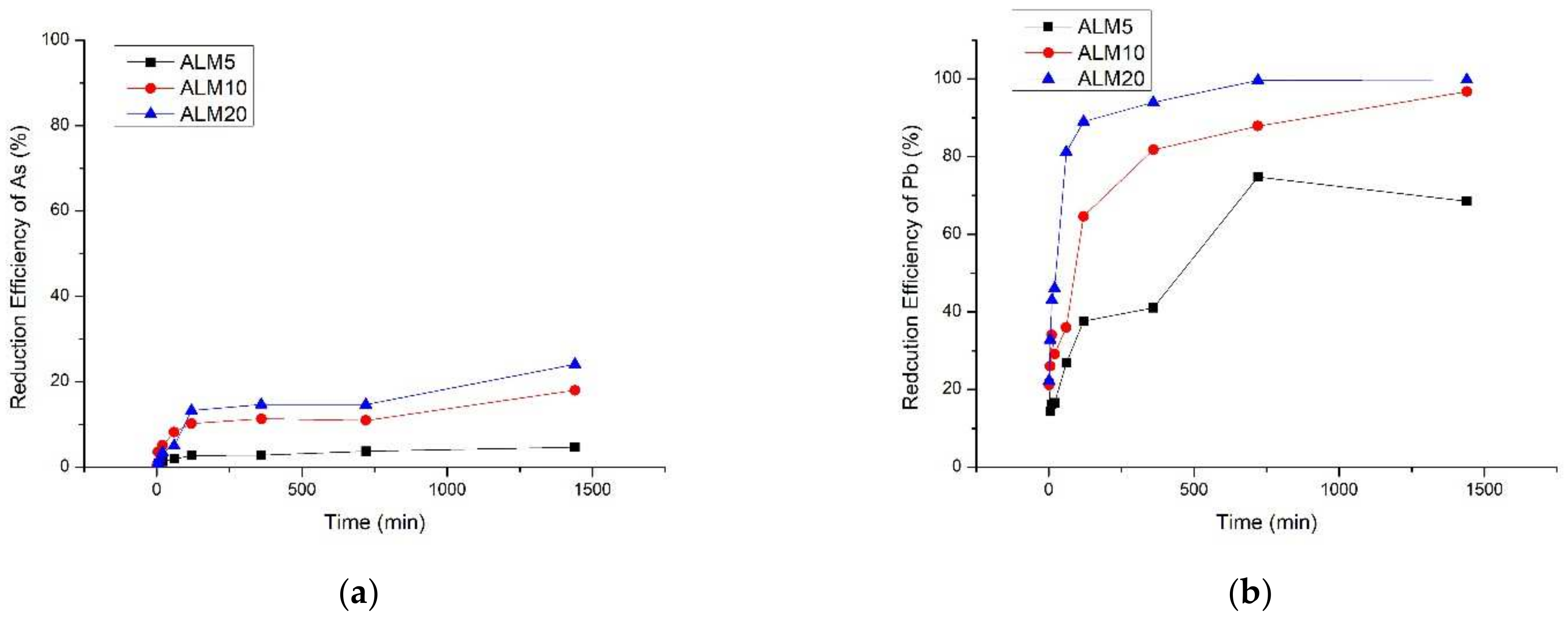
2.2. Determining Application Rate of Soil Amendment
2.3. Field Experiment Setup
2.4. Soil and Plant Sampling
2.5. Chemical and Heavy Metal Analysis in Soil and Plant
2.6. Statistical Analysis
3. Results and Discussion
3.1. Properties of ALM and Paddy Soil
3.2. Determining Optimum Application Rate of Amendments
3.3. Changes of Chemical Properties in Paddy Soil after Application of Amendments
3.4. Effect of Amendments on Bioavailability of Heavy Metals in Soil
3.5. Heavy Metal Accumulation in Rice
3.6. Correlation Analysis between Chemical Properties and Heavy Metal Concentration in Soil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergqvist, C.; Greger, M. Arsenic accumulation and speciation in plants from different habitats. Appl. Geochem. 2012, 27, 615–622. [Google Scholar] [CrossRef]
- Islam, S.; Rahman, M.M.; Islam, M.R.; Naidu, R. Arsenic accumulation in rice: Consequences of rice genotypes and management practices to reduce human health risk. Environ. Int. 2016, 96, 139–155. [Google Scholar] [CrossRef]
- Kumarathilaka, P.; Seneweera, S.; Meharg, A.; Bundschuh, J. Arsenic accumulation in rice (Oryza sativa L.) is influenced by environment and genetic factors. Sci. Total Environ. 2018, 642, 485–496. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Pandey, M.; Ghate, T.; Kumar, V.; Upadhyay, M.K.; Majumdar, A.; Sanjukta, A.K.; Agrawal, A.K.; Bose, S.; Srivastava, S.; et al. Chemical intervention for enhancing growth and reducing grain arsenic accumulation in rice. Environ. Pollut. 2021, 276, 116719. [Google Scholar] [CrossRef]
- Bartzas, G.; Tsakiridis, P.E.; Komnitsas, K. Nickel industry: Heavy metal(loid)s contamination—Sources, environmental impacts and recent advances on waste valorization. Curr. Opin. Environ. Sci. Health 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X.; Deng, J.; Li, G.; Li, Z.; Jiang, J.; Wu, Q.; Duan, L. Emission characteristics of heavy metals from a typical copper smelting plant. J. Hazard. Mater. 2022, 424, 127311. [Google Scholar] [CrossRef]
- Xu, L.; Dai, H.; Skuza, L.; Wei, S. Comprehensive exploration of heavy metal contamination and risk assessment at two common smelter sites. Chemosphere 2021, 285, 131350. [Google Scholar] [CrossRef]
- Zerizghi, T.; Guo, Q.; Tian, L.; Wei, R.; Zhao, C. An integrated approach to quantify ecological and human health risks of soil heavy metal contamination around coal mining area. Sci. Total Environ. 2021, 814, 152653. [Google Scholar] [CrossRef]
- Jiang, C.; Zhao, Q.; Zheng, L.; Chen, X.; Li, C.; Ren, M. Distribution, source and health risk assessment based on the Monte Carlo method of heavy metals in shallow groundwater in an area affected by mining activities, China. Ecotoxicol. Environ. Saf. 2021, 224, 112679. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Song, J.; Tan, M.L.; Kung, H.T.; Johnson, V.C. Pollutant source, ecological and human health risks assessment of heavy metals in soils from coal mining areas in Xinjiang, China. Environ. Res. 2021, 202, 111702. [Google Scholar] [CrossRef]
- Liang, W.; Wang, G.; Peng, C.; Tan, J.; Wan, J.; Sun, P.; Li, Q.; Ji, X.; Zhang, Q.; Wu, Y.; et al. Recent advances of carbon-based nano zero valent iron for heavy metals remediation in soil and water: A critical review. J. Hazard. Mater. 2022, 426, 127993. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Lim, S.S.; Park, H.J.; Yang, H.I.; Park, S.I.; Kwak, J.H.; Choi, W.J. Fly ash and zeolite decrease metal uptake but do not improve rice growth in paddy soils contaminated with Cu and Zn. Environ. Int. 2019, 129, 551–564. [Google Scholar] [CrossRef]
- Li, H.; Xu, H.; Zhou, S.; Yu, Y.; Li, H.; Zhou, C.; Chen, Y.; Li, Y.; Wang, M.; Wang, G. Distribution and transformation of lead in rice plants grown in contaminated soil amended with biochar and lime. Ecotoxicol. Environ. Saf. 2018, 165, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Suriyagoda, L.D.B.; Dittert, K.; Lambers, H. Mechanism of arsenic uptake, translocation and plant resistance to accumulate arsenic in rice grains. Agric. Ecosyst. Environ. 2018, 253, 23–37. [Google Scholar] [CrossRef]
- Xu, D.-M.; Fu, R.-B.; Wang, J.-X.; Shi, Y.-X.; Guo, X.-P. Chemical stabilization remediation for heavy metals in contaminated soils on the latest decade: Available stabilizing materials and associated evaluation methods—A critical review. J. Clean. Prod. 2021, 321, 1–22. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Blsson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Nemati, K.; Abu Bakar, N.K.; Abas, M.R.; Sobhanzadeh, E. Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J. Hazard. Mater. 2011, 192, 402–410. [Google Scholar] [CrossRef]
- Cui, L.; Pan, G.; Li, L.; Bian, R.; Liu, X.; Yan, J.; Quan, G.; Ding, C.; Chen, T.; Liu, Y.; et al. Continuous immobilization of cadmium and lead in biochar amended contaminated paddy soil: A five-year field experiment. Ecol. Eng. 2016, 93, 1–8. [Google Scholar] [CrossRef]
- Dedeke, G.A.; Iwuchukwu, P.O.; Aladesida, A.A.; Afolabi, T.A.; Ayanda, I.O. Impact of heavy metal bioaccumulation on antioxidant activities and DNA profile in two earthworm species and freshwater prawn from Ogun River. Sci. Total Environ. 2018, 624, 576–585. [Google Scholar] [CrossRef]
- He, H.; Tam, N.F.Y.; Yao, A.; Qiu, R.; Li, W.C.; Ye, Z. Growth and Cd uptake by rice (Oryza sativa) in acidic and Cd-contaminated paddy soils amended with steel slag. Chemosphere 2017, 189, 247–254. [Google Scholar] [CrossRef]
- Hu, P.; Yang, B.; Dong, C.; Chen, L.; Cao, X.; Zhao, J.; Wu, L.; Luo, Y.; Christie, P. Assessment of EDTA heap leaching of an agricultural soil highly contaminated with heavy metals. Chemosphere 2014, 117, 532–537. [Google Scholar] [CrossRef]
- Ownby, D.R.; Galvan, K.A.; Lydy, M.J. Lead and zinc bioavailability to Eisenia fetida after phosphorus amendment to repository soils. Environ. Pollut. 2005, 136, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Qi, F.; Seshadri, B.; Xu, Y.; Hou, J.; Ok, Y.S.; Dong, X.; Li, Q.; Sun, X.; Wang, L.; et al. Utilization of phosphorus loaded alkaline residue to immobilize lead in a shooting range soil. Chemosphere 2016, 162, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Abad-Valle, P.; Álvarez-Ayuso, E.; Murciego, A.; Pellitero, E. Assessment of the use of sepiolite amendment to restore heavy metal polluted mine soil. Geoderma 2016, 280, 57–66. [Google Scholar] [CrossRef]
- Ahmad, M.; Usman, A.R.A.; Al-Faraj, A.S.; Ahmad, M.; Sallam, A.; Al-Wabel, M.I. Phosphorus-loaded biochar changes soil heavy metals availability and uptake potential of maize (Zea mays L.) plants. Chemosphere 2018, 194, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Bian, R.; Chen, D.; Liu, X.; Cui, L.; Li, L.; Pan, G.; Xie, D.; Zheng, J.; Zhang, X.; Zheng, J.; et al. Biochar soil amendment as a solution to prevent Cd-tainted rice from China: Results from a cross-site field experiment. Ecol. Eng. 2013, 58, 378–383. [Google Scholar] [CrossRef]
- Francisca, F.M.; Glatstein, D.A. Environmental application of basic oxygen furnace slag for the removal of heavy metals from leachates. J. Hazard. Mater. 2020, 384, 1–7. [Google Scholar] [CrossRef]
- Garau, G.; Castaldi, P.; Santona, L.; Deiana, P.; Melis, P. Influence of red mud, zeolite and lime on heavy metal immobilization, culturable heterotrophic microbial populations and enzyme activities in a contaminated soil. Geoderma 2007, 142, 47–57. [Google Scholar] [CrossRef]
- Hussain Lahori, A.; Zhang, Z.; Guo, Z.; Mahar, A.; Li, R.; Kumar Awasthi, M.; Ali Sial, T.; Kumbhar, F.; Wang, P.; Shen, F.; et al. Potential use of lime combined with additives on (im)mobilization and phytoavailability of heavy metals from Pb/Zn smelter contaminated soils. Ecotoxicol. Environ. Saf. 2017, 145, 313–323. [Google Scholar] [CrossRef]
- Ko, M.-S.; Kim, J.-Y.; Park, H.-S.; Kim, K.-W. Field assessment of arsenic immobilization in soil amended with iron rich acid mine drainage sludge. J. Clean. Prod. 2015, 108, 1073–1080. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.; Liang, Y.; Gao, B.; Harris, W. Simultaneous immobilization of lead and atrazine in contaminated soils using dairy-manure biochar. Environ. Sci. Technol. 2011, 45, 4884–4889. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Kim, J.W.; Kim, H.S.; Lee, S.P.; Yang, J.E.; Kim, S.C. Bottom Ash Modification via Sintering Process for Its Use as a Potential Heavy Metal Adsorbent: Sorption Kinetics and Mechanism. Materials 2021, 14, 3060. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, G.; Zheng, H.; Li, F.; Ngo, H.H.; Guo, W.; Liu, C.; Chen, L.; Xing, B. Investigating the mechanisms of biochar’s removal of lead from solution. Bioresour. Technol. 2015, 177, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Li, Y.; Dong, H.; Liu, Y.; Wang, M.; Wang, G. Effects and mechanisms on the reduction of lead accumulation in rice grains through lime amendment. Ecotoxicol. Environ. Saf. 2019, 173, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Aftabtalab, A.; Rinklebe, J.; Shaheen, S.M.; Niazi, N.K.; Moreno-Jimenez, E.; Schaller, J.; Knorr, K.H. Review on the interactions of arsenic, iron (oxy)(hydr)oxides, and dissolved organic matter in soils, sediments, and groundwater in a ternary system. Chemosphere 2022, 286, 131790. [Google Scholar] [CrossRef]
- Wu, C.; Huang, L.; Xue, S.G.; Pan, W.S.; Zou, Q.; Hartley, W.; Wong, M.H. Oxic and anoxic conditions affect arsenic (As) accumulation and arsenite transporter expression in rice. Chemosphere 2017, 168, 969–975. [Google Scholar] [CrossRef]
- Huang, J.H.; Hsu, S.H.; Wang, S.L. Effects of rice straw ash amendment on Cu solubility and distribution in flooded rice paddy soils. J. Hazard. Mater. 2011, 186, 1801–1807. [Google Scholar] [CrossRef]
- Yao, A.; Ju, L.; Ling, X.; Liu, C.; Wei, X.; Qiu, H.; Tang, Y.; Morel, J.L.; Qiu, R.; Li, C.; et al. Simultaneous attenuation of phytoaccumulation of Cd and As in soil treated with inorganic and organic amendments. Environ. Pollut. 2019, 250, 464–474. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Amelung, W.; Cao, Z.; Fiedler, S.; Frenzel, P.; Jahn, R.; Kalbitz, K.; Kölbl, A.; Schloter, M. Biogeochemistry of paddy soils. Geoderma 2010, 157, 1–14. [Google Scholar] [CrossRef]
- Ma, L.Q.; Dong, Y. Effects of incubation on solubility and mobility of trace metals in two contaminated soils. Environ. Pollut. 2004, 130, 301–307. [Google Scholar] [CrossRef]
- Kumarathilaka, P.; Seneweera, S.; Meharg, A.; Bundschuh, J. Arsenic speciation dynamics in paddy rice soil-water environment: Sources, physico-chemical, and biological factors—A review. Water Res 2018, 140, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Panaullah, G.M.; Alam, T.; Hossain, M.B.; Loeppert, R.H.; Lauren, J.G.; Meisner, C.A.; Ahmed, Z.U.; Duxbury, J.M. Arsenic toxicity to rice (Oryza sativa L.) in Bangladesh. Plant Soil 2008, 317, 31–39. [Google Scholar] [CrossRef]
- Shrivastava, A.; Barla, A.; Singh, S.; Mandraha, S.; Bose, S. Arsenic contamination in agricultural soils of Bengal deltaic region of West Bengal and its higher assimilation in monsoon rice. J. Hazard. Mater. 2017, 324, 526–534. [Google Scholar] [CrossRef]
- Dahal, B.M.; Fuerhacker, M.; Mentler, A.; Karki, K.B.; Shrestha, R.R.; Blum, W.E. Arsenic contamination of soils and agricultural plants through irrigation water in Nepal. Environ. Pollut. 2008, 155, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.-W.; Baveye, P.C.; Kim, K.-B.; Kang, D.-H.; Lee, S.-Y.; Son, J.; Kim, D.-H.; Yoon, Y.-C.; Yu, C. Effect of postmining land use on the spatial distribution of metal(loid)s and their transport in agricultural soils: Analysis of a case study of Chungyang, South Korea. J. Geochem. Explor. 2016, 170, 157–166. [Google Scholar] [CrossRef]
- Ma, L.; Wang, L.; Jia, Y.; Yang, Z. Accumulation, translocation and conversion of six arsenic species in rice plants grown near a mine impacted city. Chemosphere 2017, 183, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, X.; Wang, M.; Sun, X. Genotypic differences among rice cultivars in lead accumulation and translocation and the relation with grain Pb levels. Ecotoxicol. Environ. Saf. 2013, 90, 35–40. [Google Scholar] [CrossRef]
- Liu, K.; Li, C.; Tang, S.; Shang, G.; Yu, F.; Li, Y. Heavy metal concentration, potential ecological risk assessment and enzyme activity in soils affected by a lead-zinc tailing spill in Guangxi, China. Chemosphere 2020, 251, 126415. [Google Scholar] [CrossRef]
- Xu, D.; Ji, P.; Wang, L.; Zhao, X.; Hu, X.; Huang, X.; Zhao, H.; Liu, F. Effect of modified fly ash on environmental safety of two soils contaminated with cadmium and lead. Ecotoxicol. Environ. Saf. 2021, 215, 112175. [Google Scholar] [CrossRef]
- Chiang, Y.W.; Ghyselbrecht, K.; Santos, R.M.; Meesschaert, B.; Martens, J.A. Synthesis of zeolitic-type adsorbent material from municipal solid waste incinerator bottom ash and its application in heavy metal adsorption. Catal. Today 2012, 190, 23–30. [Google Scholar] [CrossRef]
- Guo, R.; Yao, W.; Ma, H.; Yuan, J. Two-step hydrothermal synthesis of nano-kaolinite from fly ash: Thermodynamics and mechanism. J. Clean. Prod. 2020, 271, 1–10. [Google Scholar] [CrossRef]
- Ji, Z.; Pei, Y. Geopolymers produced from drinking water treatment residue and bottom ash for the immobilization of heavy metals. Chemosphere 2019, 225, 579–587. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ogata, F.; Nakamura, T.; Kawasaki, N. Synthesis of novel zeolites produced from fly ash by hydrothermal treatment in alkaline solution and its evaluation as an adsorbent for heavy metal removal. J. Environ. Chem. Eng. 2020, 8, 1–6. [Google Scholar] [CrossRef]
- IBM Cooperation. Statistical Package for Social Sciences; Version 20.0; IBM: Armonk, NY, USA, 2019. [Google Scholar]
- Luo, H.; Wu, Y.; Zhao, A.; Kumar, A.; Liu, Y.; Cao, B.; Yang, E.-H. Hydrothermally synthesized porous materials from municipal solid waste incineration bottom ash and their interfacial interactions with chloroaromatic compounds. J. Clean. Prod. 2017, 162, 411–419. [Google Scholar] [CrossRef]
- Pena, R.; Guerrero, A.; Goni, S. Hydrothermal treatment of bottom ash from the incineration of municipal solid waste: Retention of Cs(I), Cd(II), Pb(II) and Cr(III). J. Hazard. Mater. 2006, 129, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhou, X.; Zeng, M.; Liao, B.H.; Liu, L.; Yang, W.T.; Wu, Y.M.; Qiu, Q.Y.; Wang, Y.J. Effects of combined amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on contaminated paddy soil. Ecotoxicol. Environ. Saf. 2014, 101, 226–232. [Google Scholar] [CrossRef]
- Lewis, C.; Lennon, A.M.; Eudoxie, G.; Sivapatham, P.; Umaharan, P. Plant metal concentrations in Theobroma cacao as affected by soil metal availability in different soil types. Chemosphere 2021, 262, 127749. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test rextractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 2008, 15, 1409–1416. [Google Scholar] [CrossRef]
- Bissen, M.; Frimmel, F.H. Arsenic—A review. Part I: Occurrence, toxicity, speciation, mobility. Acta Hydrochim. Hydrobiol. 2003, 31, 9–18. [Google Scholar] [CrossRef]
- Stopelli, E.; Duyen, V.T.; Mai, T.T.; Trang, P.T.K.; Viet, P.H.; Lightfoot, A.; Kipfer, R.; Schneider, M.; Eiche, E.; Kontny, A.; et al. Spatial and temporal evolution of groundwater arsenic contamination in the Red River delta, Vietnam: Interplay of mobilisation and retardation processes. Sci. Total Environ. 2020, 717, 137143. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, T.; Khan, S.; Muhammad, S.; Amin, S. Arsenic speciation, mechanisms, and factors affecting rice uptake and potential human health risk: A systematic review. Environ. Technol. Innov. 2021, 22, 1–12. [Google Scholar] [CrossRef]
- Liu, C.Y.; Chen, C.L.; Gong, X.F.; Zhou, W.B.; Yang, J.Y. Progress in research of iron plaque on root surface of wetland plants. Shengtai Xuebao Acta Ecol. Sin. 2014, 34, 2470–2480. [Google Scholar] [CrossRef][Green Version]
- Udeigwe, T.K.; Eze, P.N.; Teboh, J.M.; Stietiya, M.H. Application, chemistry, and environmental implications of contaminant-immobilization amendments on agricultural soil and water quality. Environ. Int. 2011, 37, 258–267. [Google Scholar] [CrossRef]
- Peng, J.F.; Song, Y.H.; Yuan, P.; Cui, X.Y.; Qiu, G.L. The remediation of heavy metals contaminated sediment. J. Hazard. Mater. 2009, 161, 633–640. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, X.-Y.; Lei, M.; Yang, J.; Ma, J.; Qiao, P.-W.; Chen, T.-B. Migration and transformation of arsenic: Contamination control and remediation in realgar mining areas. Appl. Geochem. 2017, 77, 44–51. [Google Scholar] [CrossRef]
- Piracha, M.A.; Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Arif, M.S.; Rizwan, M.S.; Aziz, A.; Tu, S.; Albasher, G.; Alkahtani, S.; et al. Alteration in soil arsenic dynamics and toxicity to sunflower (Helianthus annuus L.) in response to phosphorus in different textured soils. Chemosphere 2022, 287, 132406. [Google Scholar] [CrossRef]
| ALM | |||
|---|---|---|---|
| pH | (H2O, 1:5 w/v) | 8.68 ± 0.11 | |
| EC | dS m−1 | 1.29 ± 0.03 | |
| TOC † | g kg−1 | 45.4 ± 0.4 | |
| TN † | g kg−1 | 0.5 ± 0.1 | |
| Surface area | m2 g−1 | 7.8477 | |
| Density | g cm−3 | 0.82 | |
| Heavy metals | As | mg kg−1 | 1.26 ± 0.61 |
| Cd | mg kg−1 | 0.22 ± 0.02 | |
| Pb | mg kg−1 | 7.41 ± 1.37 | |
| Cu | mg kg−1 | 5.13 ± 0.05 | |
| Zn | mg kg−1 | 14.52 ± 4.49 |
| Soil | Optimum Range/Threshold Value † | |||
|---|---|---|---|---|
| pH | (H2O, 1:5 w/v) | 7.30 ± 0.02 | 6.0–7.0 | |
| EC § | dS m−1 | 1.82 ± 0.02 | <2.0 | |
| SOM § | % | 3.12 ± 0.01 | 2.5–3.0 | |
| P2O5 | mg kg−1 | 83.0 ± 12.5 | 80–120 | |
| CEC § | cmol kg−1 | 14.7 ± 0.23 | 10–15 | |
| Heavy metals | As | mg kg−1 | 350.9 ± 11.2 | 25 |
| Cd | mg kg−1 | 5.33 ± 0.95 | 4 | |
| Pb | mg kg−1 | 207.5 ± 15.3 | 200 | |
| Cu | mg kg−1 | 33.54 ± 5.32 | 150 | |
| Zn | mg kg−1 | 168.9 ± 26.4 | 300 | |
| Treatment | pH | EC | SOM | P2O5 | CEC |
|---|---|---|---|---|---|
| dS m−1 | % | mg kg−1 | cmol kg−1 | ||
| Control | 7.29 ± 0.06 a | 0.88 ± 0.05 b | 4.15 ± 0.12 a | 78.2 ± 13.2 a | 14.62 ± 0.22 b |
| ALM10 | 6.93 ± 0.03 a | 1.03 ± 0.11 b | 3.68 ± 0.08 ab | 54.8 ± 19.4 b | 13.09 ± 0.18 b |
| ALM10+L | 7.11 ± 0.05 a | 1.28 ± 0.16 a | 4.08 ± 0.15 a | 62.4 ± 8.9 ab | 19.12 ± 0.15 a |
| ALM10+FeO | 7.12 ± 0.02 a | 0.89 ± 0.06 b | 3.16 ± 0.04 b | 61.2 ± 17.8 ab | 10.20 ± 0.17 c |
| Treatments | As | Pb |
|---|---|---|
| mg kg−1 | mg kg−1 | |
| Control | 1.63 ± 0.03 a | 12.99 ± 0.36 a |
| ALM10 | 1.26 ± 0.06 b | 6.18 ± 0.26 c |
| ALM10+L | 1.51 ± 0.07 ab | 4.46 ± 0.15 d |
| ALM10+FeO | 0.77 ± 0.04 c | 10.33 ± 0.32 b |
| Treatments | As | Pb |
|---|---|---|
| mg kg−1 | mg kg−1 | |
| Control | 1.92 ± 0.33 a | 1.58 ± 0.18 a |
| ALM10 | 1.32 ± 0.17 c | 0.60 ± 0.04 b |
| ALM10+L | 1.67 ± 0.21 b | 0.32 ± 0.07 c |
| ALM10+FeO | 0.92 ± 0.14 d | 0.45 ± 0.09 bc |
| As | Pb | |
|---|---|---|
| pH | 0.913 ** (0.001) | −0.882 ** (0.001) |
| EC | 0.605 * (0.037) | −0.723 ** (0.008) |
| SOM | −0.848 ** (0.001) | −0.575 (0.051) |
| P2O5 | −0.871 ** (0.004) | −0.251 (0.061) |
| CEC | 0.267 (0.401) | −0.031 (0.924) |
| As | 1.000 | −0.693 * (0.012) |
| Pb | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.-K.; Kim, J.-W.; Lee, S.-P.; Yang, J.-E.; Kim, S.-C. Effect of Combined Soil Amendment on Immobilization of Bioavailable As and Pb in Paddy Soil. Toxics 2022, 10, 90. https://doi.org/10.3390/toxics10020090
Hong Y-K, Kim J-W, Lee S-P, Yang J-E, Kim S-C. Effect of Combined Soil Amendment on Immobilization of Bioavailable As and Pb in Paddy Soil. Toxics. 2022; 10(2):90. https://doi.org/10.3390/toxics10020090
Chicago/Turabian StyleHong, Young-Kyu, Jin-Wook Kim, Sang-Phil Lee, Jae-E. Yang, and Sung-Chul Kim. 2022. "Effect of Combined Soil Amendment on Immobilization of Bioavailable As and Pb in Paddy Soil" Toxics 10, no. 2: 90. https://doi.org/10.3390/toxics10020090
APA StyleHong, Y.-K., Kim, J.-W., Lee, S.-P., Yang, J.-E., & Kim, S.-C. (2022). Effect of Combined Soil Amendment on Immobilization of Bioavailable As and Pb in Paddy Soil. Toxics, 10(2), 90. https://doi.org/10.3390/toxics10020090






