A Review of Methods Used to Detect Methamphetamine from Indoor Air and Textiles in Confined Spaces
Abstract
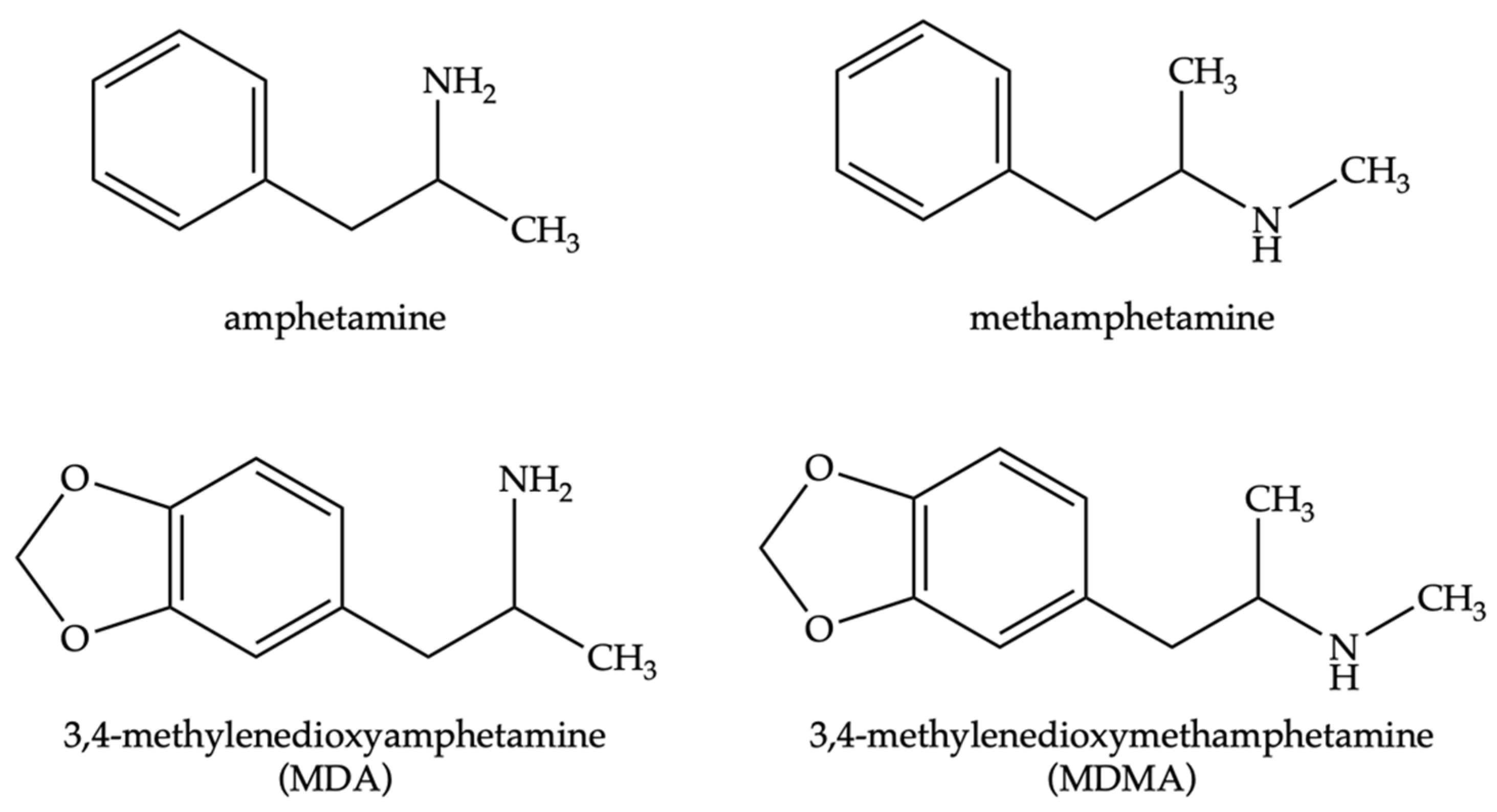
1. Introduction
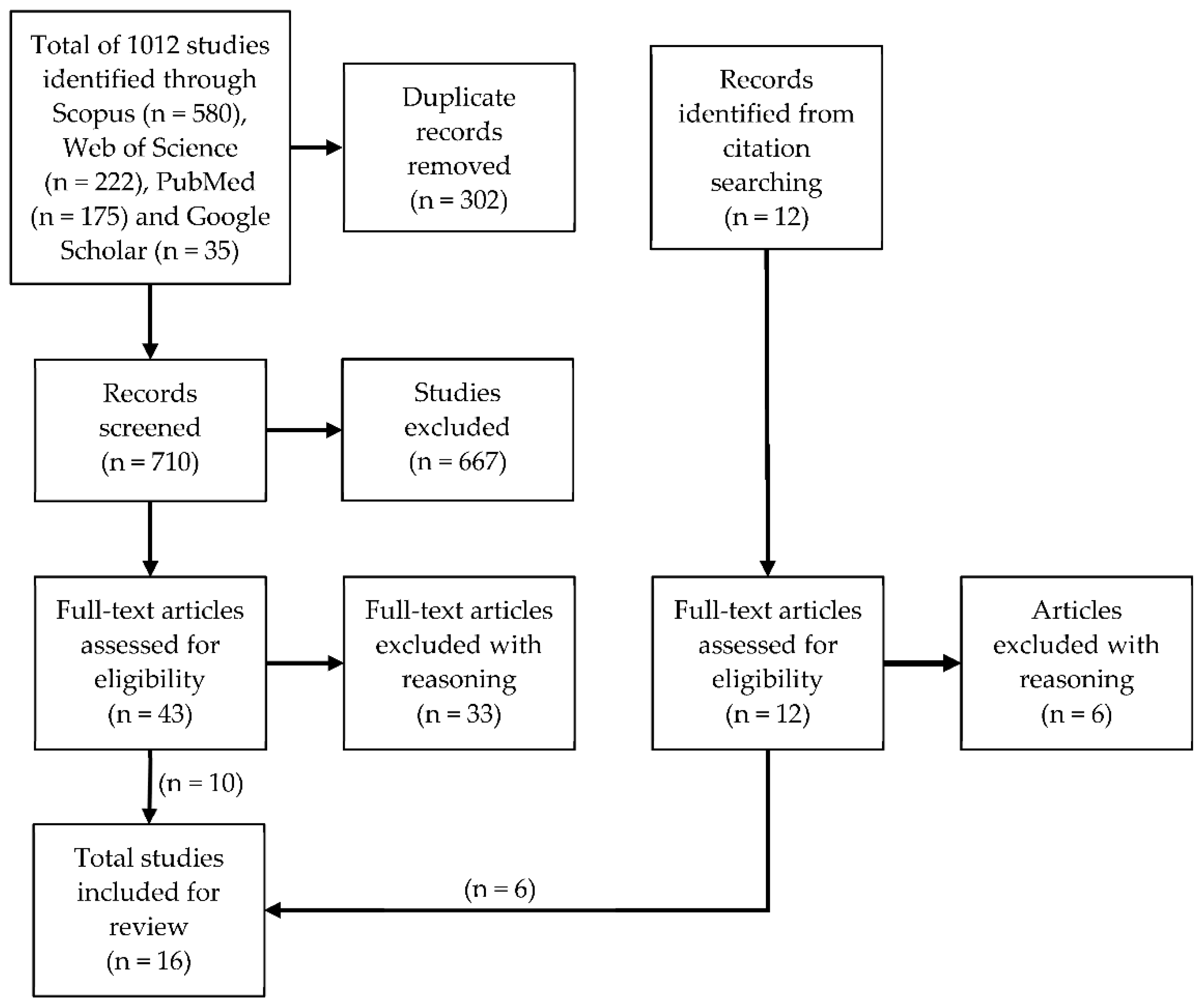
2. Materials and Methods
3. Results
4. Discussion
4.1. Extraction and Detection of Indoor Airborne Methamphetamine from Manufacture
4.2. Extraction and Detection of Indoor Airborne Methamphetamine from Smoking
4.3. Clothing and Textile Methamphetamine Extraction and Detection
4.4. Detection Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Australian Institute of Health and Welfare. Alcohol, Tobacco & Other Drugs in Australia. Available online: https://www.aihw.gov.au/reports/alcohol/alcohol-tobacco-other-drugs-australia/contents/drug-types/meth-amphetamine-and-other-stimulants (accessed on 25 May 2022).
- UNODC. Annual Drug Seizures. Available online: https://dataunodc.un.org/data/drugs/Annual%20Drug%20Seizures (accessed on 20 October 2022).
- Prakash, M.D.; Tangalakis, K.; Antonipillai, J.; Stojanovska, L.; Nurgali, K.; Apostolopoulos, V. Methamphetamine: Effects on the brain, gut and immune system. Pharmacol. Res. 2017, 120, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Thrash, B.; Thiruchelvan, K.; Ahuja, M.; Suppiramaniam, V.; Dhanasekaran, M. Methamphetamine-induced neurotoxicity: The road to Parkinson’s disease. Pharm. Rep. 2009, 61, 966–977. [Google Scholar] [CrossRef]
- Paratz, E.D.M.; Cunningham, N.J.M.F.; MacIsaac, A.I.M.D. The Cardiac Complications of Methamphetamines. Heart Lung Circ. 2015, 25, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhu, L.; Shen, Q.; Bai, X.; Di, X. Recent Advances in Methamphetamine Neurotoxicity Mechanisms and Its Molecular Pathophysiology. Behav. Neurol. 2015, 2015, 103969. [Google Scholar] [CrossRef] [PubMed]
- Australian Crime Commission. Clandestine Drug Laboratory Remediation Guidelines; Attorney-General’s Department, Commonwealth of Australia: Canberra, Australia, 2011. [Google Scholar]
- Australian Criminal Intelligence Commission. Amphetamine-Type Stimulants; Australian Criminal Intelligence Commission: Canberra, Australia, 2017. [Google Scholar]
- UNODC. Drug Market Trends: Cocaine, Amphetamine-Type Stimulants; United Nations Publication, Sales No. E.21.XI.8; United Nations: Vienna, Austria, 2021; pp. 47–73. [Google Scholar]
- Bitter, J.L. The persistence of illicit drug smoke residues and their recovery from common household surfaces: Recovery of drug smoke residues. Drug Test. Anal. 2017, 9, 603–612. [Google Scholar] [CrossRef]
- Martyny, J.W.; Arbuckle, S.L.; McCammon, C.S.; Erb, N.; Van Dyke, M. Methamphetamine contamination on environmental surfaces caused by simulated smoking of methamphetamine. J. Chem. Health Saf. 2008, 15, 25–31. [Google Scholar] [CrossRef]
- Martyny, J.W.; Arbuckle, S.L.; McCammon, C.S.; Esswein, E.J.; Erb, N.; Van Dyke, M. Chemical concentrations and contamination associated with clandestine methamphetamine laboratories. J. Chem. Health Saf. 2007, 14, 40–52. [Google Scholar] [CrossRef]
- Wright, J.; Symons, B.; Angell, J.; Ross, K.E.; Walker, S. Current practices underestimate environmental exposures to methamphetamine: Inhalation exposures are important. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 45–52. [Google Scholar] [CrossRef]
- Poppendieck, D.; Morrison, G.; Corsi, R. Desorption of a methamphetamine surrogate from wallboard under remediation conditions. Atmos. Environ. 2015, 106, 477–484. [Google Scholar] [CrossRef]
- Serrano, K.A.; Martyny, J.W.; Kofford, S.; Contreras, J.R.; Van Dyke, M.V. Decontamination of Clothing and Building Materials Associated with the Clandestine Production of Methamphetamine. J. Occup. Environ. Hyg. 2012, 9, 185–197. [Google Scholar] [CrossRef]
- Van Dyke, M.; Erb, N.; Arbuckle, S.; Martyny, J. A 24-Hour Study to Investigate Persistent Chemical Exposures Associated with Clandestine Methamphetamine Laboratories. J. Occup. Environ. Hyg. 2008, 6, 82–89. [Google Scholar] [CrossRef]
- Van Dyke, M.; Martyny, J.W.; Serrano, K.A. Methamphetamine Residue Dermal Transfer Efficiencies from Household Surfaces. J. Occup. Environ. Hyg. 2014, 11, 249–258. [Google Scholar] [CrossRef]
- Wright, J.; Walker, G.S.; Ross, K.E. Contamination of Homes with Methamphetamine: Is Wipe Sampling Adequate to Determine Risk? Int. J Environ Res. Public Health 2019, 16, 3568. [Google Scholar] [CrossRef]
- Ross, G.H.; Sternquist, M.C. Methamphetamine exposure and chronic illness in police officers: Significant improvement with sauna-based detoxification therapy. Toxicol. Ind. Health 2012, 28, 758–768. [Google Scholar] [CrossRef]
- Witter, R.Z.; Martyny, J.W.; Mueller, K.; Gottschall, B.; Newman, L.S. Symptoms Experienced by Law Enforcement Personnel During Methamphetamine Lab Investigations. J. Occup. Environ. Hyg. 2007, 4, 895–902. [Google Scholar] [CrossRef]
- Wright, J.; Kenneally, M.; Ross, K.; Walker, G.S. Environmental methamphetamine exposures and health effects in 25 case studies. Toxics 2020, 8, 61. [Google Scholar] [CrossRef]
- Wright, J.; Kenneally, M.E.; Edwards, J.W.; Walker, G.S. Adverse Health Effects Associated with Living in a Former Methamphetamine Drug Laboratory—Victoria, Australia, 2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 65, 1470–1473. [Google Scholar] [CrossRef]
- McFadden, D.; Kub, J.; Fitzgerald, S. Occupational Health Hazards to First Responders from Clandestine Methamphetamine Labs. J. Addict. Nurs. 2006, 17, 169–173. [Google Scholar] [CrossRef]
- Ciesielski, A.L.; Wagner, J.R.; Alexander-Scott, M.; Smith, J.; Snawder, J. Surface Contamination Generated by “One-Pot” Methamphetamine Production. ACS Chem. Health Saf. 2021, 28, 49–54. [Google Scholar] [CrossRef]
- Madireddy, S.B.; Bodeddula, V.R.; Mansani, S.K.; Wells, M.J.M.; Boles, J.O. Wipe sampling of amphetamine-type stimulants and recreational drugs on selected household surfaces with analysis by ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Hazard. Mater. 2013, 254–255, 46–56. [Google Scholar] [CrossRef]
- Lim Abdullah, A.F.; Miskelly, G.M. Recoveries of trace pseudoephedrine and methamphetamine residues from impermeable household surfaces: Implications for sampling methods used during remediation of clandestine methamphetamine laboratories. Talanta 2010, 81, 455–461. [Google Scholar] [CrossRef]
- Morrison, G.; Shakila, N.V.; Parker, K. Accumulation of gas-phase methamphetamine on clothing, toy fabrics, and skin oil. Indoor Air 2015, 25, 405–414. [Google Scholar] [CrossRef]
- Chien, Y.-C.; Chang, C.-P.; Liu, Z.-Z. Volatile organics off-gassed among tobacco-exposed clothing fabrics. J. Hazard. Mater. 2011, 193, 139–148. [Google Scholar] [CrossRef]
- Noble, R.E. Environmental tobacco smoke uptake by clothing fabrics. Sci. Total Environ. 2000, 262, 1–3. [Google Scholar] [CrossRef]
- Ueta, I.; Saito, Y.; Teraoka, K.; Miura, T.; Jinno, K. Determination of Volatile Organic Compounds for a Systematic Evaluation of Third-Hand Smoking. Anal. Sci. 2010, 26, 569–574. [Google Scholar] [CrossRef]
- Environmental Health Australia. Australian Voluntary Code of Practice Assessment, Remediation and Validation: Former Clandestine Drug Laboratories and Other Methamphetamine Contaminated Properties; Environmental Health Australia: Brisbane, Australia, 2019. [Google Scholar]
- Ministry of Health. Guidelines for the Remediation of Clandestine Methamphetamine Laboratory Sites; Ministry of Health (MoH): Wellington, New Zealand, 2010. [Google Scholar]
- Darke, S.; Kaye, S.; McKetin, R.; Duflou, J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev. 2008, 27, 253–262. [Google Scholar] [CrossRef]
- Degenhardt, L.; Sara, G.; McKetin, R.; Roxburgh, A.; Dobbins, T.; Farrell, M.; Burns, L.; Hall, W.D. Crystalline methamphetamine use and methamphetamine-related harms in Australia. Drug Alcohol Rev. 2017, 36, 160–170. [Google Scholar] [CrossRef]
- Heal, D.J.; Smith, S.L.; Gosden, J.; Nutt, D.J. Amphetamine, past and present—A pharmacological and clinical perspective. J. Psychopharmacol. 2013, 27, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Dragan, A.-M.; Parrilla, M.; Feier, B.; Oprean, R.; Cristea, C.; De Wael, K. Analytical techniques for the detection of amphetamine-type substances in different matrices: A comprehensive review. Trends Anal. Chem. 2021, 145, 116447. [Google Scholar] [CrossRef]
- Khorablou, Z.; Shahdost-fard, F.; Razmi, H.; Yola, M.L.; Karimi-Maleh, H. Recent advances in developing optical and electrochemical sensors for analysis of methamphetamine: A review. Chemosphere 2021, 278, 130393. [Google Scholar] [CrossRef]
- Boles, T.H.; Wells, M.J.M. Analysis of amphetamine and methamphetamine as emerging pollutants in wastewater and wastewater-impacted streams. J. Chromatogr. A 2010, 1217, 2561–2568. [Google Scholar] [CrossRef]
- Kraemer, T.; Maurer, H.H. Determination of amphetamine, methamphetamine and amphetamine-derived designer drugs or medicaments in blood and urine. J. Chromatogr. B Biomed. Sci. Appl. 1998, 713, 163–187. [Google Scholar] [CrossRef]
- Raynor, P.C.; Carmody, T. Meth Labs Sampling: Air and HVAC Systems; Minnesota Pollution Control Agency: St. Paul, MO, USA, 2006. [Google Scholar]
- McKenzie, E.J.; Miskelly, G.M.; Butler, P.A.G. Detection of methamphetamine in indoor air using dynamic solid phase microextraction: A supplementary method to surface wipe sampling. Anal. Methods 2013, 5, 5418. [Google Scholar] [CrossRef][Green Version]
- McKenzie, E.J.; Miskelly, G.M.; Butler, P.A.G. Dynamic solid phase microextraction analysis for airborne methamphetamine: Quantitation using isotopically substituted methamphetamine. Anal. Methods 2013, 5, 4391–4396. [Google Scholar] [CrossRef]
- McKenzie, E.J. Chemical Contamination in Former Clandestine Methamphetamine Laboratories. Ph.D. Thesis, University of Auckland, Auckland, New Zealand, 2014. [Google Scholar]
- Al-Dirbashi, O.Y.; Ikeda, K.; Takahashi, M.; Kuroda, N.; Ikeda, S.; Nakashima, K. Drugs of abuse in a non-conventional sample; detection of methamphetamine and its main metabolite, amphetamine in abusers’ clothes by HPLC with UV and fluorescence detection. Biomed. Chromatogr. 2001, 15, 457–463. [Google Scholar] [CrossRef]
- Talaty, N.; Mulligan, C.C.; Justes, D.R.; Jackson, A.U.; Noll, R.J.; Cooks, R.G. Fabric analysis by ambient mass spectrometry for explosives and drugs. Analyst 2008, 133, 1532–1540. [Google Scholar] [CrossRef]
- Keasey, S.J. Testing for the Presence of Methamphetamine Residues on Clothing from Suspected Clandestine Labs. Master’s Thesis, University of Alabama at Birmingham, Birmingham, AL, USA, 2011. [Google Scholar]
- Salocks, C.B.; Hui, X.; Lamel, S.; Hafeez, F.; Qiao, P.; Sanborn, J.R.; Maibach, H.I. Dermal exposure to methamphetamine hydrochloride contaminated residential surfaces II. Skin surface contact and dermal transfer relationship. Food Chem. Toxicol. 2014, 66, 1–6. [Google Scholar] [CrossRef]
- Koziel, J.A.; Pawliszyn, J. Air Sampling and Analysis of Volatile Organic Compounds with Solid Phase Microextraction. J. Air Waste Manag. Assoc. 2001, 51, 173–184. [Google Scholar] [CrossRef]
- enHealth. enHealth Guidance on: Clandestine Drug Laboratories and Public Health Risks; enHealth: Canberra, Australia, 2017. [Google Scholar]
- Martyny, J.; Arbuckle, S.; McCammon, C.; Erb, N. Chemical Exposures Associated with Clandestine Methamphetamine Laboratories Using the Anhydrous Ammonia Method of Production; National Jewish Medical and Research Center: Denver, CO, USA, 2004. [Google Scholar]
- Martyny, J.; Arbuckle, S.; McCammon, C.; Erb, N. Methamphetamine Contamination on Environmental Surfaces Caused by Simulated Smoking of Methamphetamine; National Jewish Medical and Research Center: Denver, CO, USA, 2004. [Google Scholar]
- Martyny, J.; Erb, N.; Arbuckle, S.; Van Dyke, M. A 24-Hour Study to Investigate Chemical Exposures Associated with Clandestine Methamphetamine Laboratories; National Jewish Medical and Research Centre, Division of Environmental and Occupational Health Sciences: Denver, CO, USA, 2005. [Google Scholar]
- Martyny, J.W.; Arbuckle, S.; McCammon, C.; Esswein, E.; Erb, N. Chemical Exposures Associated with Clandestine Methamphetamine Laboratories; National Jewish Medical and Research Center: Denver CO, USA, 2004. [Google Scholar]
- Martyny, J.W.; Van Dyke, M.; McCammon, C.S.; Erb, N.; Arbuckle, S. Chemical Exposures Associated with Clandestine Methamphetamine Laboratories Using the Hypophosphorous and Phosphorous Flake Method of Production; Division of Environmental and Occupational Health Sciences, National Jewish Medical and Research Center: Denver CO, USA, 2005. [Google Scholar]
- Li, H. Adsorption and Desorption Capacity of Methamphetamine in Gypsum Drywall. Master’s Thesis, Missouri University of Science and Technology, Rolla, MO, USA, 2014. [Google Scholar]
- NIOSH. 9111—METHAMPHETAMINE on Wipes by Liquid Chromatography/Mass Spectrometry; NIOSH Manual of Analytical Methods (NMAM); Centers for Disease Control and Prevention (CDC): Washington, DC, USA, 2011. [Google Scholar]
- NIOSH. 5600—Organophosphorous Pesticides; NIOSH Manual of Analytical Methods (NMAM); Centers for Disease Control and Prevention (CDC): Washington, DC, USA, 2016. [Google Scholar]
- Salocks, C.; Golub, M.; Kaufman, F. Development of a Reference Dose (RfD) for Methamphetamine; California Office of Environmental Health Hazard Assessment, Integrated Risk Assessment Branch: Sacramento, CA, USA, 2009. [Google Scholar]
- Harper, M. Sorbent trapping of volatile organic compounds from air. J. Chromatogr. A 2000, 885, 129–151. [Google Scholar] [CrossRef]
- Gorlo, D.; Zygmunt, B.; Dudek, M.; Jaszek, A.; Pilarczyk, M.; Namiesnik, J. Application of solid-phase microextraction to monitoring indoor air quality. Fresenius J. Anal. Chem. 1999, 363, 696–699. [Google Scholar] [CrossRef]
- Larroque, V.; Desauzier, V.; Mocho, P. Development of a solid phase microextraction (SPME) method for the sampling of VOC traces in indoor air. J. Environ. Monit. 2006, 8, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Belardi, R.P.; Pawliszyn, J.B. Application of chemically modified fused silica fibers in the extraction of organics from water matrix samples and their rapid transfer to capillary columns. Water Pollut. Res. J. Can. 1989, 24, 179–191. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Pawliszyn, J. Solid Phase MicroExtraction: Theory and Practice, 1st ed.; Wiley-VCH: New York City, NY, USA, 1997; p. 264. [Google Scholar]
- Koester, C.J.; Andresen, B.D.; Grant, P.M. Optimum Methamphetamine Profiling with Sample Preparation by Solid-Phase Microextraction. J. Forensic Sci. 2002, 47, 1–6. [Google Scholar] [CrossRef]
- Kuwayama, K.; Tsujikawa, K.; Miyaguchi, H.; Kanamori, T.; Iwata, Y.; Inoue, H.; Saitoh, S.; Kishi, T. Identification of impurities and the statistical classification of methamphetamine using headspace solid phase microextraction and gas chromatography–mass spectrometry. Forensic Sci. Int. 2006, 160, 44–52. [Google Scholar] [CrossRef]
- Brown, H.; Kirkbride, K.P.; Pigou, P.E.; Walker, G.S. New developments in SPME, Part 1: The use of vapor-phase deprotonation and on-fiber derivatization with alkylchloroformates in the analysis of preparations containing amphetamines. J. Forensic Sci. 2003, 48, 1231–1238. [Google Scholar] [CrossRef]
- Gura, S.; Guerra-Diaz, P.; Lai, H.; Almirall, J.R. Enhancement in sample collection for the detection of MDMA using a novel planar SPME (PSPME) device coupled to ion mobility spectrometry (IMS). Drug Test. Anal. 2009, 1, 355–362. [Google Scholar] [CrossRef]
- EPA. Voluntary Guidelines for Methamphetamine and Fentanyl Laboratory Cleanup; United States Environmental Protection Agency EPA, Office of Land and Emergency Management: Washington, DC, USA, 2021. [Google Scholar]
- Standards New Zealand. Testing and Decontamination of Methamphetamine-Contaminated Properties; Standards New Zealand: Wellington, New Zealand, 2017. [Google Scholar]
- Nair, M.V.; Miskelly, G.M. Capillary microextraction: A new method for sampling methamphetamine vapour. Forensic Sci. Int. 2016, 268, 131–138. [Google Scholar] [CrossRef]
- Wiebelhaus, N.; Hamblin, D.N.; Kreitals, N.M.; Almirall, J.R. Differentiation of marijuana headspace volatiles from other plants and hemp products using capillary microextraction of volatiles (CMV) coupled to gas-chromatography–mass spectrometry (GC–MS). Forensic Chem. 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Fan, W.; Almirall, J. High-efficiency headspace sampling of volatile organic compounds in explosives using capillary microextraction of volatiles (CMV) coupled to gas chromatography–mass spectrometry (GC-MS). Anal. Bioanal. Chem. 2014, 406, 2189–2195. [Google Scholar] [CrossRef]
- Tarifa, A.; Almirall, J.R. Fast detection and characterization of organic and inorganic gunshot residues on the hands of suspects by CMV-GC–MS and LIBS. Sci. Justice 2015, 55, 168–175. [Google Scholar] [CrossRef]
- Gura, S.; Tarifa, A.; Mulloor, J.; Torres, M.N.; Almirall, J.R. Capillary microextraction of volatiles device for enhanced BTEX vapors sampling based on a phenyl modified PDMS sol-gel adsorption phase. Anal. Chim. Acta 2018, 1014, 27–40. [Google Scholar] [CrossRef]
- Nair, M.V.; Miskelly, G.M. Determination of airborne methamphetamine via capillary microextraction of volatiles (CMV) with on-sorbent derivatisation using o-pentafluorobenzyl chloroformate. Forensic Chem. 2019, 14, 100161. [Google Scholar] [CrossRef]
- Russell, M.; Nicolle, S.; Mayo, E.; Chappell, A. Deposition of methamphetamine residues produced by simulated smoking. Forensic Sci. Int. 2022, 338, 111407. [Google Scholar] [CrossRef]
- Buchert, J.; Pere, J.; Johansson, L.S.; Campbell, J.M. Analysis of the Surface Chemistry of Linen and Cotton Fabrics. Text. Res. J. 2001, 71, 626–629. [Google Scholar] [CrossRef]
- Hansen, S.; Atwood, K.B. Polyester Fibers. In Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- NIOSH. 9106—METHAMPHETAMINE and Illicit Drugs, Precursors and Adulterants on Wipes by Liquid-Liquid Extraction; NIOSH Manual of Analytical Methods (NMAM); Centers for Disease Control and Prevention (CDC): Washington, DC, USA, 2011. [Google Scholar]
- Reynolds, J.; Siso, M.; Perkins, J. Backup Data Report for NIOSH 9106, Methamphetamine and Illicit Drugs, Precursors and Adulterants on Wipes by Liquid-Liquid Extraction, Abridged; Prepared under NIOSH Contract 200-2001-0800; DataChem Laboratories, Inc., Centers for Disease Control and Prevention (CDC): Washington, DC, USA, 2005. [Google Scholar]
- Rodes, C.E.; Newsome, J.R.; Vanderpool, R.W.; Antley, J.T.; Lewis, R.G. Experimental methodologies and preliminary transfer factor data for estimation of dermal exposures to particles. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 123–139. [Google Scholar] [CrossRef]
- Petrick, L.; Destaillats, H.; Zouev, I.; Sabach, S.; Dubowski, Y. Sorption, desorption, and surface oxidative fate of nicotine. Phys. Chem. Chem. Phys. 2010, 12, 10356–10364. [Google Scholar] [CrossRef]
- Piadé, J.J.; D’André, S.; Sanders, E.B. Sorption Phenomena of Nicotine and Ethenylpyridine Vapors on Different Materials in a Test Chamber. Environ. Sci. Technol. 1999, 33, 2046–2052. [Google Scholar] [CrossRef]
- Ciesielski, A.L.; Green, M.K.; Wagner, J.R. Characterization of One Pot methamphetamine laboratories using GC-MS and LC-MS/MS. Forensic Chem. 2020, 19, 100244. [Google Scholar] [CrossRef]
- Concheiro, M.; Simões, S.M.d.S.S.; Quintela, Ó.; de Castro, A.; Dias, M.J.R.; Cruz, A.; López-Rivadulla, M. Fast LC–MS/MS method for the determination of amphetamine, methamphetamine, MDA, MDMA, MDEA, MBDB and PMA in urine. Forensic Sci. Int. 2006, 171, 44–51. [Google Scholar] [CrossRef]
- González-Mariño, I.; Quintana, J.B.; Rodríguez, I.; Cela, R. Determination of drugs of abuse in water by solid-phase extraction, derivatisation and gas chromatography–ion trap-tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 1748–1760. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H. Derivatization reactions for the determination of amines by gas chromatography and their applications in environmental analysis. J. Chromatogr. A 1996, 733, 19–34. [Google Scholar] [CrossRef]
- Kataoka, H. 2.1.2.—Gas Chromatography of Amines as Various Derivatives. J. Chromatogr. Libr. 2005, 70, 364–404. [Google Scholar]
- Dobos, A.; Hidvégi, E.; Somogyi, G.P. Comparison of Five Derivatizing Agents for the Determination of Amphetamine-Type Stimulants in Human Urine by Extractive Acylation and Gas Chromatography-Mass Spectrometry. J. Anal. Toxicol. 2012, 36, 340–344. [Google Scholar] [CrossRef]
- Mohamed, K.M.; Bakdash, A. Comparison of 3 Derivatization Methods for the Analysis of Amphetamine-Related Drugs in Oral Fluid by Gas Chromatography-Mass Spectrometry. Anal. Chem. Insights 2017, 2017, 1177390117727533. [Google Scholar] [CrossRef]
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Simple and Rapid Determination of Amphetamine, Methamphetamine, and Their Methylenedioxy Derivatives in Urine by Automated In-Tube Solid-Phase Microextraction Coupled with Liquid Chromatography-Electrospray Ionization Mass Spectrometry. J. Anal. Toxicol. 2000, 24, 257–265. [Google Scholar] [CrossRef]
- Tatsuno, M.; Nishikawa, M.; Katagi, M.; Tsuchihashi, H. Simultaneous Determination of Illicit Drugs in Human Urine by Liquid Chromatography-Mass Spectrometry. J. Anal. Toxicol. 1996, 20, 281–286. [Google Scholar] [CrossRef][Green Version]
| Search Terms | |
|---|---|
| Key Words | amphetamine OR amfetamine OR methamphetamine OR metamfetamine OR methylamphetamine OR “clandestine laborator *” air OR “indoor air” OR airborne OR vapo?r OR “vapo?r phase” OR “air phase” OR “gas phase” OR gaseous OR volatile OR volatili?ation OR “free base” OR “meth * smok *” OR cloth * OR fabric OR textile OR upholstery |
| AND title/abstract/keywords | |
| Sample Type | Air Extraction Method | Media Desorption | Detection Method | State, Country | Reference |
|---|---|---|---|---|---|
| Two former clandestine laboratories. | Personal sampling pump (1.5 L/min flow rate) connected to a silica gel sorbent tube followed by glass fiber filter in a clear styrene cassette. | 15 mL methanol | LC-MS Agilent 1100 LC-MSD | Minnesota, USA | Raynor and Carmody 2006 [40] |
| Suspected clandestine laboratories and controlled methamphetamine manufacture in abandoned structures using red phosphorus, hypophosphorous, phosphorus flake, and anhydrous ammonia methods. | Personal sampling pump (2 L/min flow rate) with 37 mm sampling cassettes and acid-treated glass fiber filters. | Method under development at time of paper publication. | GC-MS Make not specified | Colorado, USA | Martyny et al. 2007 [12] |
| Controlled “smoking” of methamphetamine in a dwelling. | Personal sampling pump (2 L/min flow rate) with acid-treated glass fiber filters. | As specified in NIOSH draft method 9106, a method used for methamphetamine analysis on wipes by liquid-liquid extraction. | GC-MS Make not specified | Colorado, USA | Martyny et al. 2008 [11] |
| Controlled methamphetamine manufacture in a home using a red phosphorus method. | Personal sampling pump (2 or 2.5 L/min flow rate) with closed-face, acid-treated glass fiber filter cassette and with an aluminum cyclone filter cassette. Sioutas Personal Cascade Impactor (9 L/min flow) with acid-treated glass fiber media. | As specified in NIOSH draft method 9106. | GC-MS Make not specified | Colorado, USA | Van Dyke et al. 2008 [16] |
| Suspected former clandestine laboratories. | Dynamic SPME field sampler coupled to an air sampling pump (sampling for 5–30 min at 1 L/min flow rate). | SPME fiber introduced into GC inlet set at 250 °C. | GC-MS HP 6890 GC + HP 5973 MS Positive Ion Mode, 70 eV | New Zealand | McKenzie, Miskelly and Butler 2013 [41,42], McKenzie 2014 [43] * |
| Contaminated home from known methamphetamine manufacture. | Personal sampling pump (1 L/min flow rate) with ORBO™-49P (XAD®-2 resin) sampling tubes. | Based on NIOSH method 9111, a method used for methamphetamine analysis on wipe samples. | LC-MS Agilent 1290 Infinity UHPLC system 6460/6470 Triple Quadrupole, LC-MS electrospray source | Australia | Wright et al. 2021 [13] |
| Sample Type | Sample Composition and Contamination | Extraction Method | Detection Method | State, Country | Reference |
|---|---|---|---|---|---|
| Clothing | 100% cotton garment as control spiked with 0.8–1492.4 ng methamphetamine/0.1 g sample. Methamphetamine users’ clothing. | Liquid extraction of swatches using 1 mL chloroform:propan-2-ol (3:1, v/v). | HPLC-FL system Shimadzu LC-10ADvp pump with Shimadzu RF_10AXL spectrofluorometer HPLC-UV system Tosoh CCPD pump with Waters 484 absorbance detector | Japan | Al-Dirbashi et al. 2001 [44] |
| Clothing | Protective clothing worn during controlled methamphetamine manufacture in a property using red phosphorus, hypophosphorous, phosphorus flake, and anhydrous ammonia methods. | Method under development at the laboratory at time of the paper publication. | GC-MS Make not specified | Colorado, USA | Martyny et al. 2007 [12] |
| Textile | 100% cotton and 100% polyester samples spiked with 2.5 ng each of cocaine, heroin, and methamphetamine. | Not applicable. | DESI-MS Prosolia Omni Spray™ ion source and Thermo Electron LTQ MS | Indiana, USA | Talaty et al. 2008 [45] |
| Textile | Carpet in a property after controlled methamphetamine manufacture using a red phosphorus method. | Carpet vacuumed using a Eureka Sanitare Commercial vacuum cleaner fitted with a Mitest Dust collection device. Samples then underwent extraction according to NIOSH draft method 9106, a method used for methamphetamine analysis of wipes using liquid-liquid extraction. | GC-MS Make not specified | Colorado, USA | Van Dyke et al. 2008 [16] |
| Clothing | Cotton sweatshirt spiked with known concentrations of methamphetamine. Fabric and clothing (including jeans, t-shirts, undergarments, socks, and car seat covers) from suspected or known methamphetamine users or cooks. | Liquid extraction of swatches using different solvents. | GC-MS Agilent Technologies 6890N GC 5975 with Agilent Mass Selective Detector | Alabama, USA | Keasey 2011 [46] |
| Clothing | Cotton denim (tight weave), cotton blanket (looser weave), fire department turnout jackets, law enforcement ballistic vest, Nomex coveralls and polyester/cotton coveralls contaminated with laboratory-generated methamphetamine aerosol. | Liquid extraction of swatches using NIOSH method 9111, a method used for methamphetamine analysis of wipe samples. | LC-MS Make not specified | Colorado, USA | Serrano et al. 2012 [15] |
| Textile | Loosely woven upholstery fabric (19% cotton, 79% olefin, 2% rayon). Transfer of methamphetamine from fabric to skin. | Not applicable | Radioactivity using a PerkinElmer Tri-Carb 2900 TR liquid scintillation spectrometer | California, USA | Salocks et al. 2014 [47] |
| Textile | Low-pile, synthetic carpet contaminated with methamphetamine from simulated “smoking”. Cotton glove used to determine the dermal transfer of methamphetamine from a contaminated surface. | Liquid extraction of swatches and surface wipe sampling media using NIOSH method 9111. | LC-MS Make not specified | Colorado, USA | Van Dyke, Martyny, and Serrano 2014 [17] |
| Clothing/Textile | Polyester baby blankets, polyester baby toy ‘book’, polyester woman’s shirts, cotton baby T-shirt and cotton/polyester blend upholstery which were exposed to methamphetamine gas. | Liquid extraction of swatches with 6.5 mL ethyl acetate with 10 µL of 1000 ppm internal standard bromofluorobenzene (BFB) solution in ethyl acetate. | GC-MS Agilent, model not specified | Missouri, USA | Morrison, Shakila, and Parker 2015 [27] |
| Textile | Carpet and rugs from contaminated property. | Liquid extraction of surface wipe samples or bulk samples using NIOSH method 9111. | LC-MS Agilent 1290 Infinity UHPLC system with 6460/6470 Triple Quadrupole LC-MS electrospray source | Australia | Wright et al. 2019 [18] |
| Textile | Soft toys from contaminated property. | Personal sampling pump (1 L/min flow rate) with ORBO™-49P (XAD®-2 resin) sampling tubes followed extraction using NIOSH method 9111. | LC-MS Agilent 1290 Infinity UHPLC system with 6460/6470 Triple Quadrupole LC-MS electrospray source | Australia | Wright et al. 2021 [13] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerry, G.L.; Ross, K.E.; Wright, J.L.; Walker, G.S. A Review of Methods Used to Detect Methamphetamine from Indoor Air and Textiles in Confined Spaces. Toxics 2022, 10, 710. https://doi.org/10.3390/toxics10110710
Kerry GL, Ross KE, Wright JL, Walker GS. A Review of Methods Used to Detect Methamphetamine from Indoor Air and Textiles in Confined Spaces. Toxics. 2022; 10(11):710. https://doi.org/10.3390/toxics10110710
Chicago/Turabian StyleKerry, Gemma L., Kirstin E. Ross, Jackie L. Wright, and G. Stewart Walker. 2022. "A Review of Methods Used to Detect Methamphetamine from Indoor Air and Textiles in Confined Spaces" Toxics 10, no. 11: 710. https://doi.org/10.3390/toxics10110710
APA StyleKerry, G. L., Ross, K. E., Wright, J. L., & Walker, G. S. (2022). A Review of Methods Used to Detect Methamphetamine from Indoor Air and Textiles in Confined Spaces. Toxics, 10(11), 710. https://doi.org/10.3390/toxics10110710






