Adsorption Characteristics of Dimethylated Arsenicals on Iron Oxide–Modified Rice Husk Biochar
Abstract
1. Introduction
2. Materials and Methods
2.1. BC
2.2. Synthesis of FeBC
2.3. Equilibrium Adsorption Isotherm Experiments
2.4. XAS
3. Results and Discussion
3.1. Characteristics of FeBC
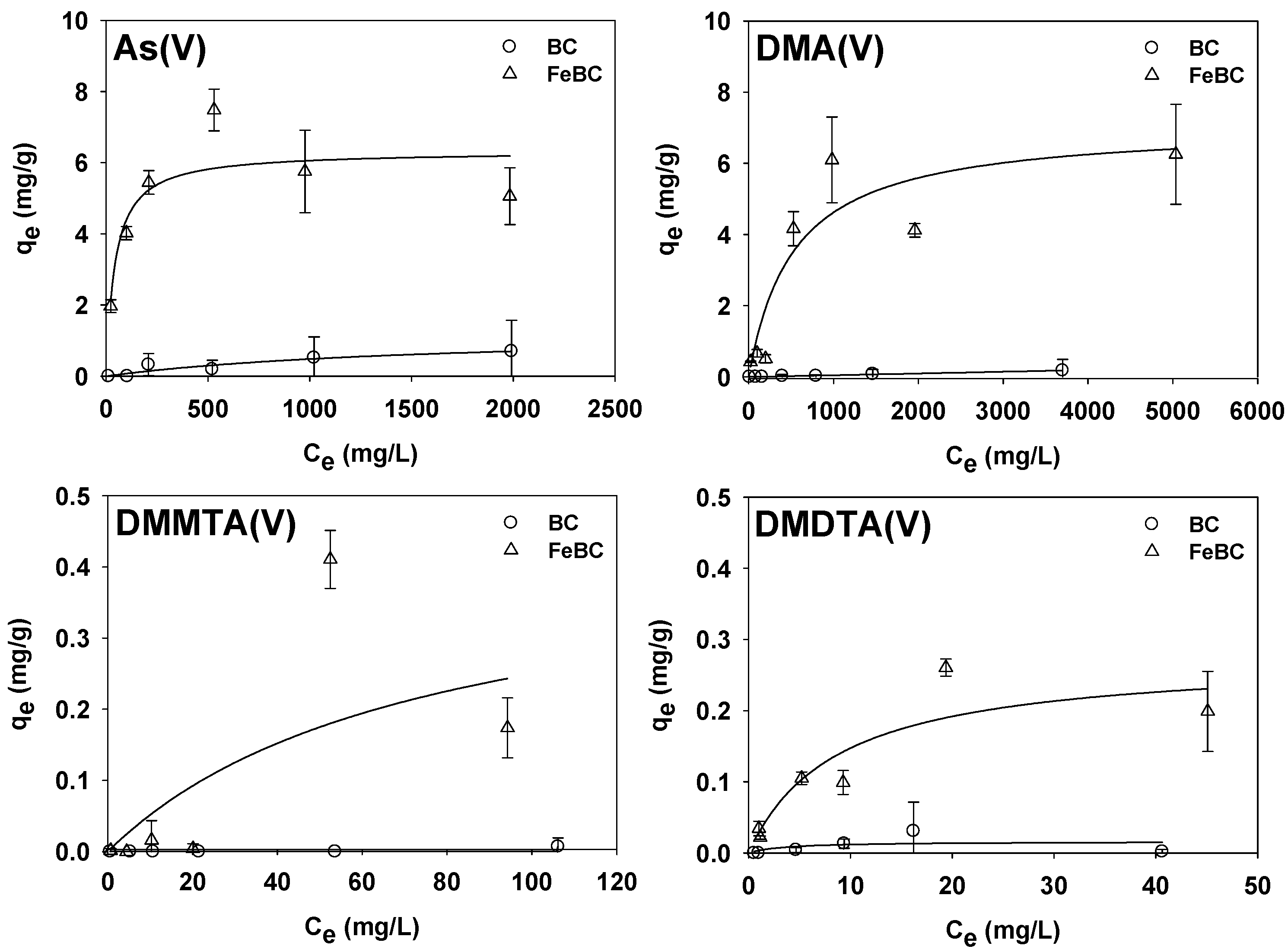
3.2. Comparison of Adsorption Characteristics of Arsenicals on BC and FeBC
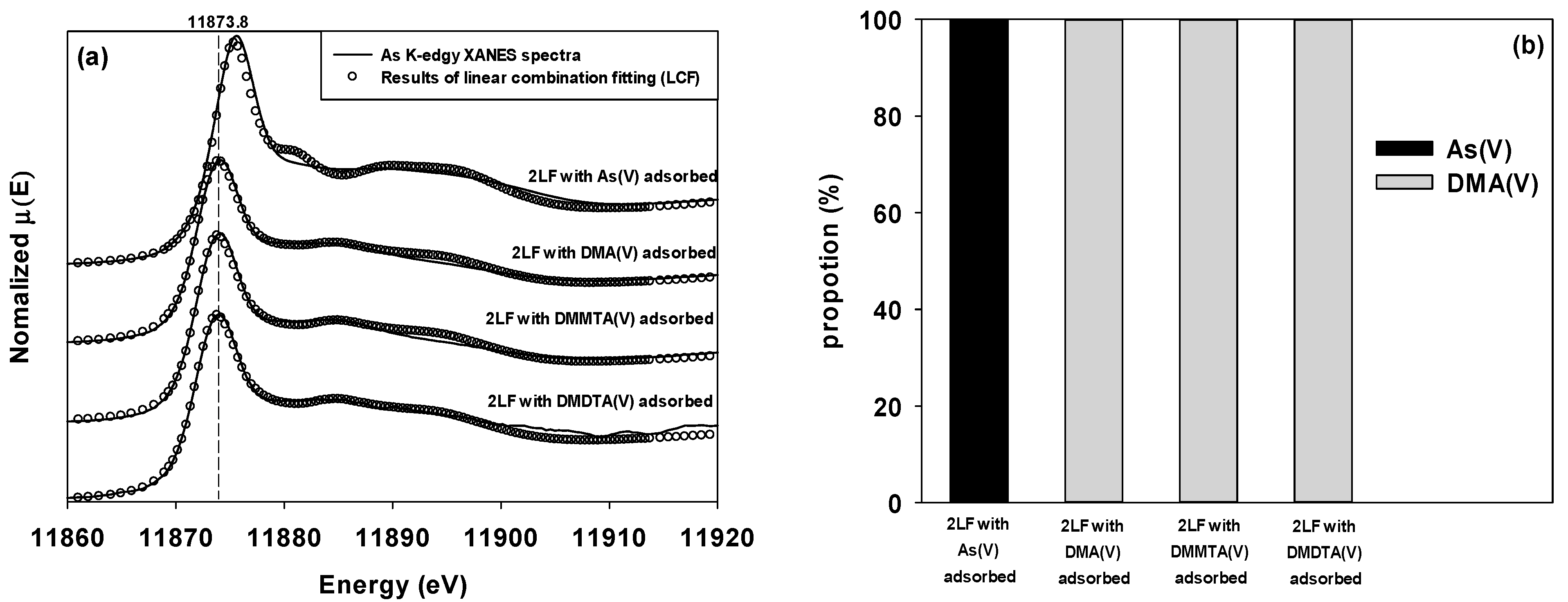
3.3. Chemical Forms of Adsorbed Dimethylated Arsenicals on Two-Line Ferrihydrite
3.4. Environmental Implication
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammed Abdul, K.S.; Jayasinghe, S.S.; Chandana, E.P.S.; Jayasumana, C.; De Silva, P.M. Arsenic and human health effects: A Review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef]
- Hughes, M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002, 133, 1–16. [Google Scholar] [CrossRef]
- Bondada, B.R.; Tu, S.; Ma, L.Q. Absorption of foliar-applied arsenic by the arsenic hyperaccumulating fern (Pteris vittata L.). Sci. Total Environ. 2004, 332, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Vithanage, M.; Herath, I.; Joseph, S.; Bundschuh, J.; Bolan, N.; Ok, Y.S.; Kirkham, M.B.; Rinklebe, J. Interaction of arsenic with biochar in soil and water: A critical review. Carbon 2017, 113, 219–230. [Google Scholar] [CrossRef]
- Parsons, C.T.; Couture, R.-M.; Omoregie, E.O.; Bardelli, F.; Greneche, J.-M.; Roman-Ross, G.; Charlet, L. The impact of oscillating redox conditions: Arsenic immobilisation in contaminated calcareous floodplain soils. Environ. Pollut. 2013, 178, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, S.H.; Chung, H.; An, J.; Nam, K. Effect of organic substrate and FE oxides transformation on the mobility of arsenic by biotic reductive dissolution under repetitive redox conditions. Chemosphere 2022, 305, 135431. [Google Scholar] [CrossRef]
- Garcia-Manyes, S. Arsenic speciation in contaminated soils. Talanta 2002, 58, 97–109. [Google Scholar] [CrossRef]
- Han, Y.-S.; Park, J.-H.; Kim, S.-J.; Jeong, H.Y.; Ahn, J.S. Redox transformation of soil minerals and arsenic in arsenic-contaminated soil under cycling redox conditions. J. Hazard. Mater. 2019, 378, 120745. [Google Scholar] [CrossRef]
- Thomas, D.J. Arsenic methylation—Lessons from three decades of research. Toxicology 2021, 457, 152800. [Google Scholar] [CrossRef]
- Katsoyiannis, I.; Zouboulis, A. Removal of arsenic from contaminated water sources by sorption onto iron-oxide-coated polymeric materials. Water Res. 2002, 36, 5141–5155. [Google Scholar] [CrossRef]
- Naranmandura, H.; Ibata, K.; Suzuki, K.T. Toxicity of dimethylmonothioarsinic acid toward human epidermoid carcinoma A431 cells. Chem. Res. Toxicol. 2007, 20, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-G.; Kim, Y.-E.; Chae, C.; An, J.; Yoon, H.-O. Dimethylmonothioarsinic acid and dimethyldithioarsinic acid in the environment: Sorption characteristics on 2-line ferrihydrite and acute toxicity to daphnia magna. Environ. Geochem. Health 2021, 44, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Planer-Friedrich, B.; London, J.; McCleskey, R.B.; Nordstrom, D.K.; Wallschläger, D. Thioarsenates in geothermal waters of Yellowstone National Park: Determination, preservation, and geochemical importance. Environ. Sci. Technol. 2007, 41, 5245–5251. [Google Scholar] [CrossRef] [PubMed]
- Hug, K.; Maher, W.A.; Stott, M.B.; Krikowa, F.; Foster, S.; Moreau, J.W. Microbial contributions to coupled arsenic and sulfur cycling in the acid-sulfide hot spring champagne pool, New Zealand. Front. Microbiol. 2014, 5, 569. [Google Scholar] [CrossRef]
- Wang, J.; Kerl, C.F.; Hu, P.; Martin, M.; Mu, T.; Brüggenwirth, L.; Wu, G.; Said-Pullicino, D.; Romani, M.; Wu, L.; et al. Thiolated arsenic species observed in rice paddy pore waters. Nat. Geosci. 2020, 13, 282–287. [Google Scholar] [CrossRef]
- Li, Y.; Low, G.K.-C.; Scott, J.A.; Amal, R. Microbial transformation of arsenic species in municipal landfill leachate. J. Hazard. Mater. 2011, 188, 140–147. [Google Scholar] [CrossRef]
- Zaccone, C.; Lobianco, D.; Raber, G.; D’Orazio, V.; Shotyk, W.; Miano, T.M.; Francesconi, K. Methylated arsenic species throughout a 4-m deep core from a free-floating peat island. Sci. Total Environ. 2018, 621, 67–74. [Google Scholar] [CrossRef]
- Dai, J.; Chen, C.; Gao, A.-X.; Tang, Z.; Kopittke, P.M.; Zhao, F.-J.; Wang, P. Dynamics of dimethylated monothioarsenate (DMMTA) in paddy soils and its accumulation in rice grains. Environ. Sci. Technol. 2021, 55, 8665–8674. [Google Scholar] [CrossRef]
- Colina Blanco, A.E.; Kerl, C.F.; Planer-Friedrich, B. Detection of thioarsenates in rice grains and rice products. J. Agric. Food Chem. 2021, 69, 2287–2294. [Google Scholar] [CrossRef]
- Luvonga, C.; Rimmer, C.A.; Yu, L.L.; Lee, S.B. Organoarsenicals in seafood: Occurrence, dietary exposure, toxicity, and risk assessment considerations—A Review. J. Agric. Food Chem. 2020, 68, 943–960. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Varnoosfaderani, S.; Hebard, A.; Yao, Y.; Inyang, M. Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour. Technol. 2013, 130, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A Review. J. Clean. Product. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.-V.N.; Manan, F.A. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Montaño-Medina, C.U.; Lopéz-Martínez, L.M.; Ochoa-Terán, A.; López-Maldonado, E.A.; Salazar-Gastelum, M.I.; Trujillo-Navarrete, B.; Pérez-Sicairos, S.; Cornejo-Bravoc, J.M. New pyridyl and aniline-functionalized carbamoylcarboxylic acids for removal of metal ions from water by coagulation-flocculation process. Chem. Eng. J. 2023, 451, 138396. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.-G.; Liu, S.-B.; Liu, H.-Y.; Zeng, G.-M.; Tan, X.-F.; Yang, C.-P.; Ding, Y.; Yan, Z.-L.; Cai, X.-X. Sorption performance and mechanisms of arsenic(v) removal by magnetic gelatin-modified biochar. Chem. Eng. J. 2017, 314, 223–231. [Google Scholar] [CrossRef]
- Chen, W.-H.; Hoang, A.T.; Nižetić, S.; Pandey, A.; Cheng, C.K.; Luque, R.; Ong, H.C.; Thomas, S.; Nguyen, X.P. Biomass-derived biochar: From production to application in removing heavy metal-contaminated water. Process Saf. Environ. Protect. 2022, 160, 704–733. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Senthil Kumar, P.; Varjani, S.J.; Saravanan, A. Advances in production and application of biochar from lignocellulosic feedstocks for remediation of environmental pollutants. Bioresour. Technol. 2019, 292, 122030. [Google Scholar] [CrossRef]
- López-Maldonadoa, E.A.; Hernández-García, H.; Zamudio-Aguilar, M.A.M.; Oropeza-Guzmánc, M.T.; Ochoa-Teránc, A.; López-Martínezd, L.M.; Martinez-Quiroze, M.; Valdez, R.; Olivas, A. Chemical issues of coffee and Tule lignins as ecofriendly materials for the effective removal of hazardous metal ions contained in metal finishing wastewater. Chem. Eng. J. 2020, 397, 125384. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of arsenic by magnetic biochar prepared from Pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Xu, Z.; Gao, B.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. Mesoporous ball-milling iron-loaded biochar for enhanced sorption of Reactive Red: Performance and mechanisms. Environ. Pollut. 2021, 290, 117992. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xu, X.; Ni, Q.; Lin, Q.; Fang, J.; Chen, Q.; Shen, X.; Lou, L. Enhanced As (v) removal from aqueous solution by biochar prepared from iron-impregnated Corn Straw. J. Chem. 2018, 2018, 5137694. [Google Scholar] [CrossRef]
- Lin, L.; Qiu, W.; Wang, D.; Huang, Q.; Song, Z.; Chau, H.W. Arsenic removal in aqueous solution by a novel Fe-Mn modified biochar composite: Characterization and mechanism. Ecotoxicol. Environ. Saf. 2017, 144, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Liu, Z.; Shi, Z. Arsenic removal from aqueous solutions by adsorption onto iron oxide/activated carbon magnetic composite. J. Environ. Health Sci. Eng. 2014, 12, 58. [Google Scholar] [CrossRef]
- Samsuri, A.W.; Sadegh-Zadeh, F.; Seh-Bardan, B.J. Adsorption of As(iii) and As(v) by Fe coated biochars and biochars produced from empty fruit bunch and rice husk. J. Environ. Chem. Eng. 2013, 1, 981–988. [Google Scholar] [CrossRef]
- Tuna, A.Ö.; Özdemir, E.; Şimşek, E.B.; Beker, U. Removal of As(v) from aqueous solution by activated carbon-based hybrid adsorbents: Impact of experimental conditions. Chem. Eng. J. 2013, 223, 116–128. [Google Scholar] [CrossRef]
- Wu, C.; Huang, L.; Xue, S.-G.; Huang, Y.-Y.; Hartley, W.; Cui, M.-Q.; Wong, M.-H. Arsenic sorption by red mud-modified biochar produced from rice straw. Environ. Sci. Pollut. Res. 2017, 24, 18168–18178. [Google Scholar] [CrossRef]
- Pedersen, H.D.; Postma, D.; Jakobsen, R. Release of arsenic associated with the reduction and transformation of iron oxides. Geochim. Cosmochim. Acta 2006, 70, 4116–4129. [Google Scholar] [CrossRef]
- Jeong, Y.; Fan, M.; Singh, S.; Chuang, C.-L.; Saha, B.; Hans van Leeuwen, J. Evaluation of iron oxide and aluminum oxide as potential arsenic(v) adsorbents. Chem. Eng. Process. Process Intensif. 2007, 46, 1030–1039. [Google Scholar] [CrossRef]
- Mamindy-Pajany, Y.; Hurel, C.; Marmier, N.; Roméo, M. Arsenic adsorption onto hematite and goethite. Comptes Rendus Chim. 2009, 12, 876–881. [Google Scholar] [CrossRef]
- Song, K.; Kim, W.; Suh, C.-Y.; Shin, D.; Ko, K.-S.; Ha, K. Magnetic iron oxide nanoparticles prepared by electrical wire explosion for arsenic removal. Powder Technol. 2013, 246, 572–574. [Google Scholar] [CrossRef]
- Luther, S.; Borgfeld, N.; Kim, J.; Parsons, J.G. Removal of arsenic from aqueous solution: A study of the effects of ph and interfering ions using iron oxide nanomaterials. Microchem. J. 2012, 101, 30–36. [Google Scholar] [CrossRef]
- Jeong, S.; Yang, K.; Jho, E.H.; Nam, K. Importance of chemical binding type between As and iron-oxide on bioaccessibility in soil: Test with synthesized two line ferrihydrite. J. Hazard. Mater. 2017, 330, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Lin, J.; Owens, G.; Chen, Z. Impact of green synthesized iron oxide nanoparticles on the distribution and transformation of as species in contaminated soil. Environ. Poll. 2020, 258, 113668. [Google Scholar] [CrossRef]
- Tuutijärvi, T.; Lu, J.; Sillanpää, M.; Chen, G. As(v) adsorption on maghemite nanoparticles. J. Hazard. Mater. 2009, 166, 1415–1420. [Google Scholar] [CrossRef]
- Kim, J.; Song, J.; Lee, S.-M.; Jung, J. Application of iron-modified biochar for arsenite removal and toxicity reduction. J. Ind. Eng. Chem. 2019, 80, 17–22. [Google Scholar] [CrossRef]
- Li, X.; Qin, Y.; Jia, Y.; Li, Y.; Zhao, Y.; Pan, Y.; Sun, J. Preparation and application of Fe/biochar (Fe-BC) catalysts in wastewater treatment: A review. Chemosphere 2021, 274, 129766. [Google Scholar] [CrossRef]
- Liu, H.; Shan, J.; Chen, Z.; Lichtfouse, E. Efficient recovery of phosphate from simulated urine by Mg/Fe bimetallic oxide modified biochar as a potential resource. Sci. Total Environ. 2021, 784, 147546. [Google Scholar] [CrossRef]
- Yi, Y.; Tu, G.; Zhao, D.; Tsang, P.E.; Fang, Z. Biomass waste components significantly influence the removal of Cr(VI) using magnetic biochar derived from four types of feedstocks and steel pickling waste liquor. Chem. Eng. J. 2019, 360, 212–220. [Google Scholar] [CrossRef]
- Reguyal, F.; Sarmah, A.K.; Gao, W. Synthesis of magnetic biochar from pine sawdust via oxidative hydrolysis of FeCl2 for the removal sulfamethoxazole from aqueous solution. J. Hazard. Mater. 2017, 321, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kim, G.; Choe, J.K.; Choi, Y. Effect of using powdered biochar and surfactant on desorption and biodegradability of phenanthrene sorbed to biochar. J. Hazard. Mater. 2019, 371, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory Preparation and Characterization; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- An, J.; Jeong, B.; Nam, K. Evaluation of the effectiveness of in situ stabilization in the field aged arsenic-contaminated soil: Chemical extractability and biological response. J. Hazard. Mater. 2019, 367, 137–143. [Google Scholar] [CrossRef]
- Park, J.; Chung, H.; Kim, S.H.; An, J.; Nam, K. Effect of neutralizing agents on the type of as co-precipitates formed by in situ Fe oxides synthesis and its impact on the bioaccessibility of as in Soil. Sci. Total. Environ. 2020, 743, 140686. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, Y.; Gao, B.; Cao, X. N-doped biochar synthesized by a facile ball-milling method for enhanced sorption of CO2 and reactive red. Chem. Eng. J. 2019, 368, 564–572. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface Chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- He, R.; Peng, Z.; Lyu, H.; Huang, H.; Nan, Q.; Tang, J. Synthesis and characterization of an iron-impregnated biochar for aqueous arsenic removal. Sci. Total. Environ. 2018, 612, 1177–1186. [Google Scholar] [CrossRef]
- Pham, T.H.; Chu, T.T.H.; Nguyen, D.K.; Le, T.K.O.; Obaid, S.A.; Alharbi, S.A.; Kim, J.; Nguyen, M.V. Alginate-modified biochar derived from rice husk waste for improvement uptake performance of lead in wastewater. Chemosphere 2022, 307, 135956. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, N.; Giri, B.S.; Chowdhary, P.; Chaturvedi, P. Removal of methylene blue dye using rice husk, cow dung and sludge biochar: Characterization, application, and kinetic studies. Bioresour. Technol. 2020, 306, 123202. [Google Scholar] [CrossRef] [PubMed]
- Cismasu, A.C.; Michel, F.M.; Tcaciuc, A.P.; Tyliszczak, T.; Brown, G.E., Jr. Composition and structural aspects of naturally occurring ferrihydrite. C. R. Geosci. 2011, 343, 210–218. [Google Scholar] [CrossRef]
- López-Maldonadoa, E.A.; Oropeza-Guzmán, M.T. Nejayote biopolyelectrolytes multifunctionality (glucurono ferulauted arabinoxylans) in the separation of hazardous metal ions from industrial wastewater. Chem. Eng. J. 2021, 423, 130210. [Google Scholar] [CrossRef]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.-R.; Li, J.-R.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H.-C. Potential applications of metal-organic frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Rivadeneira-Mendoza, B.F.; Filho, O.A.E.; Fernández-Andrade, K.J.; Curbelo, F.; Silva, F.F.D.; Luque, R.; Rodríguez-Díazhi, J.M. MOF@biomass hybrids: Trends on advanced functional materials for adsorption. Environ. Res. 2023, 216, 114424. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef]
- Rosales, E.; Meijide, J.; Pazos, M.; Sanromán, M.A. Challenges and recent advances in biochar as low-cost biosorbent: From batch assays to continuous-flow systems. Bioresour. Technol. 2017, 246, 176–192. [Google Scholar] [CrossRef]
- Jeong, S.; Kang, J.; Cho, M.; An, J.; Yoon, H.-O. New insights into surface behavior of dimethylated arsenicals on montmorillonite using X-ray absorption spectroscopy. Sci. Total Environ. 2022, 852, 158531. [Google Scholar] [CrossRef]
- Das, S.; Hendry, M.J.; Essilfie-Dughan, J. Transformation of two-line ferrihydrite to goethite and hematite as a function of pH and temperature. Environ. Sci. Technol. 2010, 45, 268–275. [Google Scholar] [CrossRef]
- Smith, P.G.; Koch, I.; Gordon, R.A.; Mandoli, D.F.; Chapman, B.D.; Reimer, K.J. X-ray absorption near-edge structure analysis of arsenic species for application to biological environmental samples. Environ. Sci. Technol. 2004, 39, 248–254. [Google Scholar] [CrossRef]
- Jing, C.; Meng, X.; Liu, S.; Baidas, S.; Patraju, R.; Christodoulatos, C.; Korfiatis, G.P. Surface complexation of organic arsenic on nanocrystalline titanium oxide. J. Colloid Interface Sci. 2005, 290, 14–21. [Google Scholar] [CrossRef]
- Maher, W.; Foster, S.; Krikowa, F.; Donner, E.; Lombi, E. Measurement of inorganic arsenic species in rice after nitric acid extraction by HPLC-ICPMS: Verification using Xanes. Environ. Sci. Technol. 2013, 47, 5821–5827. [Google Scholar] [CrossRef] [PubMed]
- Suess, E.; Scheinost, A.C.; Bostick, B.C.; Merkel, B.J.; Wallschlaeger, D.; Planer-Friedrich, B. Discrimination of thioarsenites and thioarsenates by X-ray absorption spectroscopy. Anal. Chem. 2009, 81, 8318–8326. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-T.; Lee, H.; Yoon, H.-O.; Woo, N.C. Kinetics of dimethylated thioarsenicals and the formation of highly toxic dimethylmonothioarsinic acid in environment. Environ. Sci. Technol. 2016, 50, 11637–11645. [Google Scholar] [CrossRef] [PubMed]
- Kerl, C.F.; Schindele, R.A.; Brüggenwirth, L.; Colina Blanco, A.E.; Rafferty, C.; Clemens, S.; Planer-Friedrich, B. Methylated thioarsenates and monothioarsenate differ in uptake, transformation, and contribution to total arsenic translocation in rice plants. Environ. Sci. Technol. 2019, 53, 5787–5796. [Google Scholar] [CrossRef]
- Naranmandura, H.; Carew, M.W.; Xu, S.; Lee, J.; Leslie, E.M.; Weinfeld, M.; Le, X.C. Comparative toxicity of arsenic metabolites in human bladder cancer EJ-1 cells. Chem. Res. Toxicol. 2011, 24, 1586–1596. [Google Scholar] [CrossRef]



| Sample | Surface Fe Distribution (%) Determined Using EDS Elemental Mapping | Fe Concentration (mg/kg) Determined Using Aqua Regia Digestion and AAS Analysis |
|---|---|---|
| BC | 0.2 | 527 |
| FeBC | 17.6 | 47,155 |
| Biomass Used | Contaminant | Maximum Adsorption Capacity (mg/g) | Reference | |
|---|---|---|---|---|
| Biochar | Fe/Biochar Composite | |||
| Hickory chips | Anionic dye (reactive red 120) | 2.90 | 32.0 | Feng et al. [33] |
| 2.39 | 79.4 | |||
| Corn stem | As(III) | 2.89 | 8.25 (Fe-Mn modified biochar) | Lin et al. [35] |
| Pine wood | As(V) | 0.265 | 0.429 | Wang et al. [32] |
| Corn straw | As(V) | 2.86 | 14.77 | Fan et al. [34] |
| Activated carbon | As(V) | 17.86 | 20.24 | Yao et al. [36] |
| Empty fruit bunch | As(V) | 5.5 | 15.2 | Samsuri et al. [37] |
| As(III) | 18.9 | 31.4 | ||
| Rice husk | As(V) | 19.3 | 30.7 | |
| As(III) | 7.1 | 16.9 | ||
| Activated carbon | As(V) | 0.00 | 4.663 | Tuna et al. [38] |
| Rice straw | As(V) | 0.552 | 5.923 | Wu et al. [39] |
| As Species | Absorption Energy (eV) | Reference |
|---|---|---|
| As(V) | 11,875.3 | Smith et al. [71] |
| As(V) | 11,873.5 | Jing et al. [72] |
| DMA(V) | 11,873 | |
| As(V) | 11,875 | Maher et al. [73] |
| DMA(V) | 11,873 | |
| Monothioarsenate | 11,871.3 | Suess et al. [74] |
| Dithioarsenate | 11,870.3 | |
| Tetrathioarsenate | 11,869.8 | |
| As(V) | 11,873 | Jeong et al. [69] |
| DMA(V) | 11,872 | |
| DMMTA(V) | 11,871 | |
| DMDTA(V) | 11,870 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.-G.; Kwak, I.-S.; Yoon, H.-O.; An, J. Adsorption Characteristics of Dimethylated Arsenicals on Iron Oxide–Modified Rice Husk Biochar. Toxics 2022, 10, 703. https://doi.org/10.3390/toxics10110703
Yoon S-G, Kwak I-S, Yoon H-O, An J. Adsorption Characteristics of Dimethylated Arsenicals on Iron Oxide–Modified Rice Husk Biochar. Toxics. 2022; 10(11):703. https://doi.org/10.3390/toxics10110703
Chicago/Turabian StyleYoon, Sang-Gyu, Ihn-Sil Kwak, Hye-On Yoon, and Jinsung An. 2022. "Adsorption Characteristics of Dimethylated Arsenicals on Iron Oxide–Modified Rice Husk Biochar" Toxics 10, no. 11: 703. https://doi.org/10.3390/toxics10110703
APA StyleYoon, S.-G., Kwak, I.-S., Yoon, H.-O., & An, J. (2022). Adsorption Characteristics of Dimethylated Arsenicals on Iron Oxide–Modified Rice Husk Biochar. Toxics, 10(11), 703. https://doi.org/10.3390/toxics10110703






