A Human Biomonitoring Study Assessing Glyphosate and Aminomethylphosphonic Acid (AMPA) Exposures among Farm and Non-Farm Families
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sampling Protocols
2.2. Urine Sample Collection
2.3. Urine Sample Chemical Analysis
2.4. Statistical and Data Analysis
3. Results
3.1. Descriptive and Summary Statistics
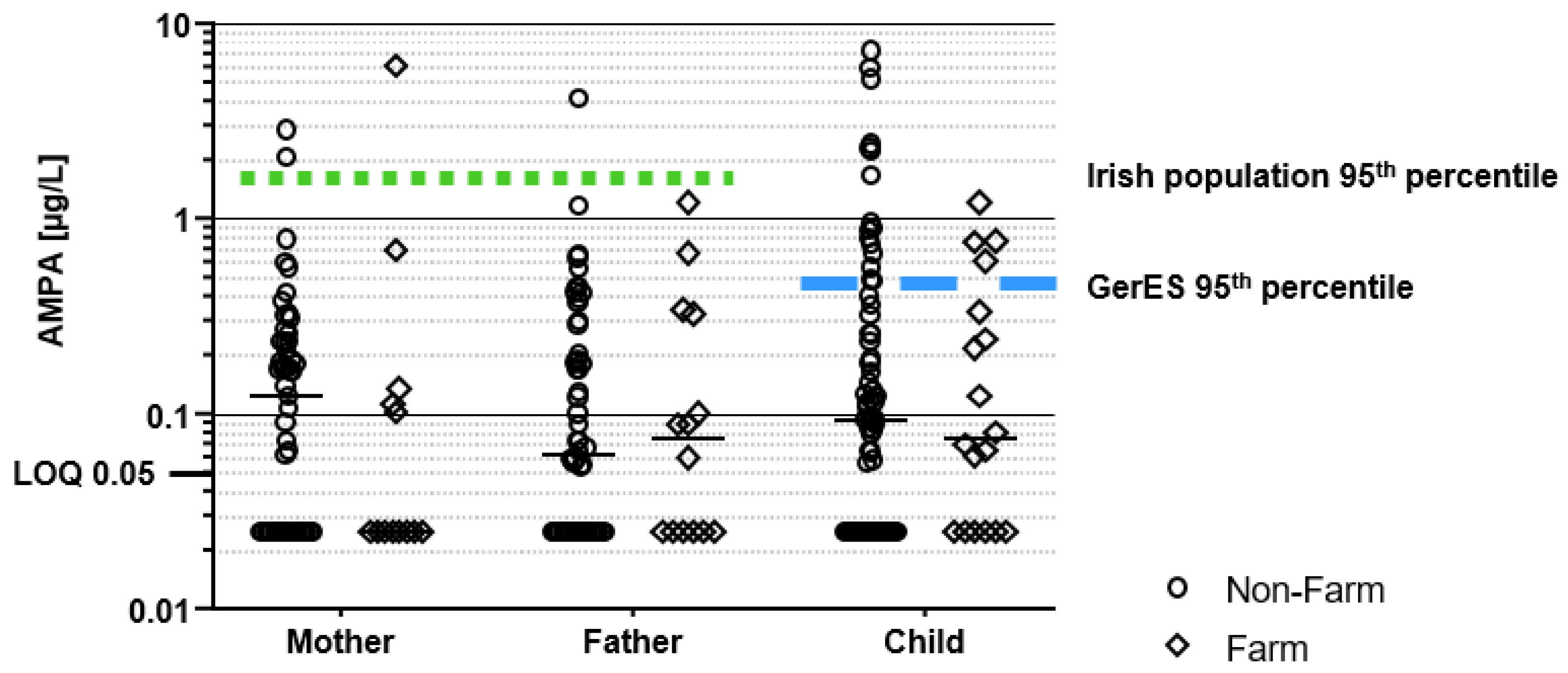
3.2. Glyphosate and AMPA Urinary Concentrations
3.3. Risk Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- EFSA. Conclusion on the Peer Review of the Pesticide Risk Assessment of the Active Substance Glyphosate; European Food Safety Authority: Parma, Italy, 2015; Available online: https://www.efsa.europa.eu/en/efsajournal/pub/4302 (accessed on 30 September 2016).
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [PubMed]
- Guyton, K.Z.; Loomis, D.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Scoccianti, C.; Mattock, H.; Straif, K. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet 2015, 16, 490–491. [Google Scholar] [CrossRef]
- Antier, C.; Kudsk, P.; Reboud, X.; Ulber, L.; Baret, P.; Messéan, A. Glyphosate Use in the European Agricultural Sector and a Framework for Its Further Monitoring. Sustainability 2020, 12, 5682. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans—Glyphosate; International Agency for Research on Cancer: Lyon, France, 2016; Available online: http://monographs.iarc.fr/ENG/Monographs/vol112/mono112-10.pdf (accessed on 27 June 2016).
- ECHA. Glyphosate Not Classified as a Carcinogen by ECHA—All News; ECHA Finland, European Chemicals Agency: Helsinki, Finland, 2017; Available online: https://echa.europa.eu/-/glyphosate-not-classified-as-a-carcinogen-by-echa (accessed on 10 October 2017).
- EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. Eur. Food Saf. Auth. (EFSA) J. 2015, 13, 4302. Available online: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2015.4302/pdf (accessed on 10 February 2018).
- ECHA. Explanatory Note—On an opinion Proposing Harmonised Classification and Labelling at EU Level of Glyphosate (ISO); N-(phosphonomethyl)glycine; European Chemicals Agency: Helsinki, Finland, 2022; Available online: https://echa.europa.eu/registry-of-clh-intentions-until-outcome/-/dislist/details/0b0236e185e41a77 (accessed on 1 July 2022).
- EFSA. Glyphosate: EFSA and ECHA Update Timelines for Assessments; European Food Safety Authority: Parma, Italy, 2022; Available online: https://www.efsa.europa.eu/en/news/glyphosate-efsa-and-echa-update-timelines-assessments (accessed on 2 August 2022).
- Zhang, L.; Rana, I.; Shaffer, R.M.; Taioli, E.; Sheppard, L. Exposure to glyphosate-based herbicides and risk for non-Hodgkin lymphoma: A meta-analysis and supporting evidence. Mutat. Res. Mutat. Res. 2019, 781, 186–206. [Google Scholar] [CrossRef]
- Jazmin, S.M.; Dheni, T.S.; Heriberto, T.J.; Joel, S.F. Glyphosate Toxicity, Oxidative Stress, Carcinogenicity and Reproductive Effects:A Review. Int. J. Recent Sci. Res. 2019, 10, 32865–32869. [Google Scholar] [CrossRef]
- Andreotti, G.; Koutros, S.; Hofmann, J.N.; Sandler, D.P.; Lubin, J.H.; Lynch, C.F.; Lerro, C.C.; De Roos, A.J.; Parks, C.G.; Alavanja, M.C.; et al. Glyphosate Use and Cancer Incidence in the Agricultural Health Study. J. Natl. Cancer Inst. 2018, 110, 509–516. [Google Scholar] [CrossRef]
- Piccoli, C.; Cremonese, C.; Koifman, R.J.; Koifman, S.; Freire, C. Pesticide exposure and thyroid function in an agricultural population in Brazil. Environ. Res. 2016, 151, 389–398. [Google Scholar] [CrossRef]
- Jayasumana, C.; Gunatilake, S.; Siribaddana, S. Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol. 2015, 16, 103. [Google Scholar] [CrossRef]
- Lebov, J.F.; Engel, L.S.; Richardson, D.; Hogan, S.L.; Hoppin, J.A.; Sandler, D.P. Pesticide use and risk of end-stage renal disease among licensed pesticide applicators in the Agricultural Health Study. Occup. Environ. Med. 2015, 73, 3–12. [Google Scholar] [CrossRef]
- Slager, R.E.; Simpson, S.L.; LeVan, T.D.; Poole, J.A.; Sandler, D.P.; Hoppin, J.A. Rhinitis Associated with Pesticide Use Among Private Pesticide Applicators in the Agricultural Health Study. J. Toxicol. Environ. Health Part A 2010, 73, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Alegakis, A.; Tzanakis, N.; Siafakas, N.; Kogevinas, M.; Lionis, C. Association of allergic rhinitis with pesticide use among grape farmers in Crete, Greece. Occup. Environ. Med. 2007, 64, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Gerona, R.R.; Proctor, C.; Friesen, M.; Ashby, J.L.; Reiter, J.L.; Lui, Z.; Winchester, P.D. Glyphosate exposure in pregnancy and shortened gestational length: A prospective Indiana birth cohort study. Environ. Health 2018, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, T.E.; Lin, Z.; Mery, L.S. An exploratory analysis of the effect of pesticide exposure on the risk of spontaneous abortion in an Ontario farm population. Environ. Health Perspect. 2001, 109, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Connolly, A.; Coggins, M.A.; Koch, H.M. Human Biomonitoring of Glyphosate Exposures: State-of-the-Art and Future Research Challenges. Toxics 2020, 8, 60. [Google Scholar] [CrossRef]
- Gillezeau, C.; van Gerwen, M.; Shaffer, R.M.; Rana, I.; Zhang, L.; Sheppard, L.; Taioli, E. The evidence of human exposure to glyphosate: A review. Environ. Health 2019, 18, 2. [Google Scholar] [CrossRef]
- Heinemeyer, G.; Connolly, A.; von Goetz, N.; Bessems, J.; de Bruin, Y.B.; Coggins, M.A.; Fantke, P.; Galea, K.S.; Gerding, J.; Hader, J.D.; et al. Towards further harmonization of a glossary for exposure science—An ISES Europe statement. J. Expo. Sci. Environ. Epidemiol. 2021, 32, 526–529. [Google Scholar] [CrossRef]
- HBM4EU. Human Biomonitoring for Europe 2020. Available online: https://www.hbm4eu.eu/about-hbm4eu/ (accessed on 24 August 2022).
- Sexton, K.; Needham, L.L.; Pirkle, J.L. Human Biomonitoring of Environmental Chemicals. Am. Sci. 2004, 92, 38–45. Available online: https://www.cdc.gov/biomonitoring/pdf/AS_article_biomonitoring.pdf (accessed on 23 October 2018). [CrossRef]
- Angerer, J.; Ewers, U.; Wilhelm, M. Human biomonitoring: State of the art. Int. J. Hyg. Environ. Health 2007, 210, 201–228. Available online: http://www.sciencedirect.com/science/article/pii/S1438463907000338 (accessed on 5 February 2022). [CrossRef]
- Bahadori, T.; Phillips, R.D.; Money, C.D.; Quackenboss, J.J.; Clewell, H.J.; Bus, J.S.; Robison, S.H.; Humphris, C.J.; Parekh, A.A.; Osborn, K.; et al. Making sense of human biomonitoring data: Findings and recommendations of a workshop. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 308–313. [Google Scholar] [CrossRef][Green Version]
- HBM4EU. HBM4EU Priority Substances. 2020. Available online: https://www.hbm4eu.eu/the-substances/ (accessed on 24 August 2022).
- Ougier, E.; Ganzleben, C.; Lecoq, P.; Bessems, J.; David, M.; Schoeters, G.; Lange, R.; Meslin, M.; Uhl, M.; Kolossa-Gehring, M.; et al. Chemical prioritisation strategy in the European Human Biomonitoring Initiative (HBM4EU)—Development and results. Int. J. Hyg. Environ. Health 2021, 236, 113778. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.A.; Saravanabhavan, G.; Werry, K.; Khoury, C. An overview of human biomonitoring of environmental chemicals in the Canadian Health Measures Survey: 2007–2019. Int. J. Hyg. Environ. Health 2017, 220, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Conrad, A.; Schröter-Kermani, C.; Hoppe, H.-W.; Rüther, M.; Pieper, S.; Kolossa-Gehring, M. Glyphosate in German adults—Time trend (2001 to 2015) of human exposure to a widely used herbicide. Int. J. Hyg. Environ. Health 2017, 220, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Lemke, N.; Murawski, A.; Schmied-Tobies, M.I.; Rucic, E.; Hoppe, H.-W.; Conrad, A.; Kolossa-Gehring, M. Glyphosate and aminomethylphosphonic acid (AMPA) in urine of children and adolescents in Germany—Human biomonitoring results of the German Environmental Survey 2014–2017 (GerES V). Environ. Int. 2021, 156, 106769. [Google Scholar] [CrossRef] [PubMed]
- NHANES. Environmental and Related Chemicals Measured in Blood, Serum or Urine in NHANES; NCEH, Ed.; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2018.
- Mesnage, R.; Moesch, C.; Grand, R.l.; Lauthier, G.; de Vendômois, J.S.; Gress, S.; Séralini, G.-E. Glyphosate Exposure in a Farmer’s Family. J. Environ. Prot. 2012, 3, 1001–1003. Available online: http://www.scirp.org/journal/PaperInformation.aspx?PaperID=22645 (accessed on 10 February 2018). [CrossRef]
- Connolly, A.; Basinas, I.; Jones, K.; Galea, K.S.; Kenny, L.; McGowan, P.; Coggins, M.A. Characterising glyphosate exposures among amenity horticulturists using multiple spot urine samples. Int. J. Hyg. Environ. Health 2018, 221, 1012–1022. [Google Scholar] [CrossRef]
- Connolly, A.; Jones, K.; Galea, K.S.; Basinas, I.; Kenny, L.; McGowan, P.; Coggins, M. Exposure assessment using human biomonitoring for glyphosate and fluroxypyr users in amenity horticulture. Int. J. Hyg. Environ. Health 2017, 220, 1064–1073. [Google Scholar] [CrossRef]
- Perry, M.J.; Mandrioli, D.; Belpoggi, F.; Manservisi, F.; Panzacchi, S.; Irwin, C. Historical evidence of glyphosate exposure from a US agricultural cohort. Environ. Health 2019, 18, 42. [Google Scholar] [CrossRef]
- Soukup, S.T.; Merz, B.; Bub, A.; Hoffmann, I.; Watzl, B.; Steinberg, P.; Kulling, S.E. Glyphosate and AMPA levels in human urine samples and their correlation with food consumption: Results of the cross-sectional KarMeN study in Germany. Arch. Toxicol. 2020, 94, 1575–1584. [Google Scholar] [CrossRef]
- Connolly, A.; Leahy, M.; Jones, K.; Kenny, L.; Coggins, M.A. Glyphosate in Irish adults—A pilot study in 2017. Environ. Res. 2018, 165, 235–236. [Google Scholar] [CrossRef]
- Mills, P.J.; Kania-Korwel, I.; Fagan, J.; McEvoy, L.K.; Laughlin, G.A.; Barrett-Connor, E. Excretion of the Herbicide Glyphosate in Older Adults Between 1993 and 2016. JAMA 2017, 318, 1610–1611. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.K.; McGuire, M.A.; Price, W.J.; Shafii, B.; Carrothers, J.M.; Lackey, K.A.; Goldstein, D.A.A.; Jensen, P.K.; Vicini, J.L. Glyphosate and aminomethylphosphonic acid are not detectable in human milk. Am. J. Clin. Nutr. 2016, 103, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Varona, M.; Henao, G.L.; Díaz, S.; Lancheros, A.; Murcia, A.; Rodríguez, N.; Alvarez, V.H. Effects of aerial applications of the herbicide glyphosate and insecticides on human health. Biomedica 2009, 29, 456–475. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, H.W. Determination of Glyphosate Residues in Human Urine Samples from 18 European Countries; Medical Laboratory: Bremen, Germany, 2013. [Google Scholar]
- Acquavella, J.F.; Alexander, B.H.; Mandel, J.S.; Gustin, C.; Baker, B.; Chapman, P.; Bleeke, M. Glyphosate biomonitoring for farmers and their families: Results from the Farm Family Exposure Study. Environ. Health Perspect. 2004, 112, 321–326. [Google Scholar] [CrossRef]
- Knudsen, L.E.; Hansen, P.W.; Mizrak, S.; Hansen, H.K.; Mørck, T.A.; Nielsen, F.; Siersma, V.; Mathiesen, L. Biomonitoring of Danish school children and mothers including biomarkers of PBDE and glyphosate. Rev. Environ. Health 2017, 32, 279–290. [Google Scholar] [CrossRef]
- Curwin, B.D.; Hein, M.J.; Sanderson, W.T.; Striley, C.; Heederik, D.; Kromhout, H.; Reynolds, S.J.; Alavanja, M.C. Urinary Pesticide Concentrations Among Children, Mothers and Fathers Living in Farm and Non-Farm Households in Iowa. Ann. Occup. Hyg. 2007, 51, 53–65. [Google Scholar] [CrossRef]
- Lozano-Kasten, F.; Sierra-Diaz, E.; Chavez, H.G.; Lucano, A.A.P.; Cremades, R.; Pinto, E.S. Seasonal Urinary Levels of Glyphosate in Children From Agricultural Communities. Dose-Response 2021, 19, 15593258211053184. [Google Scholar] [CrossRef]
- Fiddicke, U.; Pack, L.K.; Tolonen, H.; Sepai, O.; López, M.E.; Castaño, A.; Schoeters, G.; Kolossa-Gehring, M. A Phased Approach for preparation and organization of human biomonitoring studies. Int. J. Hyg. Environ. Health 2020, 232, 113684. [Google Scholar] [CrossRef]
- González-Alzaga, B.; Hernández, A.F.; Pack, L.K.; Iavicoli, I.; Tolonen, H.; Santonen, T.; Vinceti, M.; Filippini, T.; Moshammer, H.; Hensch, N.P.; et al. The questionnaire design process in the European Human Biomonitoring Initiative (HBM4EU). Environ. Int. 2022, 160, 107071. [Google Scholar] [CrossRef]
- HBM4EU. Online Library: Human Biomonitoring for Europe; Guidelines, Protocols and Questionnaires: Harmonised Questionnaires/Substance-Specific Basic Questionnaire (2nd Found Priority Substances: Acrylamide, Mycotoxins, Pesticides and Mercury). 2022. Available online: https://www.hbm4eu.eu/online-library/ (accessed on 24 August 2022).
- Connolly, A.; Koslitz, S.; Bury, D.; Brüning, T.; Conrad, A.; Kolossa-Gehring, M.; Coggins, M.A.; Koch, H.M. Sensitive and selective quantification of glyphosate and aminomethylphosphonic acid (AMPA) in urine of the general population by gas chromatography-tandem mass spectrometry. J. Chromatogr. B 2020, 1158, 122348. [Google Scholar] [CrossRef]
- Boeniger, M.F.; Lowry, L.K.; Rosenberg, J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: A review. AIHAJ 1993, 54, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Cocker, J.; Mason, H.J.; Warren, N.D.; Cotton, R.J. Creatinine adjustment of biological monitoring results. Occup. Med. 2011, 61, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary Creatinine Concentrations in the U.S. Population: Implications for Urinary Biologic Monitoring Measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Niemann, L.; Sieke, C.; Pfeil, R.; Solecki, R. A critical review of glyphosate findings in human urine samples and comparison with the exposure of operators and consumers. J. Verbr. Lebensm. 2015, 10, 3–12. [Google Scholar] [CrossRef]
- Agostoni, C.; Bresson, J.L.; Fairweather-Tait, S. EFSA Panel on Dietetic Products, Nutrition, Allergies, Scientific Opinion on Dietary Reference Values for water. EFSA J. 2010, 8, 1459. [Google Scholar] [CrossRef]
- Zoller, O.; Rhyn, P.; Zarn, J.A.; Dudler, V. Urine glyphosate level as a quantitative biomarker of oral exposure. Int. J. Hyg. Environ. Health 2020, 228, 113526. Available online: http://www.sciencedirect.com/science/article/pii/S1438463919309678 (accessed on 24 March 2022). [CrossRef]
- Weber, T.; Vogel, N.; Conolly, A.; Hoppe, H.-W.; Leahy, M.; Coggins, M.A.; Kolossa-Gehring, M. Time Trend of Glyphosate and AMPA Exposure in Germany between 2001 and 2022: Analyses of 24-h Urine Samples of the German Environmental Specimen Bank and a Comparision to Irish Adults. 2022; manuscript in preparation. [Google Scholar]
- Connolly, A.; Jones, K.; Basinas, I.; Galea, K.S.; Kenny, L.; McGowan, P.; Coggins, M.A. Exploring the half-life of glyphosate in human urine samples. Int. J. Hyg. Environ. Health 2019, 222, 205–210. [Google Scholar] [CrossRef]
- Connolly, A.; Coggins, M.A.; Galea, K.S.; Jones, K.; Kenny, L.; McGowan, P.; Basinas, I. Evaluating Glyphosate Exposure Routes and Their Contribution to Total Body Burden: A Study Among Amenity Horticulturalists. Ann. Work Expo. Health 2019, 63, 133–147. [Google Scholar] [CrossRef]
- Curwin, B.D. Bringing Work Home: Take-Home Pesticide Exposure Among Farm Families; Utrecht University: Utrecht, The Netherlands, 2006. [Google Scholar]
- Curwin, B.D.; Hein, M.J.; Sanderson, W.T.; Nishioka, M.G.; Reynolds, S.J.; Ward, E.M.; Alavanja, M.C. Pesticide Contamination Inside Farm and Nonfarm Homes. J. Occup. Environ. Hyg. 2005, 2, 357–367. [Google Scholar] [CrossRef]
- López-Gálvez, N.; Wagoner, R.; Quirós-Alcalá, L.; Van Horne, Y.O.; Furlong, M.; Avila, E.; Beamer, P. Systematic Literature Review of the Take-Home Route of Pesticide Exposure via Biomonitoring and Environmental Monitoring. Int. J. Environ. Res. Public Health 2019, 16, 2177. [Google Scholar] [CrossRef]
- Buekers, J.; Remy, S.; Bessems, J.; Govarts, E.; Rambaud, L.; Riou, M.; Tratnik, J.S.; Stajnko, A.; Katsonouri, A.; Makris, K.C.; et al. Glyphosate and AMPA in Human Urine of HBM4EU Aligned Studies: Part A Children. Toxics 2022, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Buekers, J.; Remy, S.; Bessems, J.; Govarts, E.; Rambaud, L.; Riou, M.; Halldorsson, T.I.; Ólafsdóttir, K.; Probst-Hensch, N.; Ammann, P.; et al. Glyphosate and AMPA in Human Urine of HBM4EU-Aligned Studies: Part B Adults. Toxics 2022, 10, 552. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Quraishi, S.M.; Graubard, B.I.; Weber, J.-P.; Rubertone, M.V.; Erickson, R.L. Persistent Organochlorine Pesticides and Risk of Testicular Germ Cell Tumors. JNCI J. Natl. Cancer Inst. 2008, 100, 663–671. [Google Scholar] [CrossRef] [PubMed]
- European Commission. A European Green Deal. 2022. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 24 August 2022).
- European Commission. Farm to Fork Strategy. 2022. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 24 August 2022).
- JMPR. Pesticide Residues in Food 2019—Evaluations 2019 Part I—Residues EXTRA Joint FAO/WHO Meeting; FAO and WHO Joint Meeting on Pesticide Residues (JMPR): Ottawa, ON, Canada, 2019; Available online: http://www.fao.org/publications/card/en/c/CA6010EN/ (accessed on 26 June 2020).
- Grandcoin, A.; Piel, S.; Baurès, E. AminoMethylPhosphonic acid (AMPA) in natural waters: Its sources, behavior and environmental fate. Water Res. 2017, 117, 187–197. Available online: http://www.sciencedirect.com/science/article/pii/S0043135417302452 (accessed on 27 April 2020). [CrossRef]
- Nowack, B. Environmental chemistry of phosphonates. Water Res. 2003, 37, 2533–2546. Available online: https://www.sciencedirect.com/science/article/pii/S0043135403000794 (accessed on 22 July 2022). [CrossRef]
- Bento, C.P.M.; Yang, X.; Gort, G.; Xue, S.; van Dam, R.; Zomer, P.; Mol, H.G.J.; Ritsema, C.J.; Geissen, V. Persistence of glyphosate and aminomethylphosphonic acid in loess soil under different combinations of temperature, soil moisture and light/darkness. Sci. Total Environ. 2016, 572, 301–311. [Google Scholar] [CrossRef]
- De Troeyer, K.; Casas, L.; Bijnens, E.M.; Bruckers, L.; Covaci, A.; De Henauw, S.; Hond, E.D.; Loots, I.; Nelen, V.; Verheyen, V.J.; et al. Higher proportion of agricultural land use around the residence is associated with higher urinary concentrations of AMPA, a glyphosate metabolite. Int. J. Hyg. Environ. Health 2022, 246, 114039. [Google Scholar] [CrossRef]
- Aylward, L.L.; Hays, S.M.; Zidek, A. Variation in urinary spot sample, 24 h samples, and longer-term average urinary concentrations of short-lived environmental chemicals: Implications for exposure assessment and reverse dosimetry. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 582–590. [Google Scholar] [CrossRef]
- Faniband, M. Human Exposure Biomarkers of Some Commonly Used Pesticides. Ph.D. Thesis, Faculty of Medicine, Lund University, Lund, Sweden, 2020. Available online: https://lup.lub.lu.se/search/publication/492a1c9b-c9af-40d9-8160-1a1400eebd42 (accessed on 29 July 2020).
- JMPR. Pesticides Residues in Food 2011. In Proceedings of the Joint FAO/WHO Meeting on Pesticide Residues, Glyphosate and Metabolites, Geneva, Switzerland, 20–29 September 2011. [Google Scholar]
- Esteban López, M.; Göen, T.; Mol, H.; Nübler, S.; Haji-Abbas-Zarrabi, K.; Koch, H.M.; Kasper-Sonnenberg, M.; Dvorakova, D.; Hajslova, J.; Antignac, J.-P.; et al. The European human biomonitoring platform—Design and implementation of a laboratory quality assurance/quality control (QA/QC) programme for selected priority chemicals. Int. J. Hyg. Environ. Health 2021, 234, 113740. Available online: https://www.sciencedirect.com/science/article/pii/S1438463921000559 (accessed on 16 March 2022). [CrossRef]
- EFSA. Glyphosate: EFSA and ECHA Launch Consultations; European Food Safety Authority: Parma, Italy, 2021; Available online: https://www.efsa.europa.eu/en/news/glyphosate-efsa-and-echa-launch-consultations (accessed on 21 July 2022).

| Family Type | Participants | Sample Size (No) | Age in Years AM (Range) | BMI [kg/m2] AM (Range) |
|---|---|---|---|---|
| Non-farm | Adult (Male) | 54 | 45 (29–57) | 26.5 (19.5–36.2) |
| Adult (Female) 1 | 53 | 43 (26–54) | 23.9 (17.9–38.8) | |
| Child (Male) | 37 | 11 (3–19) | 18.4 (13.7–32.6) | |
| Child (Female) 2 | 37 | 10 (3–18) | 17.5 (12.4–25.1) | |
| Farm | Adult (Male) * | 14 | 48 (39–60) | 26.5 (21.1–34) |
| Adult (Female) 1 | 13 | 43 (36–55) | 25.5 (16.3–32.4) | |
| Child (Male) | 14 | 10 (3–17) | 17 (14.1–24.1) | |
| Child (Female) | 4 | 9 (7–11) | 18.6 (14.8–22) |
| Family Type | Number of | Urine Levels (µg/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| Families | Family Members | No. | % ≥ LOQ | Range | Median | P95 | ||
| Glyphosate | ||||||||
| Father | Non-farm | 54 | 54 | 54 | 20% | <LOQ–0.17 | <LOQ | 0.11 |
| Farm * | 14 | 14 | 14 | 43% | <LOQ–3.21 | <LOQ | 2.49 | |
| Mother | Non-farm 1 | 54 | 53 | 53 | 17% | <LOQ–0.17 | <LOQ | 0.12 |
| Farm 1 | 14 | 13 | 13 | 23% | <LOQ–0.23 | <LOQ | 0.16 | |
| Children | Non-farm 2 | 54 | 74 | 75 | 36% | <LOQ–2.48 | <LOQ | 0.27 |
| Farm | 14 | 18 | 18 | 17% | <LOQ–0.23 | <LOQ | 0.17 | |
| AMPA | ||||||||
| Father | Non-farm | 54 | 54 | 54 | 59% | <LOQ–4.12 | 0.06 | 0.64 |
| Farm * | 14 | 14 | 14 | 57% | <LOQ–1.22 | 0.07 | 0.86 | |
| Mother | Non-farm 1 | 54 | 53 | 53 | 60% | <LOQ–2.86 | 0.12 | 0.67 |
| Farm 1 | 14 | 13 | 13 | 38% | <LOQ–6.01 | <LOQ | 2.82 | |
| Children | Non-farm 2 | 54 | 74 | 75 | 60% | <LOQ–7.24 | 0.09 | 2.33 |
| Farm | 14 | 18 | 18 | 67% | <LOQ–1.22 | 0.08 | 0.83 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Connolly, A.; Koch, H.M.; Bury, D.; Koslitz, S.; Kolossa-Gehring, M.; Conrad, A.; Murawski, A.; McGrath, J.A.; Leahy, M.; Brüning, T.; et al. A Human Biomonitoring Study Assessing Glyphosate and Aminomethylphosphonic Acid (AMPA) Exposures among Farm and Non-Farm Families. Toxics 2022, 10, 690. https://doi.org/10.3390/toxics10110690
Connolly A, Koch HM, Bury D, Koslitz S, Kolossa-Gehring M, Conrad A, Murawski A, McGrath JA, Leahy M, Brüning T, et al. A Human Biomonitoring Study Assessing Glyphosate and Aminomethylphosphonic Acid (AMPA) Exposures among Farm and Non-Farm Families. Toxics. 2022; 10(11):690. https://doi.org/10.3390/toxics10110690
Chicago/Turabian StyleConnolly, Alison, Holger M. Koch, Daniel Bury, Stephan Koslitz, Marike Kolossa-Gehring, André Conrad, Aline Murawski, James A. McGrath, Michelle Leahy, Thomas Brüning, and et al. 2022. "A Human Biomonitoring Study Assessing Glyphosate and Aminomethylphosphonic Acid (AMPA) Exposures among Farm and Non-Farm Families" Toxics 10, no. 11: 690. https://doi.org/10.3390/toxics10110690
APA StyleConnolly, A., Koch, H. M., Bury, D., Koslitz, S., Kolossa-Gehring, M., Conrad, A., Murawski, A., McGrath, J. A., Leahy, M., Brüning, T., & Coggins, M. A. (2022). A Human Biomonitoring Study Assessing Glyphosate and Aminomethylphosphonic Acid (AMPA) Exposures among Farm and Non-Farm Families. Toxics, 10(11), 690. https://doi.org/10.3390/toxics10110690







