Genotoxic Biomonitoring in Children Living near the El Fraile Mine Tailings in Northern Guerrero State, Mexico
Abstract
1. Introduction
2. Materials and Methods
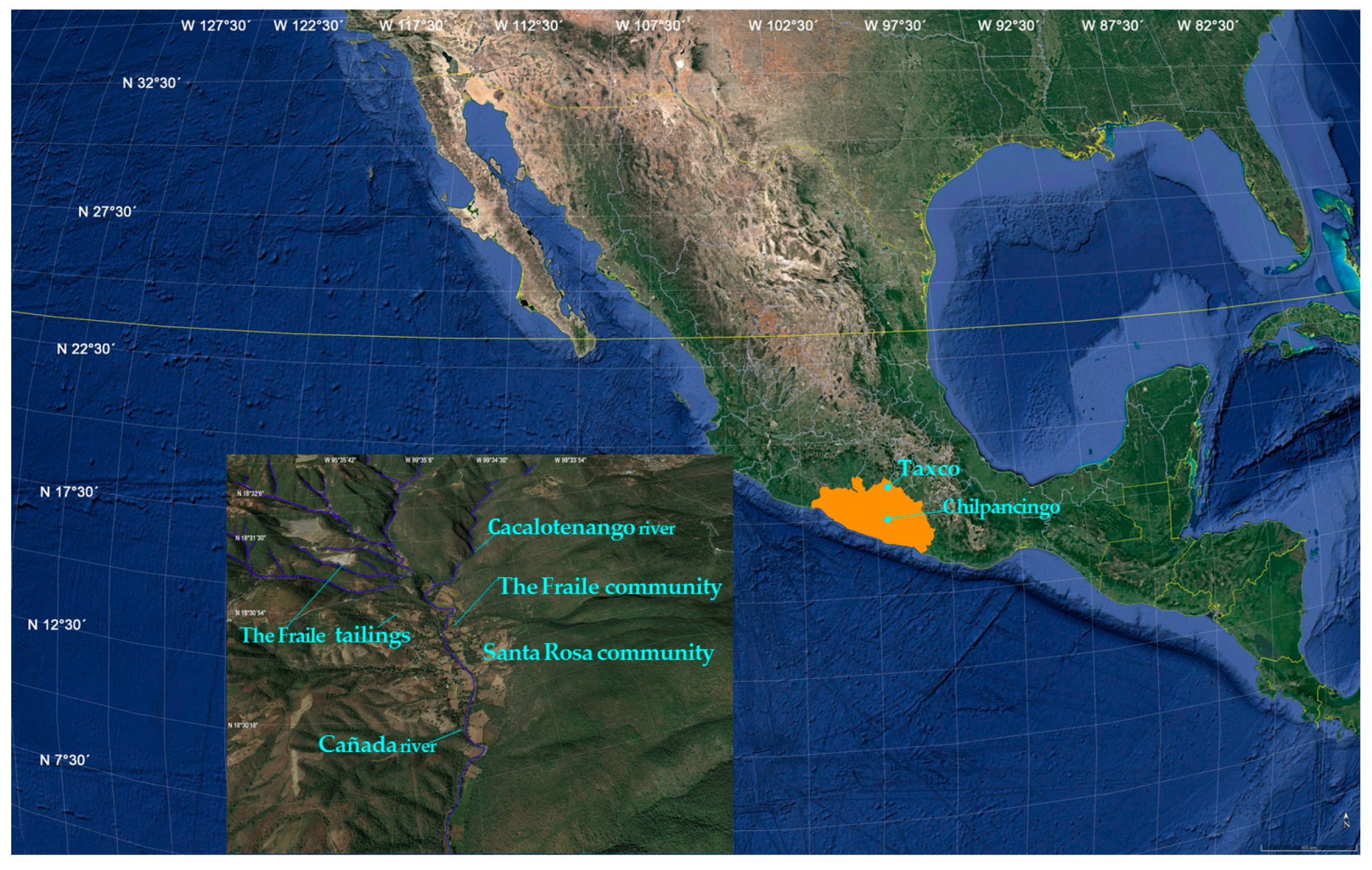
2.1. Study Areas
2.2. Study Populations
2.3. Oral Mucosa Samples Collection
2.4. Urine Collection
2.5. Alkaline Comet Assay
2.6. Detection of Urinary 8-OHdG Levels by Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Participant Children
3.2. DNA Damage in Buccal Mucosa Cells in the (El Fraile)-Exposed and (Chilpancingo)-Control Groups
3.3. Oxidative DNA Damage in the Exposure and Control Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talavera-Mendoza, O.; Armienta-Hernández, M.A.; García-Abundis, J.; Flores-Mundo, N. Geochemistry of leachates from the El Fraile sulfide tailings piles in Taxco, Guerrero, southern Mexico. Environ. Geochem. Health 2006, 28, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.M.; Nuñéz, M.E.; Gutiérrez, M.A.; Armienta, A.E.; Cineros-Gómez, A.E. Evaluation of the potential of indigenous calcareous shale for neutralization and removal of arsenic and heavy metals from acid mine drainage in the Taxco mining area, Mexico. Arch. Environ. Contam. Toxicol. 2011, 60, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.E.; Acosta-Saavedra, L.C.; Meza-Figueroa, D.; Vera, E.; Cebrian, M.E.; Ostrosky-Wegman, P.; Calderón-Aranda, E.S. Biomonitoring of metal in children living in a mine tailings zone in Southern Mexico: A pilot study. Int. J. Hyg. Environ. Health 2010, 213, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Soto-Ríos, M.L.; Rothenberg, S.; Gonsebatt, M.E.; Talavera-Mendoza, O. Cytogenotoxicity in uroepithelial cells of women exposed to mercury in a mining area. Occup. Environ. Med. 2010, 67, 620–624. [Google Scholar] [CrossRef]
- Armienta, M.A.; Talavera, O.; Morton, O.; Barrera, M. Geochemistry of Metals from Mine Tailings in Taxco, Mexico. Bull. Environ. Contam. Toxicol. 2003, 71, 387–393. [Google Scholar] [CrossRef]
- de Burbure, C.; Buchet, J.P.; Bernard, A.; Leroyer, A.; Nisse, C.; Haguenoer, J.M.; Bergamaschi, E.; Mutti, A. Biomarkers of renal effects in children and adults with low environmental exposure to heavy metals. J. Toxicol. Environ. Health A 2003, 66, 783–798. [Google Scholar] [CrossRef]
- Al Osman, M.; Yang, F.; Massey, Y.I. Exposure routes and health effects of heavy metals on children. Biometals 2019, 32, 563–573. [Google Scholar] [CrossRef]
- Bellinger, D.C. Very low lead exposures and children’s neurodevelopment. Curr. Opin. Pediatr. 2008, 20, 172–177. [Google Scholar] [CrossRef]
- Dunn, A.M.; Burns, C.; Satiler, B. Environmental Health children. J. Pediatr. Health Care 2003, 17, 223–231. [Google Scholar] [CrossRef]
- Wright, R.O. Environment, susceptibility windows, development, and child health. Curr. Opin. Pediatr. 2017, 29, 211–217. [Google Scholar] [CrossRef]
- Granot, E.; Kohen, R. Oxidative stress in childhood in health and disease states. Clin. Nutr. 2004, 23, 3–11. [Google Scholar] [CrossRef]
- Killian, B.; Yuan, T.H.; Tsai, C.H.; Chiu, T.H.T.; Chen, Y.H.; Chan, C.C. Emission-Related Heavy Metal Associated with Oxidative Stress in Children: Effect of Antioxidant Intake. Int. J. Environ. Res. Public Health 2020, 17, 3920. [Google Scholar] [CrossRef] [PubMed]
- Romaniuk, A.; Lyndin, M.; Sikora, V.; Lyndina, Y.; Romaniuk, S.; Sikora, K. Heavy metals effect on breast cancer progression. J. Occup. Med. Toxicol. 2017, 12, 32. [Google Scholar] [CrossRef]
- Loft, S.; Svoboda, P.; Kasai, H.; Tjønneland, A.; Vogel, U.; Møller, P.; Overvad, K.; Raaschou-Nielsen, O. Prospective study of 8-oxo-7,8-dihydro-2′-deoxyguanosine excretion and the risk of lung cancer. Carcinogenesis 2006, 27, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Korashy, H.M.; Attafi, I.M.; Famulski, K.S.; Bakheet, S.A.; Hafez, M.M.; Alsaad, A.M.S.; Al-Ghadeer, A.R.M. Gene expression profiling to identify the toxicities and potentially relevant human disease outcomes associated with environmental heavy metal exposure. Environ. Pollut. 2017, 221, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part I: Mechanisms involved in metal induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Marnett, L.J. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361370. [Google Scholar] [CrossRef]
- Cooke, M.S.; Olinski, R.; Loft, S. Measurement and meaning of oxidatively modified DNA lesion in Urine. Cancer Epidemiol. Biomark. Prev. 2008, 17, 13–14. [Google Scholar] [CrossRef]
- Hartwig, A.; Schwerdtle, T. Interactions by carcinogenic metal compounds with DNA repair processes: Toxicological implications. Toxicol. Lett. 2002, 127, 47–54. [Google Scholar] [CrossRef]
- Drury, J.A.; Jeffers, G.; Cooke, R.W. Urinary 8-hydroxydeoxyguanosine in infants and children. Free Radic. Res. 1998, 28, 423–438. [Google Scholar] [CrossRef]
- Poulsen, H.E.; Nadal, L.L.; Broedbaek, K.; Nielsen, P.E.; Weimann, A. Detection and interpretation of 8-oxodG and 8-oxoGua in urine, plasma and cerebrospinal fluid. Biochim. Biophys. Acta 2014, 1840, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Cahill, D.S.; Kasai, H.; Nishimura, S.; Loeb, L.A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage causes G-t and A-C substitutions. J. Biol. Chem. 1992, 267, 166–172. [Google Scholar] [CrossRef]
- Chiou, C.C.; Chang, P.Y.; Chan, E.C.; Wu, T.L.; Tsao, K.C.; Wu, J.T. Urinary 8-hydroxydeoxyguanosine and its analogues as DNA marker of oxidative stress: Development of an ELISA and measurement in both bladder and prostate cancers. Clin. Chim. Acta 2003, 334, 87–94. [Google Scholar] [CrossRef]
- Bernard, A. Biomarkers of metal toxicity in population studies: Research potential and interpretation issues. J. Toxicol. Environ. Health. Part A 2008, 71, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Segura, M.E.; Gómez-Arroyo, S.; Villalobos-Pietrini, R.; Espinosa-Ramírez, M. In vivo and in vitro promutagen activation by Vicia faba of thiocarbamate herbicides molinate and butylate to products inducing sister chromatid exchanges in human lymphocyte cultures. Mutat. Res. 1999, 438, 81–88. [Google Scholar] [CrossRef]
- Rodríguez-Romero, M.I.; Gómez-Arroyo, S.; Villalobos-Pietrini, R.; Martínez-Valenzuela, C.; Calderón-Ezquerro, M.C.; Cortés-Eslava, J.; Arenas-Huertero, F.; Calderón-Segura, M.E. Evaluation of 8-hidroxy-2′-deoxiguanosine (8-OHdG) adduct levels and DNA strand breaks in human peripheral lymphocytes in vitro exposed to polycyclic aromatic hydrocarbons with or without animal metabolic activation. Toxicol. Mech. Methods 2012, 22, 170–183. [Google Scholar] [CrossRef]
- Tsukahara, H. Biomarkers for oxidative stress: Clinical application in pediatric medicine. Curr. Med. Chem. 2007, 14, 339–351. [Google Scholar] [CrossRef]
- Martínez-Valenzuela, C.; Waliszewski, S.M.; Amador-Muñoz, O.; Meza, E.; Calderón-Segura, M.E.; Zenteno, E.; Huichapan-Martínez, J.; Caba, M.; Félix-Gastélum, R.; Longoria-Espinoza, R. Aerial pesticide application causes DNA damage in pilots from Sinaloa, Mexico. Environ. Sci. Pollut. Res. 2016, 24, 2412–2420. [Google Scholar] [CrossRef]
- Carbajal-López, Y.; Gómez-Arroyo, S.; Villalobos-Pietrini, R.; Calderón-Segura, M.E.; Martínez-Arroyo, A. Biomonitoring of agricultural workers exposed to pesticide mixtures in Guerrero state, Mexico, with comet assay and micronucleus test. Environ. Sci. Pollut. Res. 2016, 23, 2513–2520. [Google Scholar] [CrossRef]
- Zhushan, F.; Shuhua, X. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantification of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Calderón-Segura, M.E.; Gómez-Arroyo, S.; Cortés-Eslava, J.; Martínez-Valenzuela, C.; Mojica-Vázquez, L.H.; Sosa-López, M.; Flores-Ramírez, D.; Romero-Velázquez, Z.E. In Vitro cytotoxicity and genotoxicity of Furia ®180 SC (Zeta-cypermethrin) and Bulldock 125®SC (β-Cyfluthrin) pyrethroid insecticides in human peripheral blood lymphocytes. Toxicol. Mech. Methods 2018, 28, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Villaseñor, E. Transferencia de Metales Entre Suelo y Plantas de Maíz (Zea mays L.), Sembradas en Terrenos Impactados Por Jales Mineros en la Región de Taxco, Guerrero. Master’s Thesis, Universidad Autónoma de Guerrero, Iguala, Mexico, November 2006. [Google Scholar]
- Breton, J.; Sichel, F.; Bianchini, F.; Prevost, V. Measurement of 8-Hydroxy-2′deoxyguanosine by a commercially available ELISA test: Comparison with HPLC/electrochemical detection in calf thymus DNA and determination in human serum. J. Anal. Lett. 2003, 36, 123–134. [Google Scholar] [CrossRef]
- Yáñez, L.; García-Nieto, E.; Rojas, E.; Carrizales, L.; Mejía, J.; Calderón, J.; Razo, I.; Díaz-Barriga, F. DNA damage in blood cells from children exposed to arsenic and lead in a mining area. Environ. Res. 2003, 93, 231–240. [Google Scholar] [CrossRef]
- Méndez-Gómez, J.; García-Vargas, G.G.; López-Carrillo, L.; Calderón-Aranda, E.S.; Gómez, A.; Vera, E.; Valverde, M.; Cebrián, M.E.; Rojas, E. Genotoxic Effects of Environmental Exposure to Arsenic and Lead on Children in Region Lagunera, Mexico. Ann. N. Y. Acad. Sci. 2008, 1140, 358–367. [Google Scholar] [CrossRef]
- Jasso-Pineda, Y.; Díaz-Barriga, F.; Calderón, J.; Yáñez, L.; Carrizales, L.; Pérez-Maldonado, I.N. DNA damage and decreased DNA repair in peripheral blood mononuclear cells in individuals exposed to arsenic and lead in a mining site. Biol. Trace Elem. Res. 2012, 146, 141–149. [Google Scholar] [CrossRef]
- Jasso-Pineda, Y.; Díaz-Barriga, F.; Yáñez-Estrada, L.; Pérez-Vázquez, F.J.; Pérez-Maldonado, I.N. DNA damage in Mexican children living in high-risk contaminated scenarios. Sci. Total Environ. 2015, 518–519, 38–48. [Google Scholar] [CrossRef]
- Alegría-Torres, J.A.; Pérez-Rodríguez, R.Y.; García-Torres, L.; Costilla-Salazar, R.; Rocha-Amador, D. Exposure to arsenic and lead in children from Salamanca México, effects on telomeric lengthening and mitochondrial DNA. Environ. Sci. Pollut. Res. Int. 2020, 27, 6420–6428. [Google Scholar] [CrossRef]
- Kapka, L.; Baumgartner, A.; Siwińska, E.; Knudsen, L.E.; Anderson, D.; Mielzyńska, D. Environmental lead exposure increases micronuclei in children. Mutagenesis 2007, 22, 201–207. [Google Scholar] [CrossRef]
- Xu, X.; Liao, W.; Lin, Y.; Dai, Y.; Shi, Z.; Huo, X. Blood concentrations of lead, cadmium, mercury and their association with biomarkers of DNA oxidative damage in preschool children living in an e-waste recycling area. Environ. Geochem. Health 2018, 40, 1481–1494. [Google Scholar] [CrossRef]
- Moya, J.; Bearer, C.F.; Etzel, R.A. Children’s behavior and physiology and how it affects exposure to environmental contaminants. Pediatrics 2004, 113, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Sly, J.L.; Carpenter, D.O. Special vulnerability of children to environmental exposures. Rev. Environ. Health 2012, 27, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Bonassi, S.; Knudsen, L.E.; Sram, R.J.; Holland, N.; Ugolini, D.; Merlo, D.F. Children’s exposure to environmental pollutants and biomarkers of genetic damage I. Overview and critical issues. Mutat. Res. 2006, 612, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Engström, K.S.; Vahter, M.; Lindh, C.; Teichert, F.; Singh, R.; Concha, G.; Nermell, B.; Farmer, P.B.; Strömberg, U.; Broberg, K. Low 8-oxo-7,8-dihydro-2-oxi-deoxyguanosine levels and influence of genetic background in an Andean population exposed to high levels of arsenic. Mutat. Res. 2010, 683, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J. Ambient air pollution: Health hazards to children. Pediatrics 2004, 114, 1699–1707. [Google Scholar] [CrossRef]
- Sughis, M.; Nawrot, T.S.; Haufroid, V.; Nemery, B. Adverse Health Effects of Child Labor: High Exposure to Chromium and Oxidative DNA Damage in Children Manufacturing Surgical Instruments. Environ. Health Perspect. 2012, 120, 1469–1474. [Google Scholar] [CrossRef]
- Kippler, M.; Hossain, M.B.; Lindh, C.; Moore, S.E.; Kabir, I.; Vahter, M.; Broberg, K. Early life low-level cadmium exposure is positively associated with increased oxidative stress. Environ. Res. 2012, 112, 164–170. [Google Scholar] [CrossRef]
- Pizzino, G.; Bitto, A.; Interdonato, M.; Galfo, F.; Irrera, N.; Mecchio, A.; Pallio, G.; Ramistella, V.; De Luca, F.; Minutoli, L.; et al. Oxidative stress and DNA repair and detoxification gene expression in adolescents exposed to heavy metals living in the Milazzo-Valledel Mela area (Sicily, Italy). Redox Biol. 2014, 2, 686–693. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Zheng, Q.; Li, X.; Li, B.; Jin, Y.; Sun, X.; Sun, G. Association of oxidative stress with arsenic methylation in chronic arsenic-exposed children and adults. Toxicol. Appl. Pharmacol. 2008, 232, 142–149. [Google Scholar] [CrossRef]
- Hinhumpatch, P.; Navasumrit, P.; Chaisatra, K.; Promvijit, J.; Mahidol, C.; Ruchirawat, M. Oxidative DNA damage and repair in children exposed to low levels of arsenic in utero and during early childhood: Application of salivary and urinary biomarkers. Toxicol. Appl. Pharmacol. 2013, 273, 569–579. [Google Scholar] [CrossRef]
- Roy, A.; Queirolo, E.; Peregalli, F.; Mañay, N.; Martínez, G.; Kordas, K. Association of blood lead levels with urinary F2-8α Isoprostane and 8-hydroxy-2-deoxy-Guanosine concentrations in first-grade Uruguayan children. Environ. Res. 2015, 140, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kordas, K.; Roy, A.; Vahter, M.; Ravenscroft, J.; Mañay, N.; Peregalli, F.; Martínez, G.; Queirolo, E.I. Multiple-metal exposure, diet, and oxidative stress in Uruguayan school children. Environ. Res. 2018, 166, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Yamauchi, H.; Yamamoto, H.; Aminaka, M.; Murakami, H.; Kamiyama, N.; Miyamoto, Y.; Koitabashi, Y. The evaluation of oxidative DNA damage in children with brain damage using 8-hydroxydeoxyguanosine levels. Brain Dev. 2008, 30, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Kuo, C.H.; Hsu, M.L.; Wang, T.Y.; Chang, P.I.; Wu, T.H.; Huang, S. Increased Levels of 8-Hydroxy-2′-Deoxyguanosine Attributable to Carcinogenic Metal Exposure among School children. Environ. Health Perspect. 2005, 113, 1386–1390. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Zavaleta, A.P.; García-Vargas, G.; Borja-Aburto, V.H.; Acosta-Saavedra, L.C.; Vera-Aguilar, E.; Gómez-Muñoz, A.; Cebrián, M.E.; Calderón-Aranda, E.S. Nitric oxide and superoxide anion production in monocytes from children exposed to arsenic and lead in region Lagunera, Mexico. Toxicol. Appl. Pharmacol. 2004, 198, 283–290. [Google Scholar] [CrossRef]
- Leonard, S.S.; Harris, G.K.; Shi, X. Metal-induced oxidative stress and signal transduction. Free Radic. Biol. Med. 2004, 37, 1921–1942. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Miyashita, Y.; Sasaki, H.; Ebisuno, M.; Ohira, M.; Saiki, A.; Koide, N.; Oyama, T.; Takeyoshi, M.; Shirai, K. Probucol and atorvastatin decrease urinary 8-hydroxy-2′-deoxyguanosine in patients with diabetes and hypercholesterolemia. J. Atheroscler. Thromb. 2006, 13, 68–75. [Google Scholar] [CrossRef][Green Version]

| Variable | Control Group a | Exposure Group a |
|---|---|---|
| Age ranges | 6–12 | 6–12 |
| Gender | 52 girls 49 boys | 56 girls 45 boys |
| Living area | Urban | Rural |
| Diet | Not special | Not special |
| Lifestyle Children play in garden | 80% | 85% |
| Type of drinking water and zone of residence (close to or far from mine tailings) | Bottled 60% | Bottled 100% |
| Frequency of children’s contact with soil Every day | 10% | 85% |
| Frequency of consuming fruits and edible vegetables grown on site | No | 100% |
| Frequency of Cacalotenango water river use | None | 100% |
| Frequency of rain water use Every day 2–3 times/week Once a week | None | 70% |
| Children consuming meat from animals grown on site | None | 85% |
| Children consuming milk from cows grown on site | None | 80% |
| Socioeconomic status (parental education level) | Elementary, high school | Elementary school |
| Children Groups (Age Ranges) | n | Tail Intensity (%) | Tail Moment (%) | Tail Length (µm) |
|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | ||
| Exposure | 101 | 37.68 ± 4.59 * | 19.79 ± 3.50 * | 60.89 ± 6.18 * |
| 6–7 | 29 | 45.41 ± 5.40 * | 17.86 ± 5.07 * | 56.32 ± 4.36 * |
| 8–9 | 39 | 49.94 ± 4.51 ** | 24.54 ± 6.64 ** | 71.14 ± 4.98 ** |
| 10–12 | 33 | 39.94 ± 4.31 * | 17.25 ± 4.95 * | 55.81 ± 6.10 * |
| Control | 101 | 18.38 ± 1.52 | 4.24 ± 1.54 | 36.59 ± 1.86 |
| 6–7 | 30 | 16.76 ± 1.44 | 3.91 ± 2.30 | 46.13 ± 4.84 |
| 8–9 | 39 | 18.81 ± 1.38 | 4.58 ± 2.77 | 34.27 ± 4.08 |
| 10–11 | 32 | 14.53 ± 1.66 | 4.16 ± 2.65 | 44.01 ± 2.03 |
| Gender | Children’s Groups | n | Tail Intensity (%) | Tail Moment (%) | Tail Length (µm) |
|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | |||
| Exposure | |||||
| Girls | 56 | 44.33 ± 5.26 * | 22.01 ± 5.49 * | 58.73 ± 5.71 * | |
| Boys | 45 | 58.70 ± 6.44 ** | 28.93 ± 3.01 ** | 68.13 ± 4.99 ** | |
| Control | |||||
| Girls | 52 | 14.33 ± 5.04 | 5.01 ± 4.49 | 28.73 ± 4.71 | |
| boys | 49 | 17.70 ± 2.88 | 6.29 ± 3.01 | 24.13 ± 6.11 |
| Age Ranges | Children Groups | (n) | 8-OHdG Level (ng/mL) Mean ± SEM |
|---|---|---|---|
| Exposure | 101 | 5.65 ± 0.33 * | |
| 6–7 | 29 | 5.15 ± 0.67 * | |
| 8–9 | 39 | 6.95 ± 0.24 ** | |
| 10–12 | 33 | 4.35 ± 0.54 * | |
| Control | 101 | 1.64 ± 0.88 | |
| 6–7 | 30 | 1.13 ± 0.24 | |
| 8–9 | 39 | 1.88 ± 0.12 | |
| 10–12 | 32 | 1.74 ± 0.30 |
| Gender | Children Groups | n | 8-OHdG Level (ng/mL) Mean ± SEM |
|---|---|---|---|
| Exposure | |||
| Girls | 56 | 5.18 ± 0.24 * | |
| Boys | 45 | 6.88 ± 0.12 ** | |
| Control | |||
| Girls | 52 | 1.73 ± 0.24 | |
| Boys | 49 | 1.38 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderon-Segura, M.E.; Ramírez-Guzmán, A.; Talavera-Mendoza, O.; Carbajal-López, Y.; Martínez-Valenzuela, M.d.C.; Mora-Herrera, M.E.; Salinas-Alcántara, L.; Hurtado-Brito, P. Genotoxic Biomonitoring in Children Living near the El Fraile Mine Tailings in Northern Guerrero State, Mexico. Toxics 2022, 10, 674. https://doi.org/10.3390/toxics10110674
Calderon-Segura ME, Ramírez-Guzmán A, Talavera-Mendoza O, Carbajal-López Y, Martínez-Valenzuela MdC, Mora-Herrera ME, Salinas-Alcántara L, Hurtado-Brito P. Genotoxic Biomonitoring in Children Living near the El Fraile Mine Tailings in Northern Guerrero State, Mexico. Toxics. 2022; 10(11):674. https://doi.org/10.3390/toxics10110674
Chicago/Turabian StyleCalderon-Segura, María Elena, Alejandro Ramírez-Guzmán, Oscar Talavera-Mendoza, Yolanda Carbajal-López, María del Carmen Martínez-Valenzuela, Martha Elena Mora-Herrera, Liliana Salinas-Alcántara, and Patricia Hurtado-Brito. 2022. "Genotoxic Biomonitoring in Children Living near the El Fraile Mine Tailings in Northern Guerrero State, Mexico" Toxics 10, no. 11: 674. https://doi.org/10.3390/toxics10110674
APA StyleCalderon-Segura, M. E., Ramírez-Guzmán, A., Talavera-Mendoza, O., Carbajal-López, Y., Martínez-Valenzuela, M. d. C., Mora-Herrera, M. E., Salinas-Alcántara, L., & Hurtado-Brito, P. (2022). Genotoxic Biomonitoring in Children Living near the El Fraile Mine Tailings in Northern Guerrero State, Mexico. Toxics, 10(11), 674. https://doi.org/10.3390/toxics10110674








