Inhibitory Effect of α1 Receptor Antagonists on Paclitaxel-Induced Peripheral Neuropathy in a Rodent Model and Clinical Database
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Animals
2.3. Analysis of the Effects of Repeated Administration of Alpha 1 Receptor Antagonists on the Development of Peripheral Neuropathy
2.3.1. Drug Administration and Experimental Schedule
2.3.2. Von Frey Test
2.3.3. Toluidine Blue Staining
2.3.4. Statistical Processing
2.4. Analysis of the Analgesic Effects of a Single Administration of Alpha 1 Receptor Antagonists on Developed Peripheral Neuropathy
2.4.1. Drug Administration and Experimental Schedule
2.4.2. Statistical Processing
2.5. Evaluation of PIPN Suppression Effects Using a Large Adverse Event Database
2.5.1. Analysis of FAERS Data
2.5.2. Statistical Processing
3. Results
3.1. Effects of Repeated Administration of Doxazosin and Tamsulosin on Paclitaxel-Induced Mechanical Allodynia in Rats
3.2. Effects of Repeated Administration of Doxazosin and Tamsulosin on Paclitaxel-Induced Neuroaxonal Degeneration in Rat Sciatic Nerves
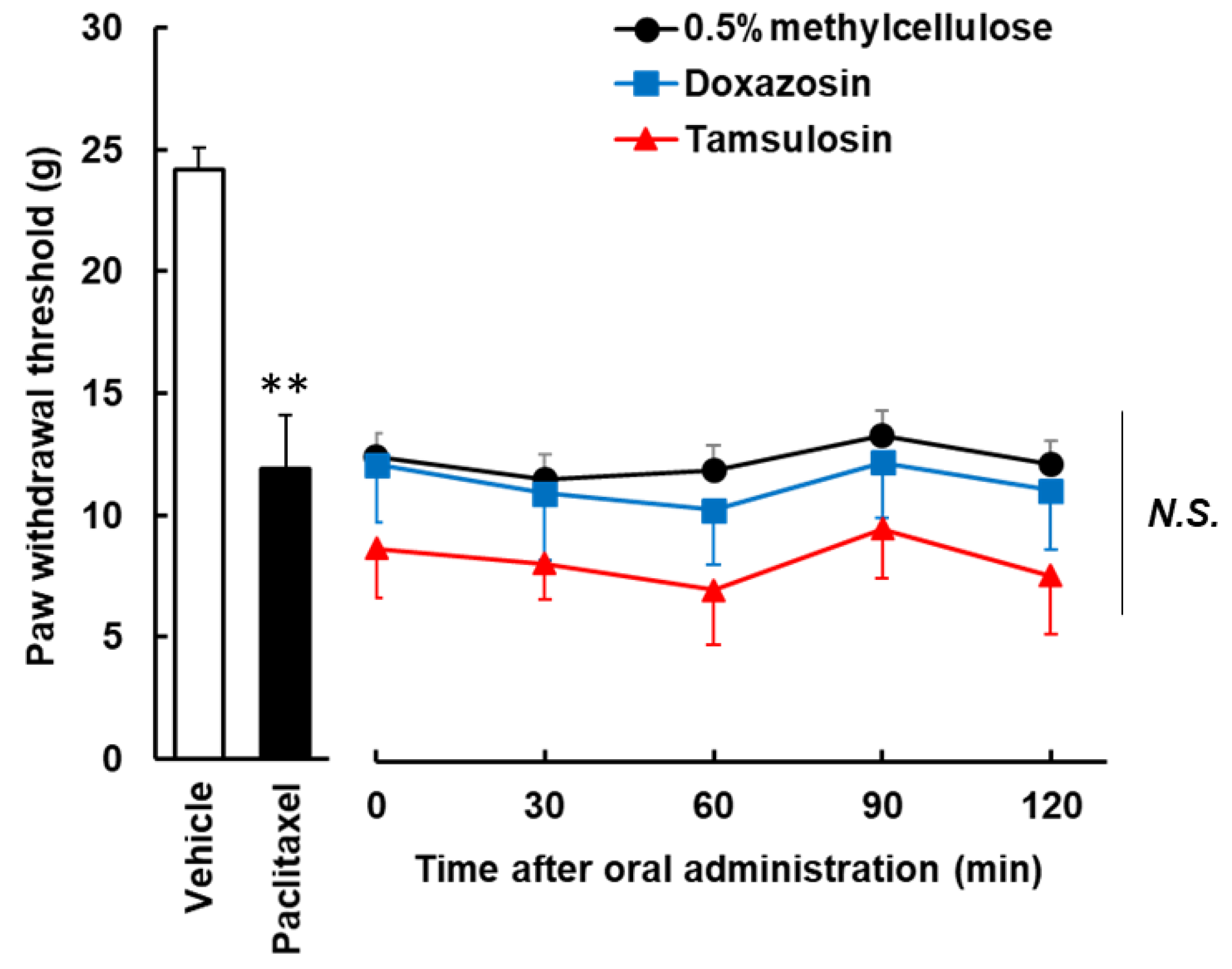
3.3. Effects of the Single Administration of Doxazosin and Tamsulosin on Developed Paclitaxel-Induced Mechanical Allodynia in Rat Sciatic Nerves
3.4. Evaluation of PIPN Suppression Using a Large Adverse Event Database
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozols, R.F.; Bundy, B.N.; Greer, B.E.; Fowler, J.M.; Clarke-Pearson, D.; Burger, R.A.; Mannel, R.S.; DeGeest, K.; Hartenbach, E.M.; Baergen, R. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A gynecologic oncology group study. J. Clin. Oncol. 2003, 21, 3194–3200. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, A.; Lück, H.-J.; Meier, W.; Adams, H.-P.; Möbus, V.; Costa, S.; Bauknecht, T.; Richter, B.; Warm, M.; Schröder, W.; et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J. Natl. Cancer. Inst. 2003, 95, 1320–1329. [Google Scholar] [CrossRef]
- Sparano, J.A.; Wang, M.; Martino, S.; Jones, V.; Perez, E.A.; Saphner, T.; Wolff, A.C.; Sledge, G.W., Jr.; Wood, W.C.; Davidson, N.E. Weekly paclitaxel in the adjuvant treatment of breast cancer. N. Engl. J. Med. 2008, 358, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Seidman, A.D.; Berry, D.; Cirrincione, C.; Harris, L.; Muss, H.; Marcom, P.K.; Gipson, G.; Burstein, H.; Lake, D.; Shapiro, C.L.; et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: Final results of cancer and leukemia group B protocol. J. Clin. Oncol. 2008, 26, 1642–1649. [Google Scholar] [PubMed]
- Gradishar, W.J.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; Hawkins, M.; O’Shaughnessy, J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil–based paclitaxel in women with breast cancer. J. Clin. Oncol. 2005, 23, 7794–7803. [Google Scholar] [CrossRef]
- Ohe, Y.; Ohashi, Y.; Tamura, T.; Nakagawa, K.; Negoro, S.; Nishiwaki, Y.; Saijo, N.; Ariyoshi, Y.; Fukuoka, M. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann. Oncol. 2007, 18, 317–323. [Google Scholar] [CrossRef]
- Socinski, M.A.; Bondarenko, I.; Karaseva, N.A.; Makhson, A.M.; Vynnychenko, I.; Okamoto, I.; Hon, J.K.; Hirsh, V.; Bhar, P.; Zhang, H.; et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non–small-cell lung cancer: Final results of a phase III trial. J. Clin. Oncol. 2012, 30, 2055–2062. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Cutsem, E.V.; Oh, S.-C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.-Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Shitara, K.; Takashima, A.; Fujitani, K.; Koeda, K.; Hara, H.; Nakayama, N.; Hironaka, S.; Nishikawa, K.; Makari, Y.; Amagai, K.; et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): An open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol. Hepatol. 2017, 2, 277–287. [Google Scholar] [CrossRef]
- Akram, T.; Maseelall, P.; Fanning, J. Carboplatin and paclitaxel for the treatment of advanced or recurrent endometrial cancer. Am. J. Obstet. Gynecol. 2005, 192, 1365–1367. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Kuroi, K.; Shimozuma, K. Neurotoxicity of taxanes: Symptoms and quality of life assessment. Breast Cancer 2004, 11, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef] [PubMed]
- Kawashiri, T.; Inoue, M.; Mori, K.; Kobayashi, D.; Mine, K.; Ushio, S.; Kudamatsu, H.; Uchida, M.; Egashira, N.; Shimazoe, T. Preclinical and clinical evidence of therapeutic agents for paclitaxel-induced peripheral neuropathy. Int. J. Mol. Sci. 2021, 22, 8733. [Google Scholar] [CrossRef] [PubMed]
- Ramer, M.S.; Bisby, M.A. Rapid sprouting of sympathetic axons in dorsal root ganglia of rats with a chronic constriction injury. Pain 1997, 70, 237–244. [Google Scholar] [CrossRef]
- Ramer, M.S.; French, G.D.; Bisby, M.A. Wallerian degeneration is required for both neuropathic pain and sympathetic sprouting into the DRG. Pain 1997, 72, 71–78. [Google Scholar] [CrossRef]
- Yen, L.D.; Bennett, G.J.; Ribeiro-da-Silva, A. Sympathetic sprouting and changes in nociceptive sensory innervation in the glabrous skin of the rat hind paw following partial peripheral nerve injury. J. Comp. Neurol. 2006, 495, 679–690. [Google Scholar] [CrossRef]
- Lee, Y.H.; Ryu, T.G.; Park, S.J.; Yang, E.J.; Jeon, B.H.; Hur, G.M.; Kim, K.J. Alpha1-adrenoceptors involvement in painful diabetic neuropathy: A role in allodynia. Neuroreport 2000, 11, 1417–1420. [Google Scholar] [CrossRef]
- Drummond, E.S.; Dawson, L.F.; Finch, P.M.; Bennett, G.J.; Drummond, P.D. Increased expression of cutaneous α1-adrenoceptors after chronic constriction injury in rats. J. Pain 2014, 15, 188–196. [Google Scholar] [CrossRef]
- Drummond, P.D. Neuronal changes resulting in up-regulation of alpha-1 adrenoceptors after peripheral nerve injury. Neural. Regen. Res. 2014, 9, 1337–1340. [Google Scholar] [CrossRef]
- Coelho, B.P.; Gaelzer, M.M.; Dos Santos Petry, F.; Hoppe, J.B.; Trindade, V.M.T.; Salbego, C.G.; Guma, F.T.C.R. Dual effect of doxazosin: Anticancer activity on SH-SY5Y neuroblastoma cells and neuroprotection on an in Vitro model of Alzheimer’s disease. Neuroscience 2019, 404, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Gros, P.; Wang, X.; Guan, J.; Lang, A.E.; Austin, P.C.; Welk, B.; Visanji, N.P.; Marras, C. Exposure to phosphoglycerate kinase 1 activators and incidence of parkinson’s disease. Mov. Disord. 2021, 36, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Yono, M.; Yoshida, M.; Yamamoto, Y.; Imanishi, A.; Fukagawa, A.; Latifpour, J.; Eto, M. Molecular mechanisms regulating urogenital expression of nitric oxide synthase in spontaneously hypertensive rats. Life. Sci. 2009, 85, 334–338. [Google Scholar] [CrossRef]
- Oyarzábal, A.; Pérez, Y.; Molina, V.; Mas, R.; Ravelo, Y.; Jiménez, S. D-004 ameliorates phenylephrine-induced urodynamic changes and increased prostate and bladder oxidative stress in rats. Transl. Androl. Urol. 2015, 4, 391–397. [Google Scholar] [PubMed]
- Smith, E.M.L.; Pang, H.; Cirrincione, C.; Fleishman, S.; Paskett, E.D.; Ahles, T.; Bressler, L.R.; Fadul, C.E.; Knox, C.; Le-Lindqwister, N.; et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy. JAMA 2013, 309, 1359–1367. [Google Scholar] [CrossRef]
- Cavaletti, G.; Tredici, G.; Braga, M.; Tazzari, S. Experimental peripheral neuropathy induced in adult rats by repeated intraperitoneal administration of taxol. Exp. Neurol. 1995, 133, 64–72. [Google Scholar] [CrossRef]
- Kim, C.H.; Ko, I.G.; Kim, S.E.; Shin, M.S.; Kang, Y.H.; Cho, J.W.; Shin, K.M.; Kim, C.J.; Lim, B.V.; Kim, K.H. Alpha1-Adrenoceptor Antagonists Improve Memory by Activating N-methyl-D-Aspartate-Induced Ion Currents in the Rat Hippocampus. Int. Neurourol. J. 2015, 19, 228–236. [Google Scholar] [CrossRef]
- Suh, H.J.; So, S.M.; Na, Y.G.; Ko, I.G.; Kim, S.E.; Sung, Y.H.; Shin, M.S.; Kim, C.J.; Cho, Y.S.; Kim, K.H. Neuroprotective effects of tamsulosin on intracerebral hemorrhage. Neural. Regen. Res. 2011, 6, 2505–2510. [Google Scholar]
- Li, T.; Yang, S.; She, X.; Yan, Q.; Zhang, P.; Zhu, H.; Wang, F.; Luo, X.; Sun, X. Modulation of α-adrenoceptor signalling protects photoreceptors after retinal detachment by inhibiting oxidative stress and inflammation. Br. J. Pharmacol. 2019, 176, 801–813. [Google Scholar] [CrossRef]
- Tung, D.; Ciallella, J.; Cheung, P.H.; Saha, S. Novel anti-inflammatory effects of doxazosin in rodent models of inflammation. Pharmacology 2013, 91, 29–34. [Google Scholar] [CrossRef]
- Niemczyk, G.; Fus, L.; Czarzasta, K.; Jesion, A.; Radziszewski, P.; Gornicka, B.; Cudnoch-Jedrzejewska, A. Expression of toll-like receptors in the animal model of bladder outlet obstruction. Biomed. Res. Int. 2020, 2020, 6632359. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, K.; Kaname, T.; Shiraishi, H.; Kawashiri, T.; Egashira, N. Polaprezinc reduces paclitaxel-induced peripheral neuropathy in rats without affecting anti-tumor activity. J. Pharmacol. Sci. 2016, 131, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Lu, N.; Cui, Y.; Yang, T.; Zhao, Z.Q.; Xin, W.J.; Liu, X.G. Prevention of paclitaxel-induced allodynia by minocycline: Effect on loss of peripheral nerve fibers and infiltration of macrophages in rats. Mol. Pain. 2010, 6, 76. [Google Scholar] [CrossRef]
- Ledeboer, A.; Jekich, B.M.; Sloane, E.M.; Mahoney, J.H.; Langer, S.J.; Milligan, E.D.; Martin, D.; Maier, S.F.; Johnson, K.W.; Leinwand, L.A.; et al. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain. Behav. Immun. 2007, 21, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, T.; Yamada, S.; Deguchi, Y.; Kimura, R. Comparative study on pharmacokinetics and in vivo alpha1-adrenoceptor binding of [3H]tamsulosin and [3H]prazosin in rats. Biol. Pharm. Bull. 1999, 22, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Nawa, H.; Niimura, T.; Hamano, H.; Yagi, K.; Goda, M.; Zamami, Y.; Ishizawa, K. Evaluation of potential complications of interstitial lung disease associated with antiandrogens using data from databases reporting spontaneous adverse effects. Front. Pharmacol. 2021, 12, 655605. [Google Scholar] [CrossRef]
- Kawashiri, T.; Kobayashi, D.; Uchida, M.; Hiromoto, S.; Inoue, M.; Ikeda, H.; Inoue, M.; Shimazoe, T. Analysis of secondary leukemia and myelodysplastic Syndrome after chemotherapy for solid organ tumors using the food and drug administration adverse event reporting system (FAERS). J. Pharm. Pharm. Sci. 2021, 24, 499–508. [Google Scholar] [CrossRef]
- Hiromoto, S.; Kawashiri, T.; Yamanaka, N.; Kobayashi, D.; Mine, K.; Inoue, M.; Uchida, M.; Shimazoe, T. Use of omeprazole, the proton pump inhibitor, as a potential therapy for the capecitabine-induced hand-foot syndrome. Sci. Rep. 2021, 11, 8964. [Google Scholar] [CrossRef]
- Shimada, K.; Hasegawa, S.; Nakao, S.; Mukai, R.; Sasaoka, S.; Ueda, N.; Kato, Y.; Abe, J.; Mori, T.; Yoshimura, T.; et al. Adverse reaction profiles of hemorrhagic adverse reactions caused by direct oral anticoagulants analyzed using the food and drug administration adverse event reporting system (FAERS) database and the japanese adverse drug event report (JADER) database. Int. J. Med. Sci. 2019, 16, 1295–1303. [Google Scholar] [CrossRef]
- Mine, K.; Kawashiri, T.; Inoue, M.; Kobayashi, D.; Mori, K.; Hiromoto, S.; Kudamatsu, H.; Uchida, M.; Egashira, N.; Koyanagi, S.; et al. Omeprazole suppresses oxaliplatin-induced peripheral neuropathy in a rodent model and clinical database. Int. J. Mol. Sci. 2022, 23, 8859. [Google Scholar] [CrossRef]
- Ng, D.Q.; Tan, C.J.; Soh, B.C.; Tan, M.M.L.; Loh, S.Y.; Tan, Y.E.; Ong, H.H.; Teng, P.P.C.; Chan, J.J.; Chay, W.Y.; et al. Impact of Cryotherapy on Sensory, Motor, and Autonomic Neuropathy in Breast Cancer Patients Receiving Paclitaxel: A Randomized, Controlled Trial. Front. Neurol. 2020, 11, 604688. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.; Kwon, N.; Beaumont, J.L.; Paice, J.A. Cold therapy to prevent paclitaxel-induced peripheral neuropathy. Support. Care Cancer 2018, 26, 3461–3469. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, H.; Hirata, T.; Nishina, M.; Yasui, D.; Ozaki, S. Cryotherapy for the prevention of weekly paclitaxel-induced peripheral adverse events in breast cancer patients. Support. Care Cancer 2020, 28, 5005–5011. [Google Scholar] [CrossRef] [PubMed]
- Beijers, A.J.M.; Bonhof, C.S.; Mols, F.; Ophorst, J.; de Vos-Geelen, J.; Jacobs, E.M.G.; van de Poll-Franse, L.V.; Vreugdenhil, G. Multicenter randomized controlled trial to evaluate the efficacy and tolerability of frozen gloves for the prevention of chemotherapy-induced peripheral neuropathy. Ann. Oncol. 2020, 31, 131–136. [Google Scholar] [CrossRef]
- Kanbayashi, Y.; Sakaguchi, K.; Ishikawa, T.; Tabuchi, Y.; Takagi, R.; Yokota, I.; Katoh, N.; Takayama, K.; Taguchi, T. Predictors of the development of nab-paclitaxel-induced peripheral neuropathy in breast cancer patients: Post hoc analysis of a prospective, phase II, self-controlled clinical trial. Med. Oncol. 2022, 39, 153. [Google Scholar] [CrossRef]
- Hiramoto, S.; Asano, H.; Miyamoto, T.; Takegami, M.; Kawabata, A. Risk factors and pharmacotherapy for chemotherapy-induced peripheral neuropathy in paclitaxel-treated female cancer survivors: A retrospective study in Japan. PLoS ONE 2021, 16, e0261473. [Google Scholar] [CrossRef]
- Hwang, L.C.; Tsai, C.H.; Chen, T.H. Overweight and obesity-related metabolic disorders in hospital employees. J. Formos. Med. Assoc. 2006, 105, 56–63. [Google Scholar] [CrossRef][Green Version]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, K.; Kawashiri, T.; Mine, K.; Inoue, M.; Kudamatsu, H.; Uchida, M.; Egashira, N.; Kobayashi, D.; Shimazoe, T. Inhibitory Effect of α1 Receptor Antagonists on Paclitaxel-Induced Peripheral Neuropathy in a Rodent Model and Clinical Database. Toxics 2022, 10, 669. https://doi.org/10.3390/toxics10110669
Mori K, Kawashiri T, Mine K, Inoue M, Kudamatsu H, Uchida M, Egashira N, Kobayashi D, Shimazoe T. Inhibitory Effect of α1 Receptor Antagonists on Paclitaxel-Induced Peripheral Neuropathy in a Rodent Model and Clinical Database. Toxics. 2022; 10(11):669. https://doi.org/10.3390/toxics10110669
Chicago/Turabian StyleMori, Kohei, Takehiro Kawashiri, Keisuke Mine, Mizuki Inoue, Hibiki Kudamatsu, Mayako Uchida, Nobuaki Egashira, Daisuke Kobayashi, and Takao Shimazoe. 2022. "Inhibitory Effect of α1 Receptor Antagonists on Paclitaxel-Induced Peripheral Neuropathy in a Rodent Model and Clinical Database" Toxics 10, no. 11: 669. https://doi.org/10.3390/toxics10110669
APA StyleMori, K., Kawashiri, T., Mine, K., Inoue, M., Kudamatsu, H., Uchida, M., Egashira, N., Kobayashi, D., & Shimazoe, T. (2022). Inhibitory Effect of α1 Receptor Antagonists on Paclitaxel-Induced Peripheral Neuropathy in a Rodent Model and Clinical Database. Toxics, 10(11), 669. https://doi.org/10.3390/toxics10110669





