Leveraging Multiple Data Streams for Prioritization of Mixtures for Hazard Characterization
Abstract
1. Introduction
1.1. Complexity of Studying Environmental Mixtures
1.1.1. Component-Based Approach
1.1.2. Whole Mixture Approach
1.1.3. Sufficiently Similar or Representative Mixtures
1.2. Exposure to Polycyclic Aromatic Hydrocarbons and Associated Health Effects
1.3. Study Objectives
2. Materials and Methods
2.1. Objective 1: Chemical Prioritization and Mixture Formation
2.1.1. Sample Site, Field Sampling, and Analysis
2.1.2. Collection of Toxicity Metrics
2.1.3. Chemical Prioritization
2.1.4. Correlation of Toxicity Metric Rankings
2.1.5. Chemical Mixture Preparation
2.2. Objective 2: Comparison of Mixture Toxicity
2.2.1. Normal Human Bronchial Epithelium Maintenance
Lactate Dehydrogenase Assay
Cell Titer Glo Assay
Mitochondrial Membrane Potential (MMP) Assay
ROS-Glo Assay
NHBE Quality Control and Statistics
2.2.2. Zebrafish Maintenance and Exposures
Morphological Assay
Zebrafish Quality Control and Statistics
2.2.3. Curve fitting and Point of Departure Calculations
3. Results
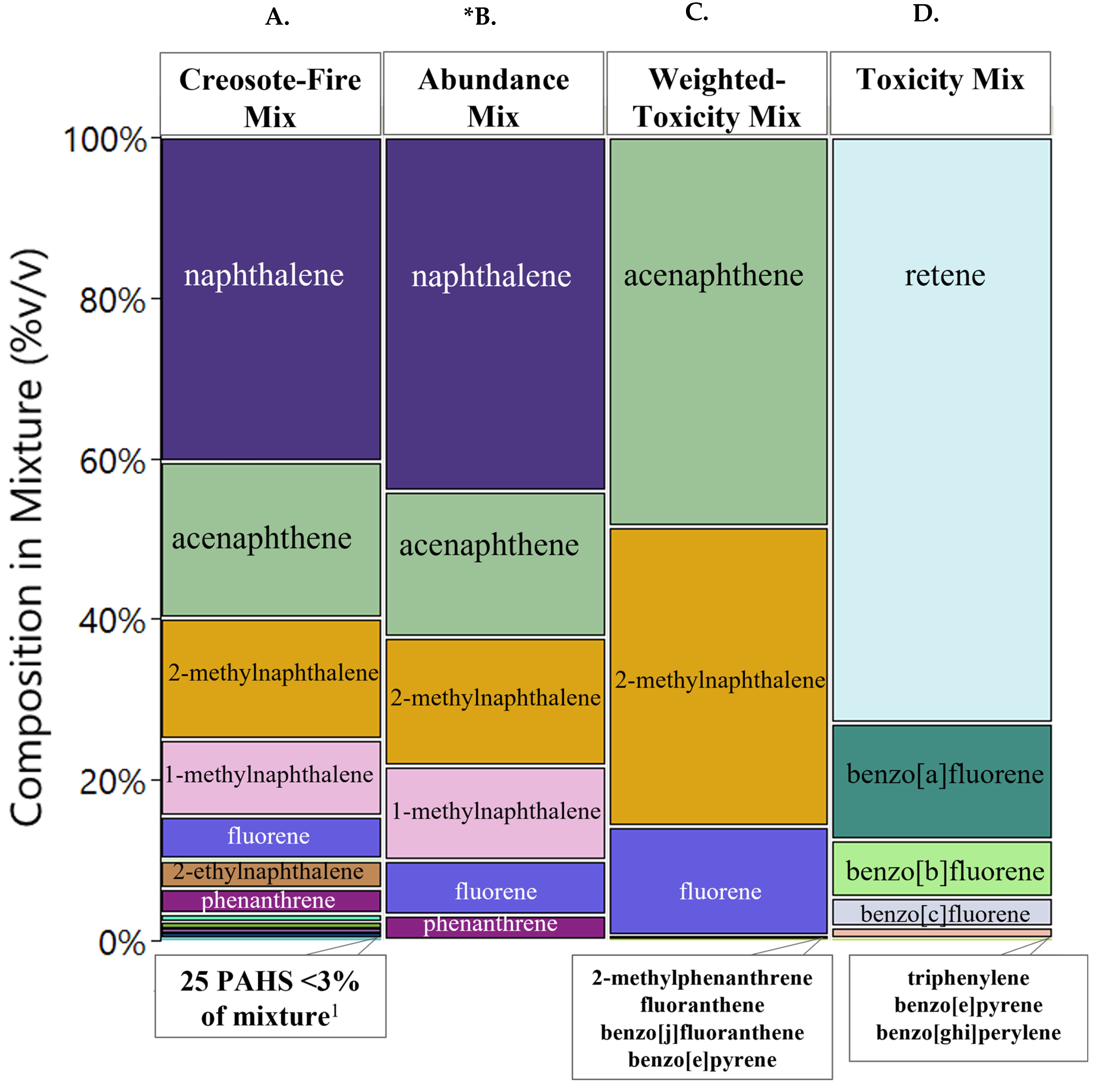
3.1. Objective 1: Chemical Prioritization and Mixture Formation
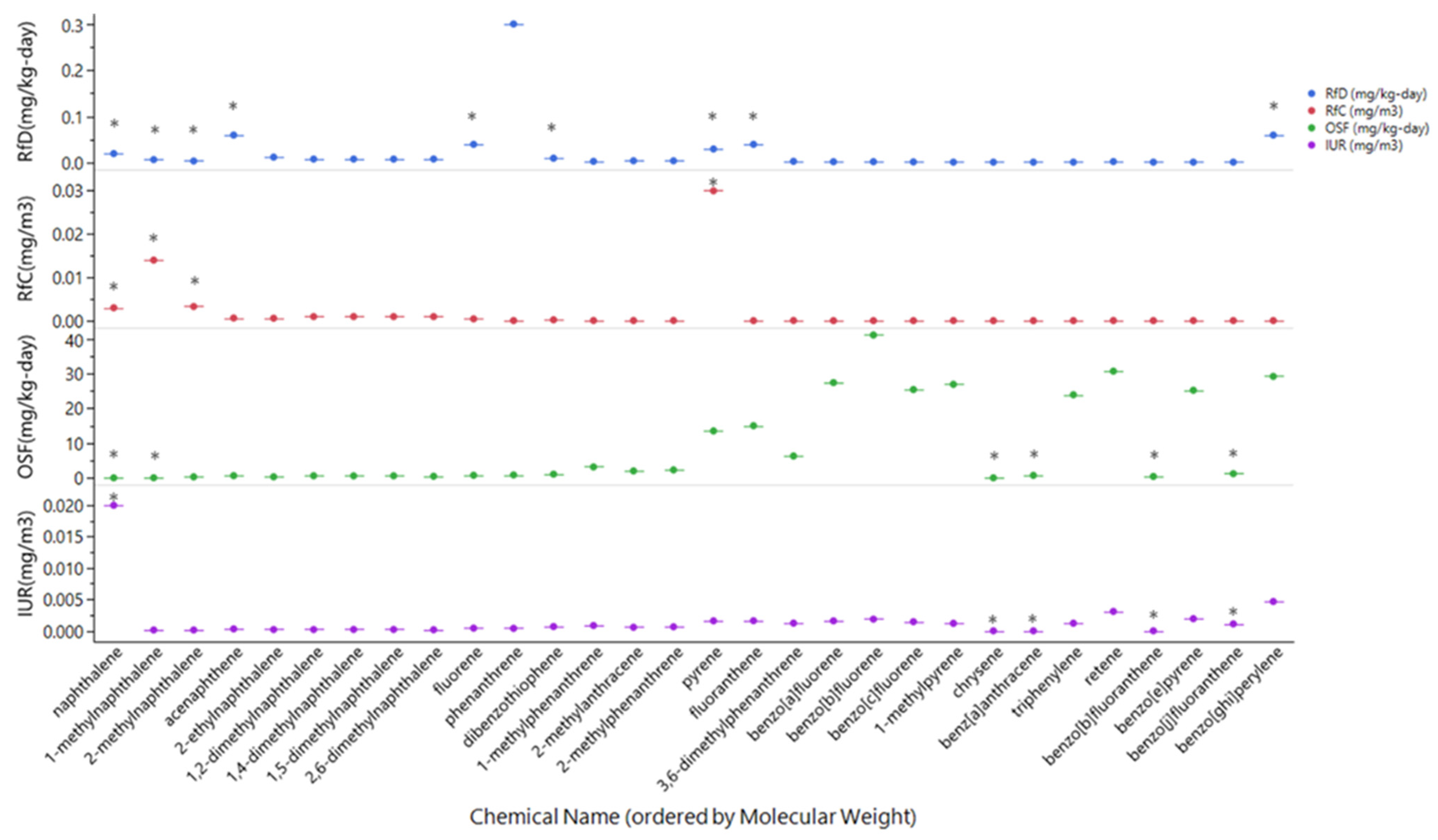
3.1.1. Availability of Toxicity Metrics and Mixture Composition
3.1.2. Comparison of Toxicity Metrics
3.2. Objective 2: Comparison of Mixture Toxicity
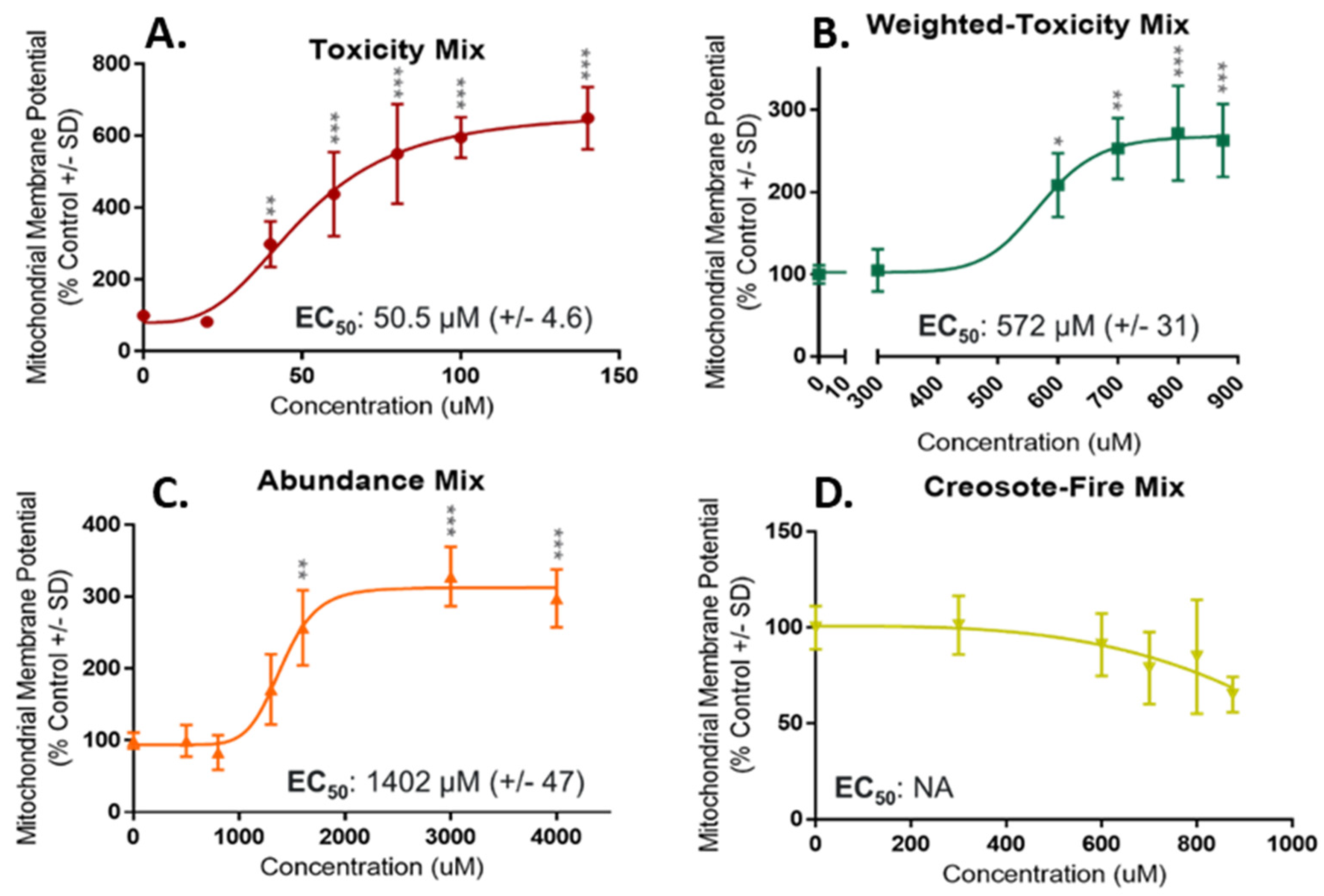
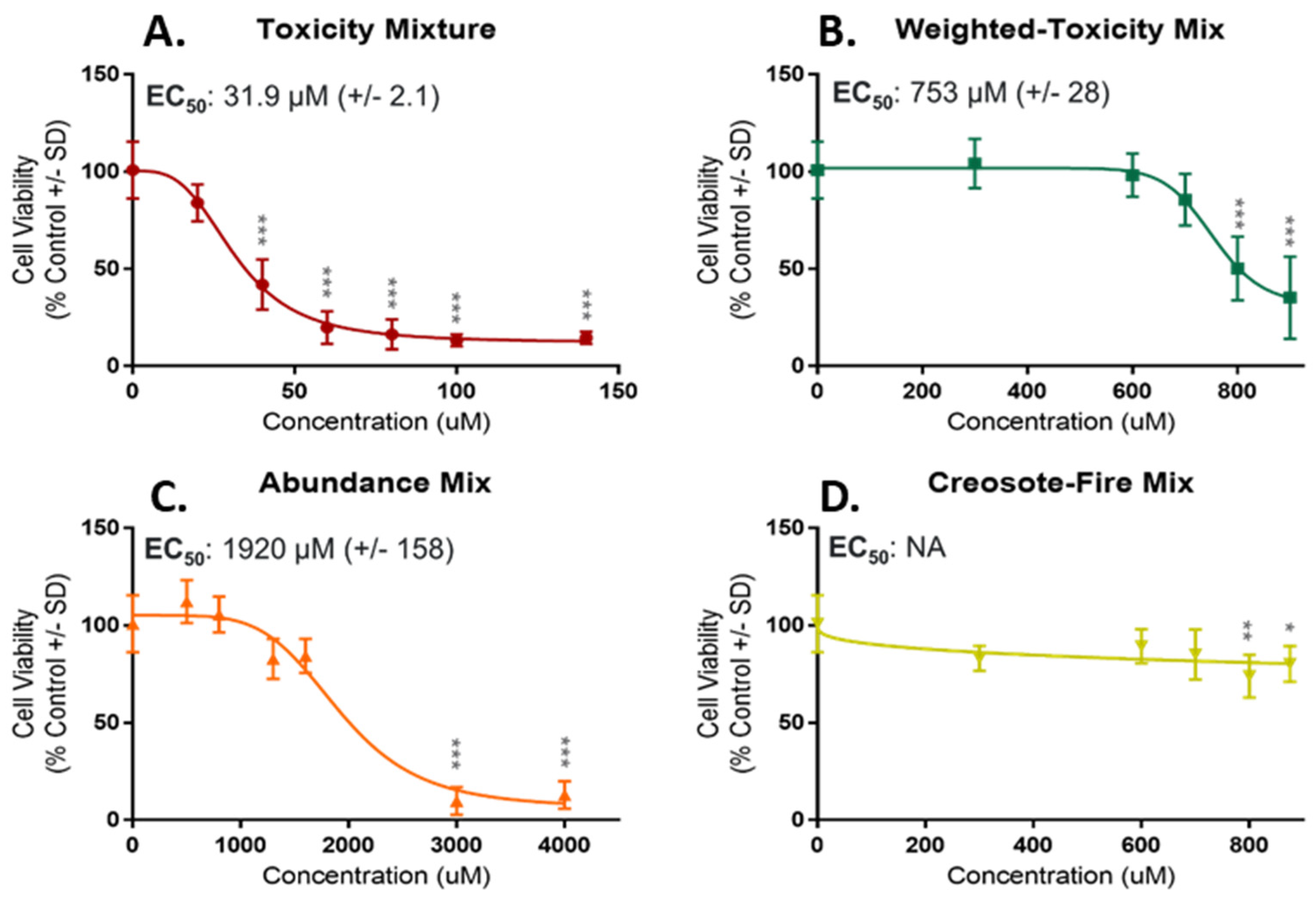
3.2.1. NHBE Bioactivity Screening and Mixture Potency
3.2.2. Zebrafish Bioactivity Screening and Mixture Potency
4. Discussion
4.1. Chemical Prioritization Appraoches
4.2. Comparison and Availability of Toxicity Values
4.3. Comparison of Mixture Composition and Toxicity
4.4. Mixture Formation Implications and Potential Mixture Interactions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carlin, D.J.; Rider, C.V.; Woychik, R.; Birnbaum, L.S. Unraveling the Health Effects of Environmental Mixtures: An NIEHS Priority. Environ. Health Perspect. 2013, 121, a6–a8. [Google Scholar] [CrossRef] [PubMed]
- Feron, V.J.; Groten, J.P. Toxicological evaluation of chemical mixtures. Food Chem. Toxicol. 2002, 40, 825–839. [Google Scholar] [CrossRef]
- Gibson, E.A.; Goldsmith, J.; Kioumourtzoglou, M.-A. Complex Mixtures, Complex Analyses: An Emphasis on Interpretable Results. Curr. Envir. Health Rep. 2019, 6, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Heys, K.A.; Shore, R.F.; Pereira, M.G.; Jones, K.C.; Martin, F.L.; Martin, F. Risk assessment of environmental mixture effects. RSC Adv. 2016, 6, 47844–47857. [Google Scholar] [CrossRef]
- Arnold, C. Mix Masters: Using a New Tool to Identify Commonly Occurring Chemical Mixtures. Environ. Health Perspect. 2017, 125, 124002. [Google Scholar] [CrossRef] [PubMed]
- Bopp, S.K.; Kienzler, A.; Richarz, A.-N.; Linden SC van der Paini, A.; Parissis, N.; Andrew, P. Regulatory assessment and risk management of chemical mixtures: Challenges and ways forward. Crit. Rev. Toxicol. 2019, 49, 174–189. [Google Scholar] [CrossRef]
- Kapraun, D.F.; Wambaugh, J.F.; Ring, C.L.; Tornero-Velez, R.S.R.W. A Method for Identifying Prevalent Chemical Combinations in the U.S. Population. Environ. Health Perspect. 2017, 125, 087017. [Google Scholar] [CrossRef]
- Arnot, J.A.; Brown, T.N.; Wania, F.; Breivik, K.; McLachlan, M.S. Prioritizing Chemicals and Data Requirements for Screening-Level Exposure and Risk Assessment. Environ. Health Perspect. 2012, 120, 1565–1570. [Google Scholar] [CrossRef]
- Marshall, S.; Gennings, C.; Teuschler, L.K.; Stork, L.G.; Tornero-Velez, R.; Crofton, K.M.; Rice, G.E. An Empirical Approach to Sufficient Similarity: Combining Exposure Data and Mixtures Toxicology Data. Risk Anal. 2013, 33, 1582–1595. [Google Scholar] [CrossRef]
- Bornehag, C.G.; Kitraki, E.; Stamatakis, A.; Panagiotidou, E.; Rudén, C.; Shu, H.; Lindh, C.; Ruegg, J.; Gennings, C. A Novel Approach to Chemical Mixture Risk Assessment—Linking Data from Population-Based Epidemiology and Experimental Animal Tests. Risk Anal. 2019, 39, 2259–2271. [Google Scholar] [CrossRef]
- Mitchell, J.; Arnot, J.A.; Jolliet, O.; Georgopoulos, P.G.; Isukapalli, S.; Dasgupta, S.; Pandian, M.; Wambaugh, J.; Egeghy, P.; Cohen Hubal, E.A.; et al. Comparison of modeling approaches to prioritize chemicals based on estimates of exposure and exposure potential. Sci. Total Environ. 2013, 458–460, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.-H.; Sedykh, A.; Mutlu, E.; Germolec, D.R.; Auerbach, S.S.; Rider, C.V. Harnessing In Silico, In Vitro, and In Vivo Data to Understand the Toxicity Landscape of Polycyclic Aromatic Compounds (PACs). Chem. Res. Toxicol. 2021, 34, 268–285. [Google Scholar] [CrossRef]
- Baldwin, A.K.; Corsi, S.R.; Stefaniak, O.M.; Loken, L.C.; Villeneuve, D.L.; Ankley, G.T.; Blackwell, B.R.; Lenaker, P.L.; Nott, M.A.; Mills, M.A. Risk-Based Prioritization of Organic Chemicals and Locations of Ecological Concern in Sediment from Great Lakes Tributaries. Environ. Toxicol. Chem. 2022, 41, 1016–1041. [Google Scholar] [CrossRef]
- Rider, C.V.; McHale, C.M.; Webster, T.F.; Lowe, L.; Goodson, W.H.; La, M.M.A.; Rice, G.; Zeise, L.; Zhang, L.; Smith, M.T. Using the Key Characteristics of Carcinogens to Develop Research on Chemical Mixtures and Cancer. Environ. Health Perspect. 2021, 129, 035003. [Google Scholar] [CrossRef]
- Altenburger, R.; Backhaus, T.; Boedeker, W.; Faust, M.; Scholze, M. Simplifying complexity: Mixture toxicity assessment in the last 20 years. Environ. Toxicol. Chem. 2013, 32, 1685–1687. [Google Scholar] [CrossRef]
- Chang, Y.; Rager, J.E.; Tilton, S.C. Linking Coregulated Gene Modules with Polycyclic Aromatic Hydrocarbon-Related Cancer Risk in the 3D Human Bronchial Epithelium. Chem. Res. Toxicol. 2021, 34, 1445–1455. [Google Scholar] [CrossRef]
- McCarthy, C.J.; Roark, S.A.; Middleton, E.T. Considerations for toxicity experiments and risk assessments with PFAS mixtures. Integr. Environ. Assess. Manag. 2021, 17, 697–704. [Google Scholar] [CrossRef]
- Hertzberg, R.C.; Mumtaz, M.M. Component-Based Risk Assessment Approaches with Additivity and Interactions. In Chemical Mixtures and Combined Chemical and Nonchemical Stressors: Exposure, Toxicity, Analysis, and Risk; Rider, C.V., Simmons, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 369–419. [Google Scholar] [CrossRef]
- Hernández, A.F.; Tsatsakis, A.M. Human exposure to chemical mixtures: Challenges for the integration of toxicology with epidemiology data in risk assessment. Food Chem. Toxicol. 2017, 103, 188–193. [Google Scholar] [CrossRef]
- Hayes, A.W.; Li, R.; Hoeng, J.; Iskandar, A.; Peistch, M.C.; Dourson, M.L. New approaches to risk assessment of chemical mixtures. Toxicol. Res. Appl. 2019, 3, 2397847318820768. [Google Scholar] [CrossRef]
- Teuschler, L.K. Deciding which chemical mixtures risk assessment methods work best for what mixtures. Toxicol. Appl. Pharmacol. 2007, 223, 139–147. [Google Scholar] [CrossRef]
- US EPA. Guidelines for the Health Risk Assessment of Chemical Mixtures. 1986. Available online: https://www.epa.gov/risk/guidelines-health-risk-assessment-chemical-mixtures (accessed on 17 May 2022).
- US EPA National Center for Environmental Assessment CO. Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures. n.d. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=20533 (accessed on 9 April 2021).
- Cizmas, L.; McDonald, T.J.; Phillips, T.D.; Gillespie, A.M.; Lingenfelter, R.A.; Kubena, L.F.; Phillips, T.D.; Donnelly, K.C. Toxicity Characterization of Complex Mixtures Using Biological and Chemical Analysis in Preparation for Assessment of Mixture Similarity. Environ. Sci. Technol. 2004, 38, 5127–5133. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Buha, A.; Constantin, C.; Wallace, D.R.; Sarigiannis, D.; Neagu, M.; Antonijevic, B.; Hayes, A.W.; Wilks, M.F.; Tsatsakis, A. Critical assessment and integration of separate lines of evidence for risk assessment of chemical mixtures. Arch. Toxicol. 2019, 93, 2741–2757. [Google Scholar] [CrossRef]
- Groten, J.P.; Feron, V.J.; Sühnel, J. Toxicology of simple and complex mixtures. Trends Pharmacol. Sci. 2001, 22, 316–322. [Google Scholar] [CrossRef]
- Bergmann, A.J.; Tanguay, R.L.; Anderson, K.A. Using passive sampling and zebrafish to identify developmental toxicants in complex mixtures. Environ. Toxicol. Chem. 2017, 36, 2290–2298. [Google Scholar] [CrossRef]
- Brack, W. (Ed.) Effect-Directed Analysis of Complex Environmental Contamination; Springer: Berlin/Heidelberg, Germany, 2011; Volume 15. [Google Scholar] [CrossRef]
- Brack, W.; Schmitt-Jansen, M.; Machala, M.; Brix, R.; Barceló, D.; Schymanski, E.; Streck, G.; Schulze, T. How to confirm identified toxicants in effect-directed analysis. Anal. Bioanal. Chem. 2008, 390, 1959–1973. [Google Scholar] [CrossRef]
- Rivera, B.N.; Wilson, L.B.; Kim, D.N.; Pande, P.; Anderson, K.A.; Tilton, S.C.; Tanguay, R.L. A Comparative Multi-System Approach to Characterizing Bioactivity of Commonly Occurring Chemicals. Int. J. Environ. Res. Public Health 2022, 19, 3829. [Google Scholar] [CrossRef]
- Rice, G.E.; Teuschler, L.K.; Bull, R.J.; Simmons, J.E.; Feder, P.I. Evaluating the Similarity of Complex Drinking-Water Disinfection By-Product Mixtures: Overview of the Issues. J. Toxicol. Environ. Health Part A 2009, 72, 429–436. [Google Scholar] [CrossRef]
- Borgert, C.J. Chemical Mixtures: An Unsolvable Riddle? Hum. Ecol. Risk Assess. Int. J. 2004, 10, 619–629. [Google Scholar] [CrossRef]
- East, A.; Anderson, R.H.; Salice, C.J. Per- and Polyfluoroalkyl Substances (PFAS) in Surface Water Near US Air Force Bases: Prioritizing Individual Chemicals and Mixtures for Toxicity Testing and Risk Assessment. Environ. Toxicol. Chem. 2021, 40, 859–870. [Google Scholar] [CrossRef]
- Jakobs, G.; Krüger, J.; Schüttler, A.; Altenburger, R.; Busch, W. Mixture toxicity analysis in zebrafish embryo: A time and concentration resolved study on mixture effect predictivity. Environ. Sci. Eur. 2020, 32, 143. [Google Scholar] [CrossRef]
- Ball, N.; Bars, R.; Botham, P.A.; Cuciureanu, A.; Cronin, M.T.D.; Doe, J.E.; Dudzina, T.; Gant, T.W.; Leist, M.; van Ravenzwaay, B. A framework for chemical safety assessment incorporating new approach methodologies within REACH. Arch. Toxicol. 2022, 96, 743–766. [Google Scholar] [CrossRef]
- Kim, K.-H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- World Health Organization. Regional Office for Europe. Air Quality Guidelines for Europe; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2000. [Google Scholar]
- Lawal, A.T. Polycyclic aromatic hydrocarbons. A review. Cogent Environ. Sci. 2017, 3, 1339841. [Google Scholar] [CrossRef]
- Nikolaou, K.; Masclet, P.; Mouvier, G. Sources and chemical reactivity of polynuclear aromatic hydrocarbons in the atmosphere—A critical review. Sci. Total Environ. 1984, 32, 103–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, S. Global atmospheric emission inventory of polycyclic aromatic hydrocarbons (PAHs) for 2004. Atmos. Environ. 2009, 43, 812–819. [Google Scholar] [CrossRef]
- Karyab, H.; Yunesian, M.; Nasseri, S.; Mahvi, A.H.; Ahmadkhaniha, R.; Rastkari, N.; Nabizadeh, R. Polycyclic Aromatic Hydrocarbons in drinking water of Tehran, Iran. J. Environ. Health Sci. Eng. 2013, 11, 25. [Google Scholar] [CrossRef]
- Li, H.; Chen, J.; Jiang, L. Elevated critical micelle concentration in soil–water system and its implication on PAH removal and surfactant selecting. Environ. Earth Sci. 2014, 71, 3991–3998. [Google Scholar] [CrossRef]
- Primbs, T.; Piekarz, A.; Wilson, G.; Schmedding, D.; Higginbotham, C.; Field, J.; Simonich, S.M. Influence of Asian and Western United States Urban Areas and Fires on the Atmospheric Transport of Polycyclic Aromatic Hydrocarbons, Polychlorinated Biphenyls, and Fluorotelomer Alcohols in the Western United States. Environ. Sci. Technol. 2008, 42, 6385–6391. [Google Scholar] [CrossRef]
- Yuan, H.; Tao, S.; Li, B.; Lang, C.; Cao, J.; Coveney, R.M. Emission and outflow of polycyclic aromatic hydrocarbons from wildfires in China. Atmos. Environ. 2008, 42, 6828–6835. [Google Scholar] [CrossRef]
- Kramer, A.L.; Campbell, L.; Donatuto, J.; Heidt, M.; Kile, M.; Massey Simonich, S.L. Impact of local and regional sources of PAHs on tribal reservation air quality in the U.S. Pacific Northwest. Sci. Total Environ. 2020, 710, 136412. [Google Scholar] [CrossRef]
- Menzie, C.A.; Potocki, B.B.; Santodonato, J. Exposure to carcinogenic PAHs in the environment. Environ. Sci. Technol. 1992, 26, 1278–1284. [Google Scholar] [CrossRef]
- Choi, H.; Harrison, R.; Komulainen, H.; Saborit, J.M.D. Polycyclic Aromatic Hydrocarbons; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic Aromatic Hydrocarbons: From Metabolism to Lung Cancer. Toxicol. Sci. 2015, 145, 5–15. [Google Scholar] [CrossRef]
- Cakmak, S.; Hebbern, C.; Cakmak, J.D.; Dales, R.E. The influence of polycyclic aromatic hydrocarbons on lung function in a representative sample of the Canadian population. Environ. Pollut. 2017, 228, 1–7. [Google Scholar] [CrossRef]
- Mu, G.; Fan, L.; Zhou, Y.; Liu, Y.; Ma, J.; Yang, S.; Wang, B.; Xiao, L.; Ye, Z.; Shi, T.; et al. Personal exposure to PM2.5-bound polycyclic aromatic hydrocarbons and lung function alteration: Results of a panel study in China. Sci. Total Environ. 2019, 684, 458–465. [Google Scholar] [CrossRef]
- Boffetta, P.; Jourenkova, N.; Gustavsson, P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control. 1997, 8, 444–472. [Google Scholar] [CrossRef]
- Winker, N.; Tuschl, H.; Kovac, R.; Weber, E. Immunological Investigations in a Group of Workers Exposed to Various Levels of Polycyclic Aromatic Hydrocarbons. J. Appl. Toxicol. 1997, 17, 23–29. [Google Scholar] [CrossRef]
- Alhamdow, A.; Lindh, C.; Albin, M.; Gustavsson, P.; Tinnerberg, H.; Broberg, K. Cardiovascular Disease-Related Serum Proteins in Workers Occupationally Exposed to Polycyclic Aromatic Hydrocarbons. Toxicol. Sci. 2019, 171, 235–246. [Google Scholar] [CrossRef]
- Perera, F.P.; Whyatt, R.M.; Jedrychowski, W.; Rauh, V.; Manchester, D.; Santella, R.M.; Ottman, R. Recent Developments in Molecular Epidemiology: A Study of the Effects of Environmental Polycyclic Aromatic Hydrocarbons on Birth Outcomes in Poland. Am. J. Epidemiol. 1998, 147, 309–314. [Google Scholar] [CrossRef]
- Dejmek, J.; Solanský, I.; Benes, I.; Lenícek, J.; Srám, R.J. The impact of polycyclic aromatic hydrocarbons and fine particles on pregnancy outcome. Environ. Health Perspect. 2000, 108, 1159–1164. [Google Scholar] [CrossRef]
- Perera, F.; Herbstman, J. Prenatal environmental exposures, epigenetics, and disease. Reprod. Toxicol. 2011, 31, 363–373. [Google Scholar] [CrossRef]
- Perera, F.P.; Chang, H.; Tang, D.; Roen, E.L.; Herbstman, J.; Margolis, A.; Huang, T.J.; Miller, R.L.; Wang, S.; Rauh, V. Early Life Exposure to Polycyclic Aromatic Hydrocarbons and ADHD Behavior Problems. PLoS ONE 2014, 9, e111670. [Google Scholar] [CrossRef] [PubMed]
- Ghetu, C.C.; Scott, R.P.; Wilson, G.; Liu-May, R.; Anderson, K.A. Improvements in identification and quantitation of alkylated PAHs and forensic ratio sourcing. Anal. Bioanal. Chem. 2021, 413, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Minick, D.J.; Anderson, K.A. Diffusive flux of PAHs across sediment–water and water–air interfaces at urban superfund sites. Environ. Toxicol. Chem. 2017, 36, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.A.; Johnson, E. Bioavailable Organochlorine Pesticides in a Semi-Arid Region of Eastern Oregon, USA, as Determined by Gas Chromatography with Electron-Capture Detection. J. AOAC Int. 2001, 84, 1371–1382. [Google Scholar] [CrossRef]
- Anderson, K.A.; Szelewski, M.J.; Wilson, G.; Quimby, B.D.; Hoffman, P.D. Modified ion source triple quadrupole mass spectrometer gas chromatograph for polycyclic aromatic hydrocarbon analyses. J. Chromatogr. A 2015, 1419, 89–98. [Google Scholar] [CrossRef]
- Paulik, L.B.; Donald, C.E.; Smith, B.W.; Tidwell, L.G.; Hobbie, K.A.; Kincl, L.; Haynes, E.N.; Anderson, K.A. Emissions of Polycyclic Aromatic Hydrocarbons from Natural Gas Extraction into Air. Environ. Sci. Technol. 2016, 50, 7921–7929. [Google Scholar] [CrossRef]
- Anastasopoulos, A.T.; Wheeler, A.J.; Karman, D.; Kulka, R.H. Intraurban concentrations, spatial variability and correlation of ambient polycyclic aromatic hydrocarbons (PAH) and PM2.5. Atmos. Environ. 2012, 59, 272–283. [Google Scholar] [CrossRef]
- Assessment UENC for, E. IRIS Assements | IRIS | US EPA. Available online: https://iris.epa.gov/AtoZ/?list_type=alpha (accessed on 14 July 2022).
- Williams, A.J.; Grulke, C.M.; Edwards, J.; McEachran, A.D.; Mansouri, K.; Baker, N.C.; Patlewicz, G.; Shah, I.; Wambaugh, J.F.; Judson, R.S.; et al. The CompTox Chemistry Dashboard: A community data resource for environmental chemistry. J. Cheminform. 2017, 9, 61. [Google Scholar] [CrossRef]
- Office of Superfund Remediation and Technology Innovation. Human Health Toxicity Values in Superfund Risk Assessments; United States Environmental Protection Agency: Washington, DC, USA, 2003.
- Wignall, J.A.; Muratov, E.; Sedykh, A.; Guyton, K.Z.; Tropsha, A.; Rusyn, I.; Chiu, W.A. Conditional Toxicity Value (CTV) Predictor: An In Silico Approach for Generating Quantitative Risk Estimates for Chemicals. Environ. Health Perspect. 2018, 126, 057008. [Google Scholar] [CrossRef]
- Shankar, P.; Geier, M.C.; Truong, L.; McClure, R.S.; Pande, P.; Waters, K.M.; Tanguay, R.L. Coupling Genome-wide Transcriptomics and Developmental Toxicity Profiles in Zebrafish to Characterize Polycyclic Aromatic Hydrocarbon (PAH) Hazard. Int. J. Mol. Sci. 2019, 20, 2570. [Google Scholar] [CrossRef]
- List of Classifications—IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 4 April 2022).
- Samburova, V.; Zielinska, B.; Khlystov, A. Do 16 Polycyclic Aromatic Hydrocarbons Represent PAH Air Toxicity? Toxics 2017, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, I.C.; LaGoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Office of Environmental Health Hazard Assessment. California EPA Air Toxics Report; California Environmental Protection Agency: Sacremento, CA, USA, 1999.
- Ritz, C.; Streibig, J.C. Bioassay Analysis Using, R.J. Stat. Softw. 2005, 12, 1–22. [Google Scholar] [CrossRef]
- Kassambara. ggcorrplot. 2019. Available online: https://cran.r-project.org/web/packages/ggcorrplot/index.html (accessed on 2 April 2022).
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Mandrell, D.; Truong, L.; Jephson, C.; Sarker, M.R.; Moore, A.; Lang, C.; Simonich, M.T.; Tanguay, R.L. Automated zebrafish chorion removal and single embryo placement: Optimizing throughput of zebrafish developmental toxicity screens. J. Lab. Autom. 2012, 17, 66–74. [Google Scholar] [CrossRef]
- Truong, L.; Harper, S.L.; Tanguay, R.L. Evaluation of Embryotoxicity Using the Zebrafish Model. In Drug Safety Evaluation: Methods and Protocols; Gautier, J.-C., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 271–279. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Truong, L.; Reif, D.M.; St Mary, L.; Geier, M.C.; Truong, H.D.; Tanguay, R.L. Multidimensional in vivo hazard assessment using zebrafish. Toxicol. Sci. 2014, 137, 212–233. [Google Scholar] [CrossRef]
- GraphPad/Prism. Available online: https://www.graphpad.com/ (accessed on 26 May 2022).
- EPA. Integrated Risk Information System. Available online: https://www.epa.gov/iris (accessed on 10 June 2018).
- Geier, M.C.; James Minick, D.; Truong, L.; Tilton, S.; Pande, P.; Anderson, K.A.; Teeguardan, J.; Tanguay, R.L. Systematic developmental neurotoxicity assessment of a representative PAH Superfund mixture using zebrafish. Toxicol. Appl. Pharmacol. 2018, 354, 115–125. [Google Scholar] [CrossRef]
- Altenburger, R.; Backhaus, T.; Boedeker, W.; Faust, M.; Scholze, M.; Grimme, L.H. Predictability of the toxicity of multiple chemical mixtures to Vibrio fischeri: Mixtures composed of similarly acting chemicals. Environ. Toxicol. Chem. 2000, 19, 2341–2347. [Google Scholar] [CrossRef]
- Olmstead, A.W.; LeBlanc, G.A. Toxicity Assessment of Environmentally Relevant Pollutant Mixtures Using a Heuristic Model. Integr. Environ. Assess. Manag. 2005, 1, 114–122. [Google Scholar] [CrossRef]
- Bates, E.R.; Sahle-Demessie, E.; Grosse, D.W. Solidification/stabilization for remediation of wood preserving sites: Treatment for dioxins, PCP, creosote, and metals. Remediat. J. 2000, 10, 51–65. [Google Scholar] [CrossRef]
- Kelly, R.L.; Bian, X.; Feakins, S.J.; Fornace, K.L.; Gunderson, T.; Hawco, N.J.; Liang, H.; Niggemann, J.; Paulson, S.E.; Pinedo-Gonzalez, P.; et al. Delivery of Metals and Dissolved Black Carbon to the Southern California Coastal Ocean via Aerosols and Floodwaters Following the 2017 Thomas Fire. J. Geophys. Res. Biogeosciences 2021, 126, e2020JG006117. [Google Scholar] [CrossRef]
- Sparks, T.L.; Wagner, J. Composition of particulate matter during a wildfire smoke episode in an urban area. Aerosol Sci. Technol. 2021, 55, 734–747. [Google Scholar] [CrossRef]
- Wentworth, G.R.; Aklilu, Y.; Landis, M.S.; Hsu, Y.-M. Impacts of a large boreal wildfire on ground level atmospheric concentrations of PAHs, VOCs and ozone. Atmos. Environ. 2018, 178, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Ahtiainen, J.; Valo, R.; Järvinen, M.; Joutti, A. Microbial Toxicity Tests and Chemical Analysis as Monitoring Parameters at Composting of Creosote-Contaminated Soil. Ecotoxicol. Environ. Saf. 2002, 53, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Melber, C. International Programme on Chemical Safety, Eds. Coal Tar Creosote; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Da Silva Junior, F.C.; Felipe MB, M.C.; de Castro DE, F.; da Silva Araujo, S.C.; Sisenando HC, N.; de Medeiros, S.R.B. A look beyond the priority: A systematic review of the genotoxic, mutagenic, and carcinogenic endpoints of non-priority PAHs. Environ. Pollut. 2021, 278, 116838. [Google Scholar] [CrossRef]
- Andersson, J.T.; Achten, C. Time to Say Goodbye to the 16 EPA PAHs? Toward an Up-to-Date Use of PACs for Environmental Purposes. Polycycl. Aromat. Compd. 2015, 35, 330–354. [Google Scholar] [CrossRef]
- Hussar, E.; Richards, S.; Lin, Z.-Q.; Dixon, R.P.; Johnson, K.A. Human Health Risk Assessment of 16 Priority Polycyclic Aromatic Hydrocarbons in Soils of Chattanooga, Tennessee, USA. Water Air Soil Pollut. 2012, 223, 5535–5548. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. 6—The interactions of microplastics and chemical pollutants. In Microplastic Pollutants; Crawford, C.B., Quinn, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 131–157. [Google Scholar] [CrossRef]
- Syed, K.; Porollo, A.; Lam, Y.W.; Grimmett, P.E.; Yadav, J.S. CYP63A2, a Catalytically Versatile Fungal P450 Monooxygenase Capable of Oxidizing Higher-Molecular-Weight Polycyclic Aromatic Hydrocarbons, Alkylphenols, and Alkanes. Appl. Environ. Microbiol. 2013, 79, 2692–2702. [Google Scholar] [CrossRef]
- Ding, Y.S.; Ashley, D.L.; Watson, C.H. Determination of 10 Carcinogenic Polycyclic Aromatic Hydrocarbons in Mainstream Cigarette Smoke. J. Agric. Food Chem. 2007, 55, 5966–5973. [Google Scholar] [CrossRef]
- Board of Environmental Studies and Toxicology. Derivation of Toxicity Values; National Academies Press (US): Washington, DC, USA, 2014. [Google Scholar]
- National Toxicology Program. A Chemical Mixture of 25 Groundwater Contaminants; National Institute of Environmental Health Sciences: Research Triangle Park, NC, USA, 1993.
- National Toxicology Program. Pesticide/Fertilizer Mixtures; National Institute of Environmental Health Sciences: Research Triangle Park, NC, USA, 1993.
- Ke, S.; Liu, Q.; Yao, Y.; Zhang, X.; Sui, G. An in vitro cytotoxicities comparison of 16 priority polycyclic aromatic hydrocarbons in human pulmonary alveolar epithelial cells HPAEpiC. Toxicol. Lett. 2018, 290, 10–18. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wheelock, Å.M.; Morin, D.; Baldwin, R.M.; Lee, M.G.; Taff, A.; Plopper, C.; Buckpitt, A.; Rohde, A. Toxicity and metabolism of methylnaphthalenes: Comparison with naphthalene and 1-nitronaphthalene. Toxicology 2009, 260, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Preuss, R.; Angerer, J.; Drexler, H. Naphthalene—An environmental and occupational toxicant. Int. Arch. Occup. Environ. Health 2003, 76, 556–576. [Google Scholar] [CrossRef] [PubMed]
- Buckpitt, A.; Boland, B.; Isbell, M.; Morin, D.; Shultz, M.; Baldwin, R.; Chan, K.; Karlsson, A.; Lin, C.; Taff, A.; et al. Naphthalene-induced respiratory tract toxicity: Metabolic mechanisms of toxicity. Drug Metab. Rev. 2002, 34, 791–820. [Google Scholar] [CrossRef] [PubMed]
- Sapkal, A.U.; Nashine, S.; Mansoor, S.; Sharma, V.R.; Ramirez, C.A.; Migon, R.Z.; Gupta, N.K.; Chwa, M.; Kuppermann, B.D.; Kenney, M.C. Protective Effects of 17β-Estradiol on Benzo(e) pyrene[B(e)P]-induced Toxicity in ARPE-19 cells. J. Ophthalmic Vis. Res. 2018, 13, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Estrago-Franco, M.F.; Moustafa, M.T.; Riazi-Esfahani, M.; Sapkal, A.U.; Piche-Lopez, R.; Patil, A.J.; Sharma, A.; Falatoonzadeh, P.; Chwa, M.; Luczy-Bachman, G.; et al. Effects of Benzo(e)pyrene on Reactive Oxygen/Nitrogen Species and Inflammatory Cytokines Induction in Human RPE Cells and Attenuation by Mitochondrial-involved Mechanism. J. Ophthalmic Vis. Res. 2016, 11, 385–393. [Google Scholar] [CrossRef]
- Platt, K.L.; Aderhold, S.; Kulpe, K.; Fickler, M. Unexpected DNA damage caused by polycyclic aromatic hydrocarbons under standard laboratory conditions. Mutat. Res. 2008, 650, 96–103. [Google Scholar] [CrossRef]
- Sharma, A.; Neekhra, A.; Gramajo, A.L.; Patil, J.; Chwa, M.; Kuppermann, B.D.; Kenney, M.C. Effects of Benzo(e)Pyrene, a toxic component of cigarette smoke, on human retinal pigment epithelial cells in vitro. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5111–5117. [Google Scholar] [CrossRef]
- Lau, H.H.S.; Baird, W.M. The co-carcinogen benzo[e]pyrene increases the binding of a low dose of the carcinogen benzo[a]pyrene to DNA in Sencar mouse epidermis. Cancer Lett. 1992, 63, 229–236. [Google Scholar] [CrossRef]
- Fang, M.; Getzinger, G.J.; Cooper, E.M.; Clark, B.W.; Garner, L.V.T.; Di Giulio, R.T.; Ferguson, P.L.; Stapleton, H.M. Effect-directed analysis of Elizabeth River porewater: Developmental toxicity in zebrafish (Danio rerio): Effect-directed analysis of teratogenesis in porewater. Environ. Toxicol. Chem. 2014, 33, 2767–2774. [Google Scholar] [CrossRef]
- Courter, L.A.; Musafia-Jeknic, T.; Fischer, K.; Bildfell, R.; Giovanini, J.; Pereira, C.; Baird, W.M. Urban Dust Particulate Matter Alters PAH-Induced Carcinogenesis by Inhibition of CYP1A1 and CYP1B1. Toxicol. Sci. 2007, 95, 63–73. [Google Scholar] [CrossRef]
- Jarvis, I.W.H.; Bergvall, C.; Morales, D.A.; Kummrow, F.; Umbuzeiro, G.A.; Westerholm, R.; Stenius, U.; Dreij, K. Nanomolar levels of PAHs in extracts from urban air induce MAPK signaling in HepG2 cells. Toxicol. Lett. 2014, 229, 25–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- White, P.A. The genotoxicity of priority polycyclic aromatic hydrocarbons in complex mixtures. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2002, 515, 85–98. [Google Scholar] [CrossRef]
- Lemieux, C.L.; Long, A.S.; Lambert, I.B.; Lundstedt, S.; Tysklind, M.; White, P.A. In Vitro Mammalian Mutagenicity of Complex Polycyclic Aromatic Hydrocarbon Mixtures in Contaminated Soils. Environ. Sci. Technol. 2015, 49, 1787–1796. [Google Scholar] [CrossRef]
- US EPA. Estimation Programs Interface SuiteTM for Microsoft® Windows; US EPA: Washington, DC, USA, 2012.


| Toxicity Metrics | Abbreviation | Sources * |
|---|---|---|
| Reference Concentration | RfC | IRIS [64]; EPA Comptox Chemicals Dashboard [65]; CTV [67] |
| Reference Dose | RfD | IRIS [64]; EPA Comptox Chemicals Dashboard [65]; CTV [67] |
| Inhalation Unit Risk | IUR | California EPA [72]; EPA Comptox Chemicals Dashboard [65]; CTV [67] |
| Oral Slope Factor | OSF | California EPA [72]; EPA Comptox Chemicals Dashboard [65]; CTV [67] |
| Cancer Potency Value | CPV | IRIS [64]; CTV [67] |
| Relative Potency Factor | RPF | IRIS [64] |
| Toxic Equivalency Factor | TEF | Samburova 2017 [70] |
| IARC Classification | IARC Class | IARC [69] |
| Zebrafish Benchmark Concentration | Zfish BMC | Shankar et al., 2019 [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, B.N.; Ghetu, C.C.; Chang, Y.; Truong, L.; Tanguay, R.L.; Anderson, K.A.; Tilton, S.C. Leveraging Multiple Data Streams for Prioritization of Mixtures for Hazard Characterization. Toxics 2022, 10, 651. https://doi.org/10.3390/toxics10110651
Rivera BN, Ghetu CC, Chang Y, Truong L, Tanguay RL, Anderson KA, Tilton SC. Leveraging Multiple Data Streams for Prioritization of Mixtures for Hazard Characterization. Toxics. 2022; 10(11):651. https://doi.org/10.3390/toxics10110651
Chicago/Turabian StyleRivera, Brianna N., Christine C. Ghetu, Yvonne Chang, Lisa Truong, Robyn L. Tanguay, Kim A. Anderson, and Susan C. Tilton. 2022. "Leveraging Multiple Data Streams for Prioritization of Mixtures for Hazard Characterization" Toxics 10, no. 11: 651. https://doi.org/10.3390/toxics10110651
APA StyleRivera, B. N., Ghetu, C. C., Chang, Y., Truong, L., Tanguay, R. L., Anderson, K. A., & Tilton, S. C. (2022). Leveraging Multiple Data Streams for Prioritization of Mixtures for Hazard Characterization. Toxics, 10(11), 651. https://doi.org/10.3390/toxics10110651