Synergistic Effect of Charge Separation and Multiple Reactive Oxygen Species Generation on Boosting Photocatalytic Degradation of Fluvastatin by ZnIn2S4/Bi2WO6 Z-Scheme Heterostructured Photocatalytst
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis
2.2.1. Synthesis of ZnIn2S4
2.2.2. Synthesis of Composite Photocatalyst
2.3. Characterization
2.4. Photoeletrochemical Measurement
2.5. Photocatalytic Experiment
2.6. ROS Analysis
2.6.1. In-Situ Capture Experiment
2.6.2. Probe Molecular Transformation
2.7. Analysis Methods
3. Results
3.1. Morphology
3.2. Crystal Phase
3.3. Specific Surface Area and Pore Size Distribution
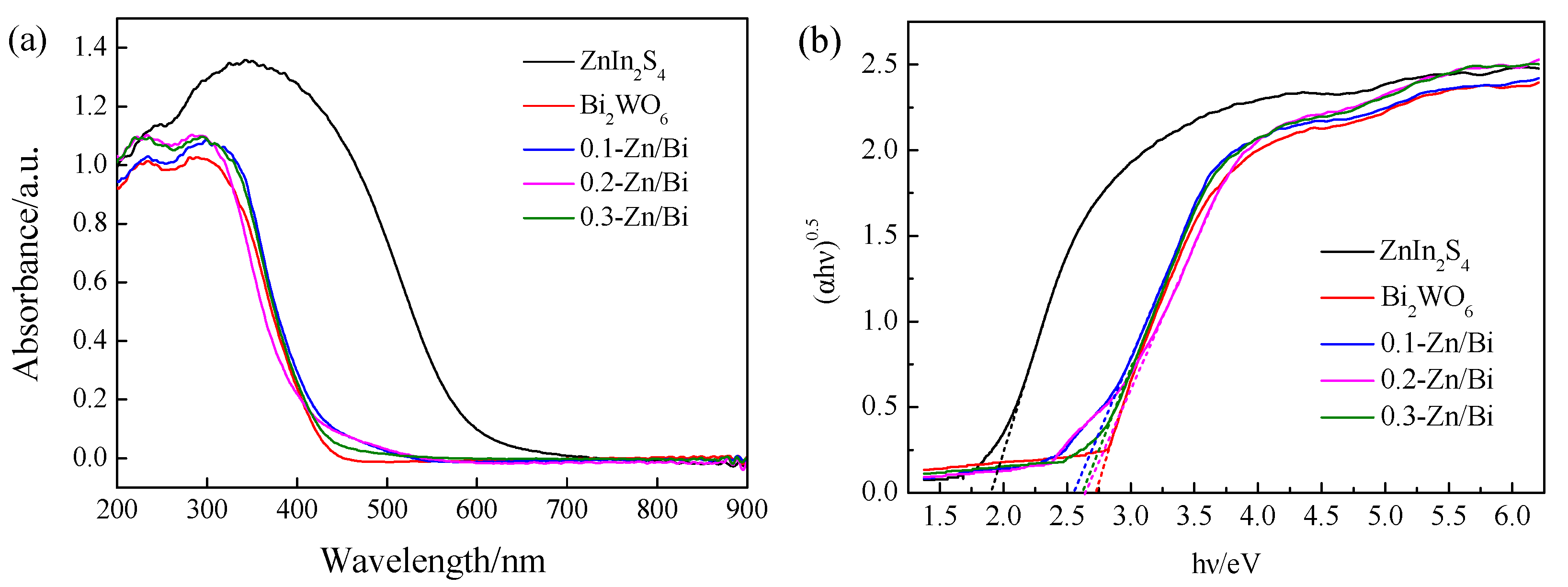
3.4. Optical Properties
3.5. Photoelectrochemical Property
3.6. Photocatalytic Performance
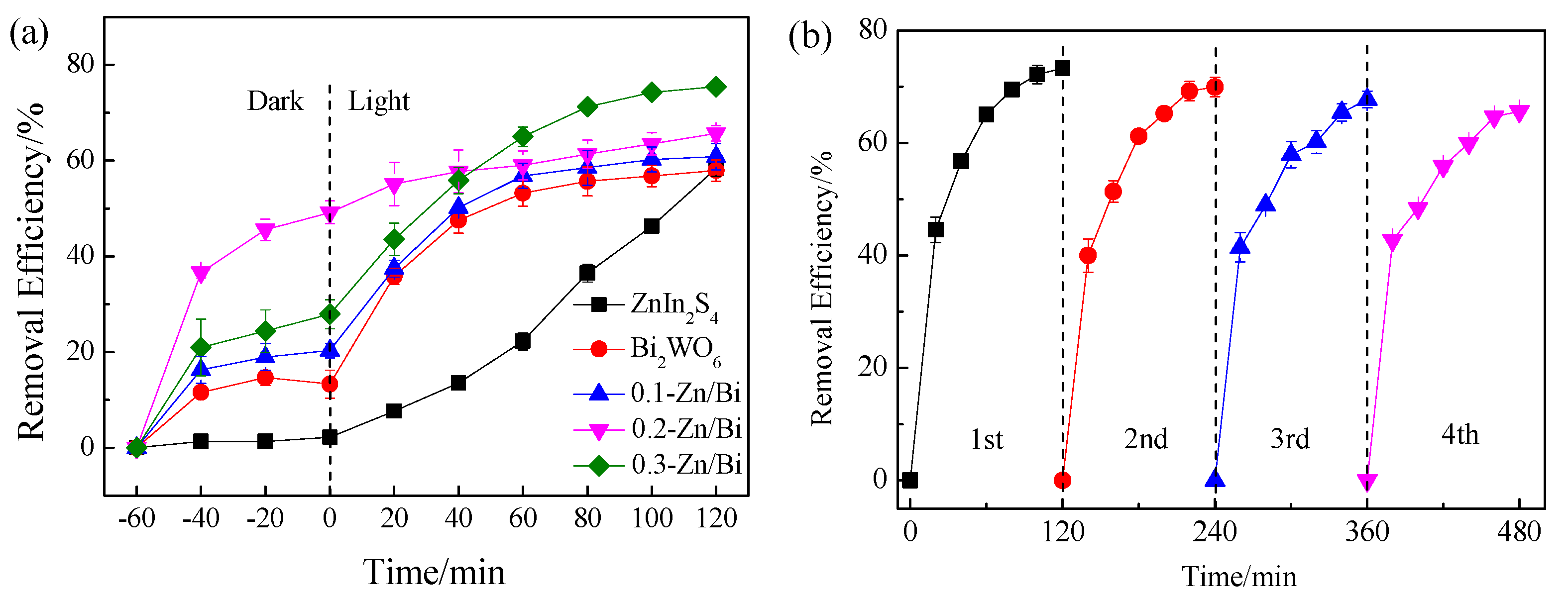
3.6.1. Removal of Fluvastatin
3.6.2. ROS Analysis
3.7. Mechanism for the Enhancement of Photocatalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jo, W.-K.; Lee, J.Y.; Natarajan, T.S. Fabrication of hierarchically structured novel redox-mediator-free ZnIn2S4 marigold flower/Bi2WO6 flower-like direct Z-scheme nanocomposite photocatalysts with superior visible light photocatalytic efficiency. Phys. Chem. Chem. Phys. 2015, 18, 1000–1016. [Google Scholar] [CrossRef]
- Shi, L.; Yin, P.; Dai, Y. Synthesis and photocatalytic performance of ZnIn2S4 nanotubes and nanowires. Langmuir 2013, 29, 12818–12822. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Taheri, A.H.; Mansourpanah, Y. Coupling TiO2 nanoparticles with UV irradiation for modification of polyethersulfone ultrafiltration membranes. J. Membr. Sci. 2008, 313, 158–169. [Google Scholar] [CrossRef]
- You, S.-J.; Semblante, G.U.; Lu, S.-C.; Damodar, R.A.; Wei, T.-C. Evaluation of the antifouling and photocatalytic properties of poly(vinylidene fluoride) plasma-grafted poly(acrylic acid) membrane with self-assembled TiO2. J. Hazard. Mater. 2012, 237–238, 10–19. [Google Scholar] [CrossRef]
- Sakarkar, S.; Muthukumran, S.; Jegatheesan, V. Factors affecting the degradation of remazol turquoise blue (RTB) dye by titanium dioxide (TiO2) entrapped photocatalytic membrane. J. Environ. Manag. 2020, 272, 111090. [Google Scholar] [CrossRef]
- Lei, Z.-D.; Wang, J.-J.; Tang, L.; Yang, X.-Y.; Xu, G. Efficient photocatalytic degradation of ibuprofen in aqueous solution using novel visible-light responsive graphene quantum dot/AgVO3 nanoribbons. J. Hazard. Mater. 2016, 312, 298–306. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J.; Muthupandian, A.; Prabhakarn, A. A review on BiVO4 photocatalyst: Activity enhancement methods for solar photocatalytic applications. Appl. Catal. A Gen. 2018, 555, 47–74. [Google Scholar]
- Tian, F.; Li, G.; Zhao, H.; Chen, F.; Li, M.; Liu, Y.; Chen, R. Residual Fe enhances the activity of BiOCl hierarchical nanostructure for hydrogen peroxide activation. J. Catal. 2019, 370, 265–273. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Li, Q.; Fan, J.; Carabineiro, S.A.; Lv, K. Recent advances on Bismuth-based Photocatalysts: Strategies and mechanisms. Chem. Eng. J. 2021, 419, 129484. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Bismuth-based photocatalytic semiconductors: Introduction, challenges and possible approaches. J. Mol. Catal. A Chem. 2016, 423, 533–549. [Google Scholar] [CrossRef]
- Guan, Y.; Wu, J.; Man, X.; Liu, Q.; Qi, Y.; He, P.; Qi, X. Rational fabrication of flower-like BiOI1-x photocatalyst by modulating efficient iodine vacancies for mercury removal and DFT study. Chem. Eng. J. 2020, 396, 125234. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, G.; Zhang, A.; Tan, Z.; Wang, R.; Wang, H.; Mei, Y.; Zhang, X.; Ran, J. Enhanced photocatalytic activity of Bi24O31Br10 microsheets constructing heterojunction with AgI for Hg0 removal. Sep. Purif. Technol. 2021, 262, 118296. [Google Scholar] [CrossRef]
- Zhan, G.S.; Ding, J.; Zhou, F.Y.; Chen, X.Q.; Wei, L.L.; Gao, Q.W.; Wang, K.; Zhao, Q.L. Construction of a visible-light-driven magnetic dual Z-scheme BiVO4/g-C3N4/NiFe2O4 photocatalyst for effective removal of ofloxacin: Mechanisms and degradation pathway. Chem. Eng. J. 2021, 405, 126704. [Google Scholar]
- Qiu, P.; Yao, J.; Chen, H.; Jiang, F.; Xie, X.C. Enhanced visible-light photocatalytic decomposition of 2,4-dichlorophenoxyacetic acid over ZnIn2S4/g-C3N4 photocatalyst. J. Hazard. Mater. 2017, 317, 158–168. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Sun, C.; Liu, X.; Miao, R.; Lv, Y. A comparison of the photolytic and photocatalytic degradation of fluvastatin. Chem. Eng. J. 2018, 358, 1296–1304. [Google Scholar] [CrossRef]
- Chachvalvutikul, A.; Luangwanta, T.; Pattisson, S.; Hutchings, G.J.; Kaowphong, S. Enhanced photocatalytic degradation of organic pollutants and hydrogen production by a visible light–responsive Bi2WO6/ZnIn2S4 heterojunction. Appl. Surf. Sci. 2021, 544, 148885–148898. [Google Scholar] [CrossRef]
- Bi, H.F.; Liu, J.S.; Wu, Z.Y.; Zhu, K.J.; Suo, H.; Lv, X.L.; Fu, Y.L.; Jian, R.; Sun, Z.B. Construction of Bi2WO6/ZnIn2S4 with Z-scheme structure for efficient photocatalytic performance. Chem. Phys. Lett. 2021, 769, 138449–138507. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.Z.; Hao, J.Y.; Zhang, T.Y.; Sun, Q.; Wang, Y. Boosted charge transfer in dual Z-scheme BiVO4@ZnIn2S4/Bi2Sn2O7 heterojunctions: Towards superior photocatalytic properties for organic pollutant degradation. Chemosphere 2021, 276, 130226–130236. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. ZnIn2S4 grown on nitrogen-doped hollow carbon spheres: An advanced catalyst for Cr(VI) reduction. J. Hazard. Mater. 2020, 391, 122205. [Google Scholar] [CrossRef]
- He, Y.; Wang, D.; Li, X.; Fu, Q.; Yin, L.; Yang, Q.; Chen, H. Photocatalytic degradation of tetracycline by metal-organic frameworks modified with Bi2WO6 nanosheet under direct sunlight. Chemosphere 2021, 284, 131386. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Z.; Liu, H.; Wang, Y. Cd0.2Zn0.8S@UiO-66-NH2 nanocomposites as efficient and stable visible-light-driven photocatalyst for H2 evolution and CO2 reduction. Appl. Catal. B Environ. 2017, 200, 448–457. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Z.Z.; Zhang, Z.; Ao, D. Highly efficient photocatalytic H2 evolution from water over CdLa2S4/mesoporous g-C3N4 hybrids under visible light irradiation. Appl. Catal. B Environ. 2016, 192, 234–241. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, Z.; Lv, H.; Guang, J.; Li, S.; Jiang, J. Hydrothermal synthesis of hierarchical flower-like Bi2WO6 microspheres with enhanced visible-light photoactivity. Mater. Lett. 2015, 157, 158–162. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Du, M.; Xing, Z.P.; Li, Z.Z.; Pan, K.; Zhou, W. Defect-rich and electron-rich mesoporous Ti-MOFs based NH2-MIL-125(Ti)@ZnIn2S4/CdS hierarchical tandem heterojunctions with improved charge separation and enhanced solar-driven photocatalytic performance. Appl. Catal. B Environ. 2020, 262, 118202–118213. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Ao, D. Construction of heterostructured ZnIn2S4@NH2-MIL-125(Ti) nanocomposites for visible-light-driven H2 production. Appl. Catal. B Environ. 2018, 221, 433–442. [Google Scholar] [CrossRef]
- Gao, B.; Liu, L.; Liu, J.; Yang, F. Photocatalytic degradation of 2,4,6-tribromophenol on Fe2O3 or FeOOH doped ZnIn2S4 heterostructure: Insight into degradation mechanism. Appl. Catal. B Environ. 2014, 147, 929–939. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Zhang, Z.W.; Li, Y.X.; Song, Q.; Zhang, J.Q.; Liu, D. Multi-pathway photoelectron migration in globular flower-like In2O3/AgBr/Bi2WO6 synthesized by microwave-assisted method with enhanced photocatalytic activity. J. Mol. Catal. A Chem. 2016, 414, 27–36. [Google Scholar] [CrossRef]
- Jo, W.K.; Natarajan, T.S. Fabrication and efficient visible light photocatalytic properties of novel zinc indium sulfide (ZnIn2S4)-graphitic carbon nitride (g-C3N4)/bismuth vanadate (BiVO4) nanorod-based ternary nanocomposites with enhanced charge separation via Z-scheme transfer. J. Colloid. Interf. Sci. 2016, 482, 58–72. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness: Application to inorganic chemistry. Inorg. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]
- Liu, B.B.; Liu, X.J.; Liu, J.Y.; Feng, C.J.; Li, Z.; Li, C.; Gong, Y.Y.; Pan, L.K.; Xu, S.Q.; Sun, C.Q. fficient charge separation between UiO-66 and ZnIn2S4 flowerlike 3D microspheres for photoelectronchemical properties. Appl. Catal. B Environ. 2018, 226, 234–241. [Google Scholar] [CrossRef]
- Peng, X.; Ye, L.; Ding, Y.; Yi, L.; Zhang, C.; Wen, Z. Nanohybrid photocatalysts with ZnIn2S4 nanosheets encapsulated UiO-66 octahedral nanoparticles for visible-light-driven hydrogen generation. Appl. Catal. B Environ. 2019, 260, 118152. [Google Scholar] [CrossRef]
- Liu, T.T.; Wang, L.; Lu, X.; Fan, J.M.; Cai, X.X.; Gao, B.; Miao, R.; Wang, J.X.; Lv, Y.T. Comparative study of the photocatalytic performance for the degradation of different dyes by ZnIn2S4: Adsorption, active species, and pathways. RSC Adv. 2017, 7, 12292–12301. [Google Scholar] [CrossRef]
- Qin, K.; Zhao, Q.; Yu, H.; Xia, X.; Li, J.; He, S.; Wei, L.; An, T. A review of bismuth-based photocatalysts for antibiotic degradation: Insight into the photocatalytic degradation performance, pathways and relevant mechanisms. Environ. Res. 2021, 199, 111360. [Google Scholar] [CrossRef]
- Geng, H.M.; Ying, P.Z.; Li, K.; Zhao, Y.L.; Gu, X.Q. In2S3/ZnIn2S4 heterojunction nanosheet arrays on FTO substrates for photoelectrochemical water splitting. Appl. Surf. Sci. 2021, 563, 150289–150296. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.; Jiang, W.; Yao, W.; Yang, H.; Zhu, Y. Self-assembled perylene diimide based supramolecular heterojunction with Bi2WO6 for efficient visible-light-driven photocatalysis. Appl. Catal. B Environ. 2018, 232, 175–181. [Google Scholar] [CrossRef]
- Li, X.; Garlisi, C.; Guan, Q.; Anwer, S.; Al-Ali, K.; Palmisano, G.; Zheng, L. A review of material aspects in developing direct Z-scheme photocatalysts. Mater. Today 2021, 47, 75–107. [Google Scholar] [CrossRef]







| Photocatalyst | SBET (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| ZnIn2S4 | 86.88 | 0.27 | 9.99 |
| Bi2WO6 | 19.64 | 0.51 | 34.11 |
| 0.1-Zn/Bi | 32.87 | 0.19 | 18.80 |
| 0.2-Zn/Bi | 37.36 | 0.75 | 40.10 |
| 0.3-Zn/Bi | 37.97 | 0.50 | 24.78 |
| Photocatalyst | Element | Element Electronegativity (eV) [29] | Molar Ratio | χ (eV) | Eg (eV) | EVB (eV) | ECB (eV) |
|---|---|---|---|---|---|---|---|
| ZnIn2S4 | Zn | 4.45 | 1 | 4.84 | 1.88 | +1.28 | −0.60 |
| In | 3.10 | 2 | |||||
| S | 6.22 | 3.9 | |||||
| Bi2WO6 | Bi | 4.69 | 2.3 | 6.30 | 2.72 | +3.16 | +0.44 |
| W | 4.40 | 1 | |||||
| O | 77.54 | 5.8 | |||||
| 0.1-Zn/Bi | 2.56 | ||||||
| 0.2-Zn/Bi | 2.65 | ||||||
| 0.3-Zn/Bi | 2.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Yang, F.; Wang, L.; Pei, L.; Hu, Y.; Li, R.; Hou, K.; Ren, T. Synergistic Effect of Charge Separation and Multiple Reactive Oxygen Species Generation on Boosting Photocatalytic Degradation of Fluvastatin by ZnIn2S4/Bi2WO6 Z-Scheme Heterostructured Photocatalytst. Toxics 2022, 10, 555. https://doi.org/10.3390/toxics10100555
Liu T, Yang F, Wang L, Pei L, Hu Y, Li R, Hou K, Ren T. Synergistic Effect of Charge Separation and Multiple Reactive Oxygen Species Generation on Boosting Photocatalytic Degradation of Fluvastatin by ZnIn2S4/Bi2WO6 Z-Scheme Heterostructured Photocatalytst. Toxics. 2022; 10(10):555. https://doi.org/10.3390/toxics10100555
Chicago/Turabian StyleLiu, Tingting, Fanyu Yang, Liming Wang, Liang Pei, Yushan Hu, Ru Li, Kang Hou, and Tianlong Ren. 2022. "Synergistic Effect of Charge Separation and Multiple Reactive Oxygen Species Generation on Boosting Photocatalytic Degradation of Fluvastatin by ZnIn2S4/Bi2WO6 Z-Scheme Heterostructured Photocatalytst" Toxics 10, no. 10: 555. https://doi.org/10.3390/toxics10100555
APA StyleLiu, T., Yang, F., Wang, L., Pei, L., Hu, Y., Li, R., Hou, K., & Ren, T. (2022). Synergistic Effect of Charge Separation and Multiple Reactive Oxygen Species Generation on Boosting Photocatalytic Degradation of Fluvastatin by ZnIn2S4/Bi2WO6 Z-Scheme Heterostructured Photocatalytst. Toxics, 10(10), 555. https://doi.org/10.3390/toxics10100555











