Prenatal PM2.5 Exposure in Relation to Maternal and Newborn Telomere Length at Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Studied
2.2. PM2.5 Exposure
2.3. Telomere Length Measurement
2.4. Statistical Analysis
2.5. Covariates
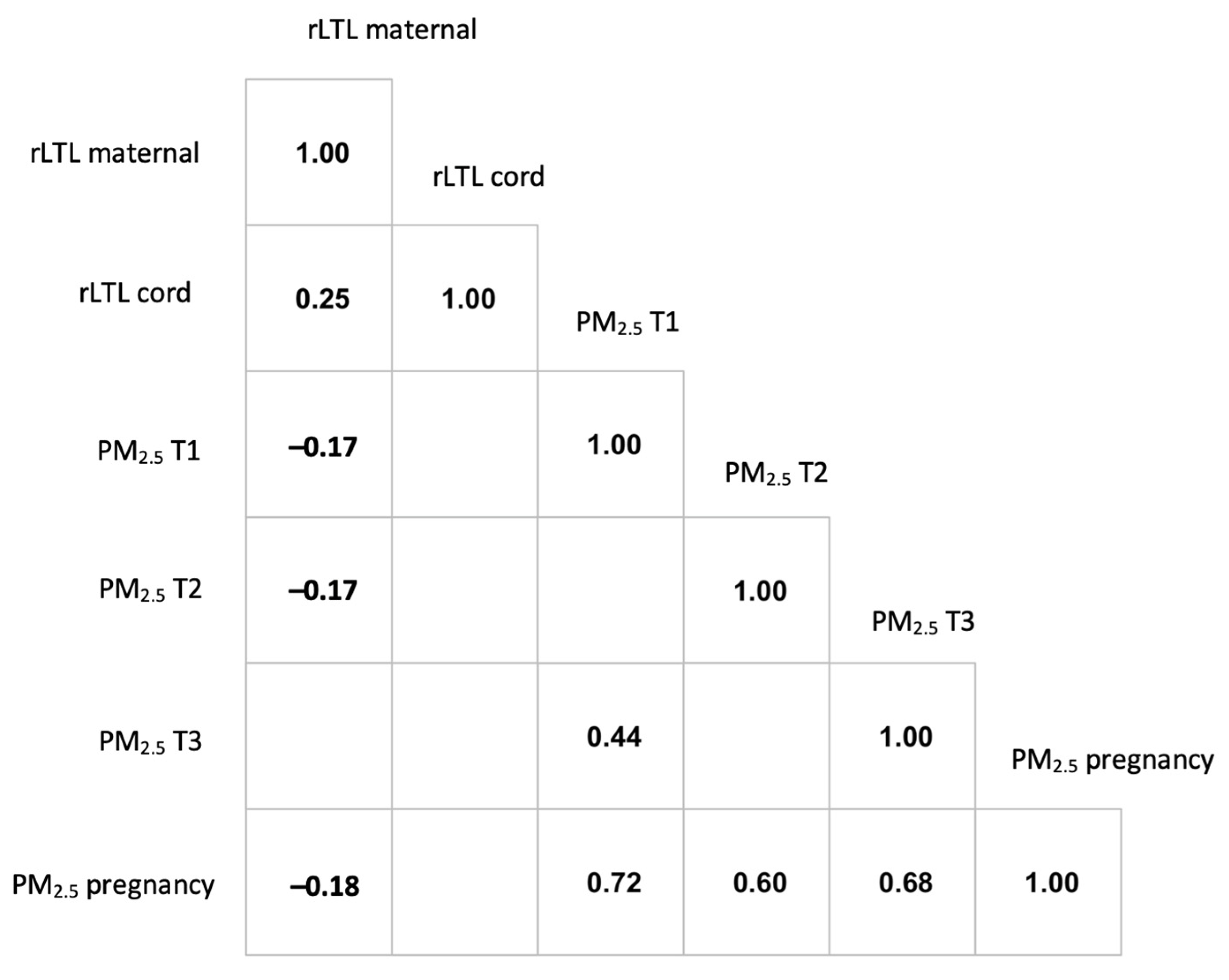
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forouzanfar, M.H.; GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Varshavsky, J.; Smith, A.; Wang, A.; Hom, E.; Izano, M.; Huang, H.; Padula, A.; Woodruff, T. Heightened susceptibility: A review of how pregnancy and chemical exposures influence maternal health. Reprod. Toxicol. 2020, 92, 14–56. [Google Scholar] [CrossRef]
- Bové, H.; Bongaerts, E.; Slenders, E.; Bijnens, E.M.; Saenen, N.D.; Gyselaers, W.; Van Eyken, P.; Plusquin, M.; Roeffaers, M.B.J.; Ameloot, M.; et al. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. 2019, 10, 3866. [Google Scholar] [CrossRef]
- Clemens, T.; Turner, S.; Dibben, C. Maternal exposure to ambient air pollution and fetal growth in North-East Scotland: A population-based study using routine ultrasound scans. Environ. Int. 2017, 107, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Giorgis-Allemand, L.; Bernard, C.; Aguilera, I.; Andersen, A.-M.N.; Ballester, F.; Beelen, R.M.J.; Chatzi, L.; Cirach, M.; Danilevičiūtė, A.; et al. Ambient air pollution and low birthweight: A European cohort study (ESCAPE). Lancet Respir. Med. 2013, 1, 695–704. [Google Scholar] [CrossRef]
- Poirier, A.; Dodds, L.; Dummer, T.; Rainham, D.; Maguire, B.; Johnson, M. Maternal Exposure to Air Pollution and Adverse Birth Outcomes in Halifax, Nova Scotia. J. Occup. Environ. Med. 2015, 57, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Liang, S.; Yang, S.; Trevathan, E.; Huang, Z.; Yang, R.; Wang, J.; Hu, K.; Zhang, Y.; Vaughn, M.; et al. Ambient air pollution and preterm birth: A prospective birth cohort study in Wuhan, China. Int. J. Hyg. Environ. Health 2015, 219, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Siddika, N.; Balogun, H.A.; Amegah, A.K.; Jaakkola, J.J.K. Prenatal ambient air pollution exposure and the risk of stillbirth: Systematic review and meta-analysis of the empirical evidence. Occup. Environ. Med. 2016, 73, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Martens, D.S.; Cox, B.; Janssen, B.G.; Clemente, D.B.P.; Gasparrini, A.; Vanpoucke, C.; Lefebvre, W.; Roels, H.A.; Plusquin, M.; Nawrot, T.S. Prenatal Air Pollution and Newborns’ Predisposition to Accelerated Biological Aging. JAMA Pediatr. 2017, 171, 1160–1167. [Google Scholar] [CrossRef]
- De Felice, B.; Nappi, C.; Zizolfi, B.; Guida, M.; Sardo, A.D.S.; Bifulco, G.; Guida, M. Telomere shortening in women resident close to waste landfill sites. Gene 2012, 500, 101–106. [Google Scholar] [CrossRef]
- Hoxha, M.; Dioni, L.; Bonzini, M.; Pesatori, A.C.; Fustinoni, S.; Cavallo, D.; Carugno, M.; Albetti, B.; Marinelli, B.; Schwartz, J.; et al. Association between leukocyte telomere shortening and exposure to traffic pollution: A cross-sectional study on traffic officers and indoor office workers. Environ. Health 2009, 8, 41. [Google Scholar] [CrossRef]
- Perera, F.; Lin, C.-J.; Qu, L.; Tang, D. Shorter telomere length in cord blood associated with prenatal air pollution exposure: Benefits of intervention. Environ. Int. 2018, 113, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhang, C.; Silveyra, P. The Relationships Between Prenatal Smoking Exposure and Telomere Lengths in Fetuses, Infants, and Children: A Systematic Literature Review. UJAN 2020, 31, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, L.; Song, L.; Liu, B.; Liu, Y.; Bi, J.; Liu, Q.; Chen, K.; Li, Y.; Xia, W.; et al. The association between prenatal exposure to thallium and shortened telomere length of newborns. Chemosphere 2021, 265, 129025. [Google Scholar] [CrossRef] [PubMed]
- Coluzzi, E.; Colamartino, M.; Cozzi, R.; Leone, S.; Meneghini, C.; O’Callaghan, N.; Sgura, A. Oxidative Stress Induces Persistent Telomeric DNA Damage Responsible for Nuclear Morphology Change in Mammalian Cells. PLoS ONE 2014, 9, e110963. [Google Scholar] [CrossRef]
- McCracken, J.; Baccarelli, A.; Hoxha, M.; Dioni, L.; Melly, S.; Coull, B.; Suh, H.; Vokonas, P.; Schwartz, J. Annual Ambient Black Carbon Associated with Shorter Telomeres in Elderly Men: Veterans Affairs Normative Aging Study. Environ. Health Perspect. 2010, 118, 1564–1570. [Google Scholar] [CrossRef]
- Huang, Y.; Kioumourtzoglou, M.-A.; Mittleman, M.A.; Ross, Z.; Williams, M.A.; Friedman, A.M.; Schwartz, J.; Wapner, R.J.; Ananth, C.V. Air Pollution and Risk of Placental Abruption: A Study of Births in New York City, 2008–2014. Am. J. Epidemiol. 2021, 190, 1021–1033. [Google Scholar] [CrossRef]
- Wang, Y.; Perera, F.; Guo, J.; Riley, K.W.; Durham, T.; Ross, Z.; Ananth, C.V.; Baccarelli, A.; Wang, S.; Herbstman, J.B. A methodological pipeline to generate an epigenetic marker of prenatal exposure to air pollution indicators. Epigenetics 2021, 1–9. [Google Scholar] [CrossRef]
- Ross, Z.; Ito, K.; Johnson, S.; Yee, M.; Pezeshki, G.; Clougherty, J.E.; Savitz, D.; Matte, T. Spatial and temporal estimation of air pollutants in New York City: Exposure assignment for use in a birth outcomes study. Environ. Health 2013, 12, 51. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef]
- Cowell, W.; Tang, D.; Yu, J.; Guo, J.; Wang, S.; Baccarelli, A.A.; Perera, F.; Herbstman, J.B. Telomere dynamics across the early life course: Findings from a longitudinal study in children. Psychoneuroendocrinology 2021, 129, 105270. [Google Scholar] [CrossRef]
- Lindrose, A.; Drury, S. Minimum Reporting Recommendations for PCR-based Telomere Length Measurement. Open Sci. Framew. 2020. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Xu, X.; Xu, J.; Meng, W.; Pu, W. Seasonal and diurnal variations of ambient PM2.5 concentration in urban and rural environments in Beijing. Atmos. Environ. 2009, 43, 2893–2900. [Google Scholar] [CrossRef]
- Factor-Litvak, P.; Susser, E.; Aviv, A. Environmental Exposures, Telomere Length at Birth, and Disease Susceptibility in Later Life. JAMA Pediatr. 2017, 171, 1143. [Google Scholar] [CrossRef]
- Marchetto, N.M.; Glynn, R.; Ferry, M.L.; Ostojic, M.; Wolff, S.M.; Yao, R.; Haussmann, M.F. Prenatal stress and newborn telomere length. Am. J. Obstet. Gynecol. 2016, 215, 94.e1–94.e8. [Google Scholar] [CrossRef]
- Liu, B.; Song, L.; Zhang, L.; Wu, M.; Wang, L.; Cao, Z.; Xiong, C.; Zhang, B.; Li, Y.; Xia, W.; et al. Prenatal second-hand smoke exposure and newborn telomere length. Pediatr. Res. 2020, 87, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Iodice, S.; Hoxha, M.; Ferrari, L.; Carbone, I.F.; Anceschi, C.; Miragoli, M.; Pesatori, A.C.; Persico, N.; Bollati, V. Particulate Air Pollution, Blood Mitochondrial DNA Copy Number, and Telomere Length in Mothers in the First Trimester of Pregnancy: Effects on Fetal Growth. Oxidative Med. Cell. Longev. 2018, 2018, 5162905. [Google Scholar] [CrossRef] [PubMed]
- Scholten, R.H.; Møller, P.; Andersen, Z.J.; Dehlendorff, C.; Khan, J.; Brandt, J.; Ketzel, M.; Knudsen, L.E.; Mathiesen, L. Telomere length in newborns is associated with exposure to low levels of air pollution during pregnancy. Environ. Int. 2021, 146, 106202. [Google Scholar] [CrossRef]
- Lee, A.G.; Cowell, W.; Kannan, S.; Ganguri, H.B.; Nentin, F.; Wilson, A.; Coull, B.A.; Wright, R.O.; Baccarelli, A.; Bollati, V.; et al. Prenatal particulate air pollution and newborn telomere length: Effect modification by maternal antioxidant intakes and infant sex. Environ. Res. 2020, 187, 109707. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.J.; Hsu, H.-H.L.; Just, A.C.; Brennan, K.J.; Bloomquist, T.; Kloog, I.; Pantic, I.; García, A.M.; Wilson, A.; Coull, B.A.; et al. Association between prenatal particulate air pollution exposure and telomere length in cord blood: Effect modification by fetal sex. Environ. Res. 2019, 172, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, B.; Liu, B.; Wu, M.; Zhang, L.; Wang, L.; Xu, S.; Cao, Z.; Wang, Y. Effects of maternal exposure to ambient air pollution on newborn telomere length. Environ. Int. 2019, 128, 254–260. [Google Scholar] [CrossRef]
- Costantine, M.M. Physiologic and pharmacokinetic changes in pregnancy. Front. Pharmacol. 2014, 5, 65. [Google Scholar] [CrossRef]
- Pritchard, J.A. Changes in the Blood Volume During Pregnancy and Delivery. Anesthesiology 1965, 26, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Vricella, L.K. Emerging understanding and measurement of plasma volume expansion in pregnancy. Am. J. Clin. Nutr. 2017, 106, 1620S–1625S. [Google Scholar] [CrossRef]
- Koch, C.A.; Platt, J.L. T cell recognition and immunity in the fetus and mother. Cell. Immunol. 2007, 248, 12–17. [Google Scholar] [CrossRef][Green Version]
- Eisenberg, D.T.A. An evolutionary review of human telomere biology: The thrifty telomere hypothesis and notes on potential adaptive paternal effects. Am. J. Hum. Biol. 2011, 23, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Morin, A.M.; Gatev, E.; McEwen, L.M.; MacIsaac, J.L.; Lin, D.T.S.; Koen, N.; Czamara, D.; Räikkönen, K.; Zar, H.J.; Koenen, K.; et al. Maternal blood contamination of collected cord blood can be identified using DNA methylation at three CpGs. Clin. Epigenet. 2017, 9, 75. [Google Scholar] [CrossRef]
- Lin, N.; Mu, X.; Wang, G.; Ren, Y.; Su, S.; Li, Z.; Wang, B.; Tao, S. Accumulative effects of indoor air pollution exposure on leukocyte telomere length among non-smokers. Environ. Pollut. 2017, 227, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lupo, P.; Symanski, E.; Chan, W.; Mitchell, L.E.; Waller, D.; Canfield, M.A.; Langlois, P.H. Differences in exposure assignment between conception and delivery: The impact of maternal mobility. Paediatr. Perinat. Epidemiol. 2010, 24, 200–208. [Google Scholar] [CrossRef]
- Winquist, A.; Kirrane, E.; Klein, M.; Strickland, M.; Darrow, L.A.; Sarnat, S.E.; Gass, K.; Mulholland, J.; Russell, A.; Tolbert, P. Joint Effects of Ambient Air Pollutants on Pediatric Asthma Emergency Department Visits in Atlanta, 1998–2004. Epidemiology 2014, 25, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.; Peng, R.D.; Barr, C.D.; Bell, M. Protecting Human Health From Air Pollution: Shifting From a Single-pollutant to a Multipollutant Approach. Epidemiology 2010, 21, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Billionnet, C.; Sherrill, D.; Annesi-Maesano, I. Estimating the Health Effects of Exposure to Multi-Pollutant Mixture. Ann. Epidemiol. 2012, 22, 126–141. [Google Scholar] [CrossRef] [PubMed]
| Mean ± SD or N (%) | |
|---|---|
| African American | 72 (37.3) |
| Dominican | 121 (62.7) |
| <High school education or equivalent | 72 (37.3) |
| Child sex (girl) | 111 (57.5) |
| Season of conception | |
| Spring | 48 (24.9) |
| Summer | 43 (22.3) |
| Fall | 44 (22.8) |
| Winter | 58 (30.1) |
| Maternal age (years) | 25.43 ± 5.18 |
| Gestational age (weeks) | 39.39 ± 1.30 |
| Maternal rLTL at delivery | 1.02 ± 0.13 |
| Umbilical cord rLTL | 1.22 ± 0.26 |
| PM2.5 (µg/m3), median (Q1, Q3) | |
| Total pregnancy | 16.53 (15.69, 17.71) |
| First trimester | 16.68 (15.25, 18.76) |
| Second trimester | 16.59 (14.67, 18.31) |
| Third trimester | 16.78 (14.97, 18.48) |
| Cord rLTL | Cord Rltl b | Maternal rLTL | ||||
|---|---|---|---|---|---|---|
| PM2.5 Exposure | β (95% CI) | p-Value | β (95% CI) | p-Value | β (95% CI) | p-Value |
| Total pregnancy | 0.063 (−0.021, 0.147) | 0.141 | 0.083 (0.001, 0.164) | 0.047 | −0.034 (−0.074, 0.005) | 0.087 |
| 1st trimester | 0.039 (−0.039, 0.117) | 0.323 | 0.051 (−0.024, 0.127) | 0.183 | −0.022 (−0.059, 0.015) | 0.240 |
| 2nd trimester | −0.037 (−0.114, 0.039) | 0.337 | −0.017 (−0.092, 0.058) | 0.657 | −0.039 (−0.074, −0.003) | 0.034 |
| 3rd trimester | 0.042 (−0.036, 0.120) | 0.292 | 0.039 (−0.037, 0.114) | 0.313 | 0.006 (−0.031, 0.042) | 0.765 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durham, T.; Guo, J.; Cowell, W.; Riley, K.W.; Wang, S.; Tang, D.; Perera, F.; Herbstman, J.B. Prenatal PM2.5 Exposure in Relation to Maternal and Newborn Telomere Length at Delivery. Toxics 2022, 10, 13. https://doi.org/10.3390/toxics10010013
Durham T, Guo J, Cowell W, Riley KW, Wang S, Tang D, Perera F, Herbstman JB. Prenatal PM2.5 Exposure in Relation to Maternal and Newborn Telomere Length at Delivery. Toxics. 2022; 10(1):13. https://doi.org/10.3390/toxics10010013
Chicago/Turabian StyleDurham, Teresa, Jia Guo, Whitney Cowell, Kylie W. Riley, Shuang Wang, Deliang Tang, Frederica Perera, and Julie B. Herbstman. 2022. "Prenatal PM2.5 Exposure in Relation to Maternal and Newborn Telomere Length at Delivery" Toxics 10, no. 1: 13. https://doi.org/10.3390/toxics10010013
APA StyleDurham, T., Guo, J., Cowell, W., Riley, K. W., Wang, S., Tang, D., Perera, F., & Herbstman, J. B. (2022). Prenatal PM2.5 Exposure in Relation to Maternal and Newborn Telomere Length at Delivery. Toxics, 10(1), 13. https://doi.org/10.3390/toxics10010013







