A Method for the Rapid Measurement of Alkylresorcinols in Flour, Bread and Related Products Based on 1H qNMR
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Plant Material and Processed Products
2.3. Extraction and Isolation
2.4. GC-MS
2.5. High-Throughput Extraction and NMR Analysis
2.6. Precision-Recovery-LOD-LOQ
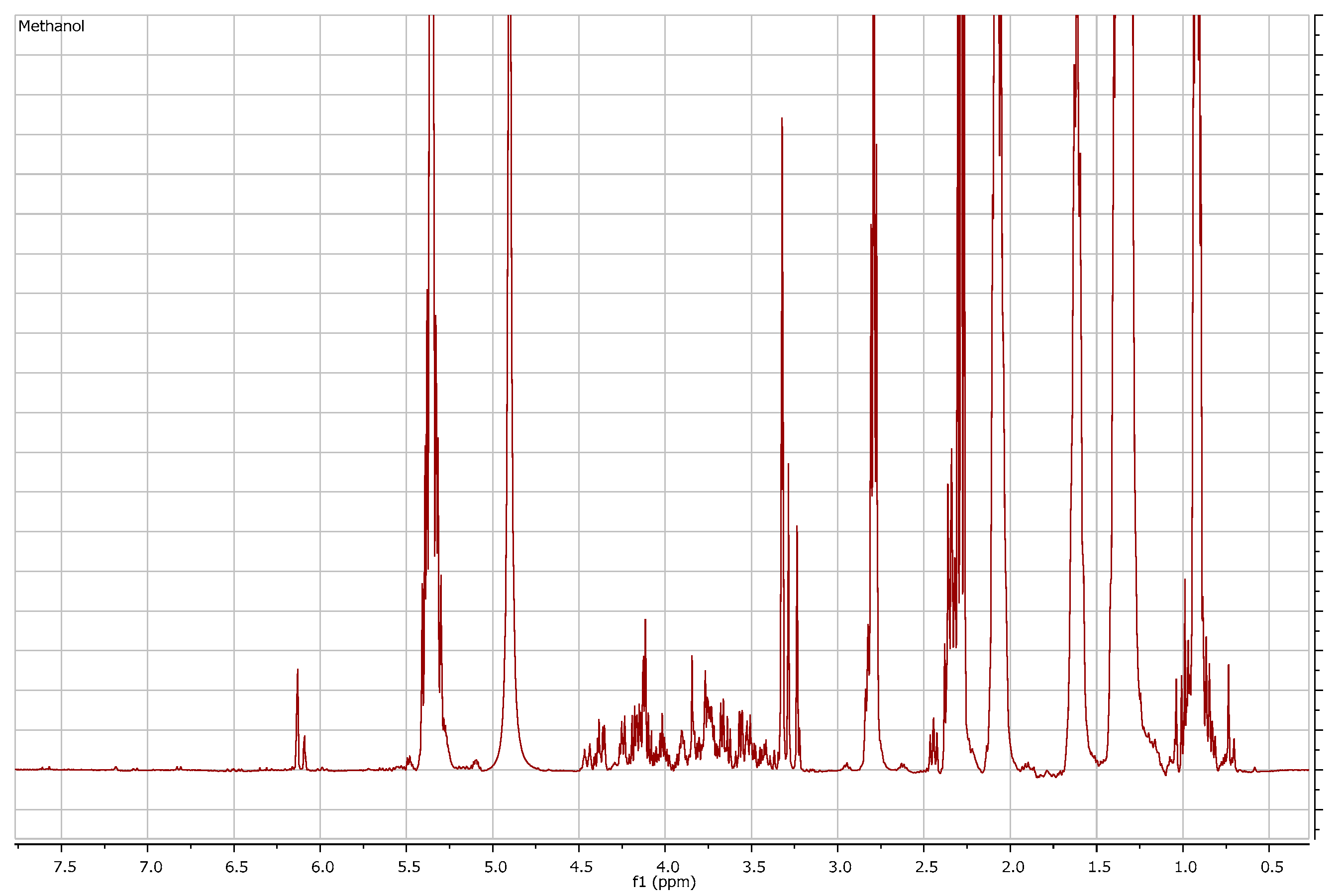
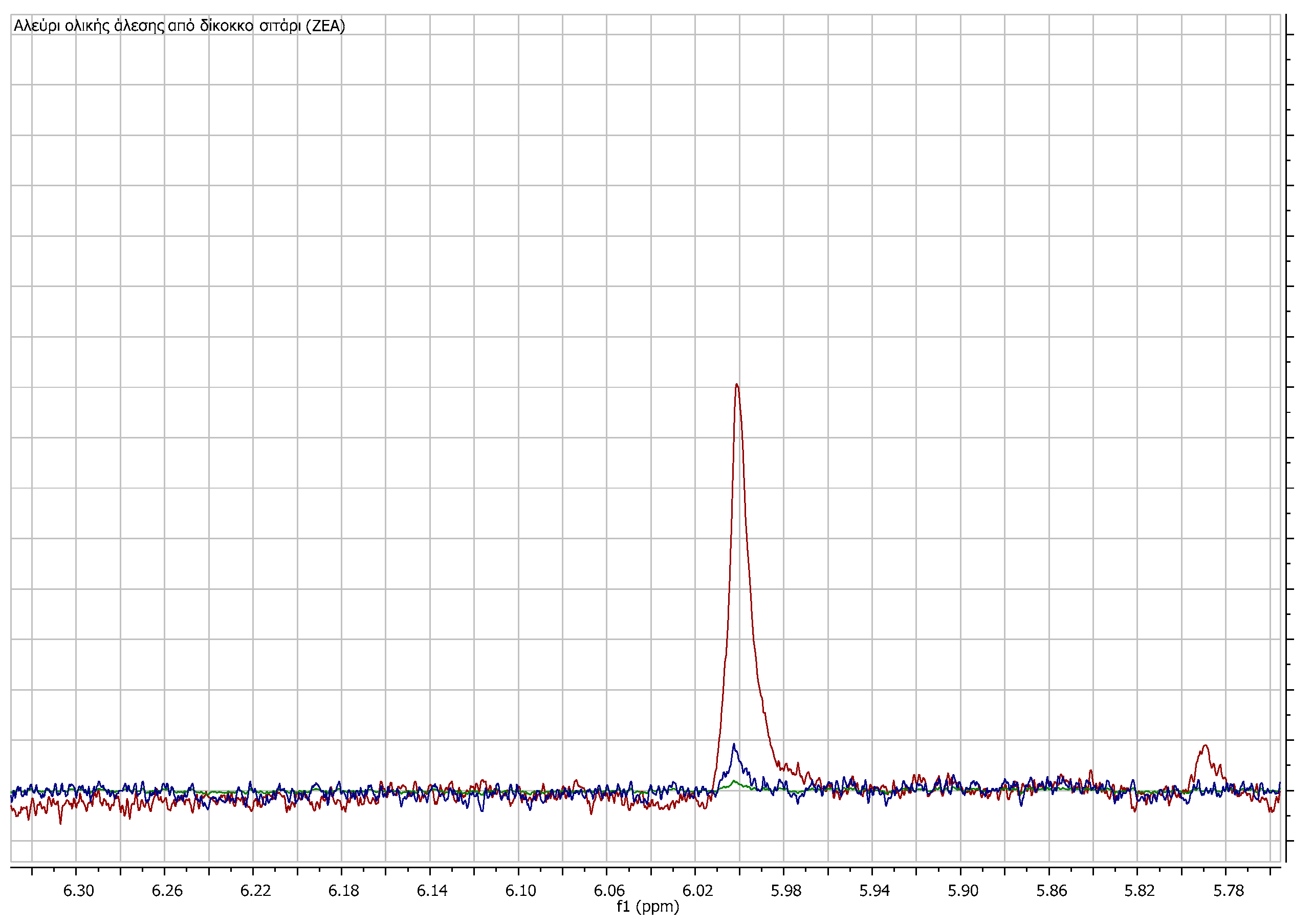
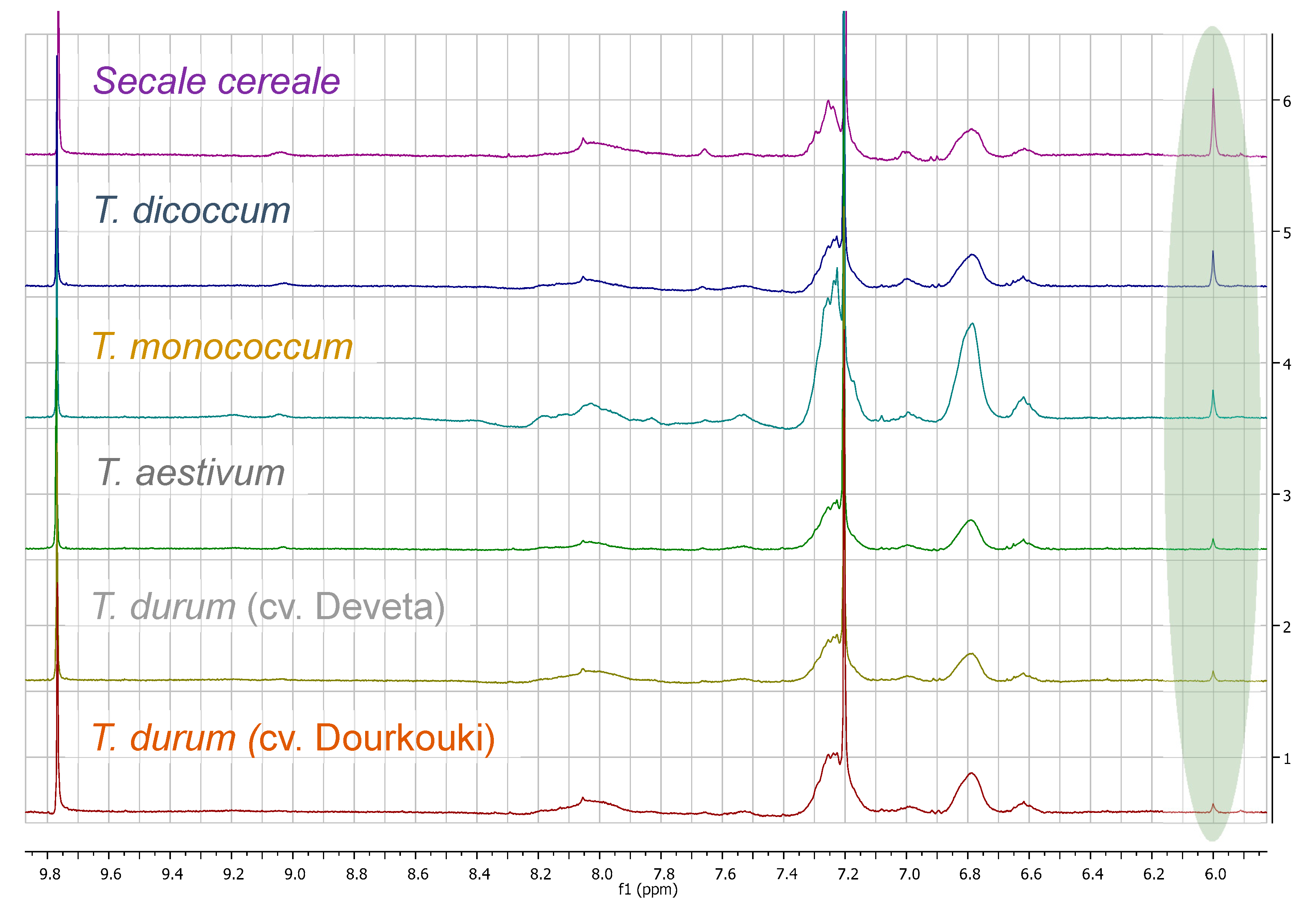
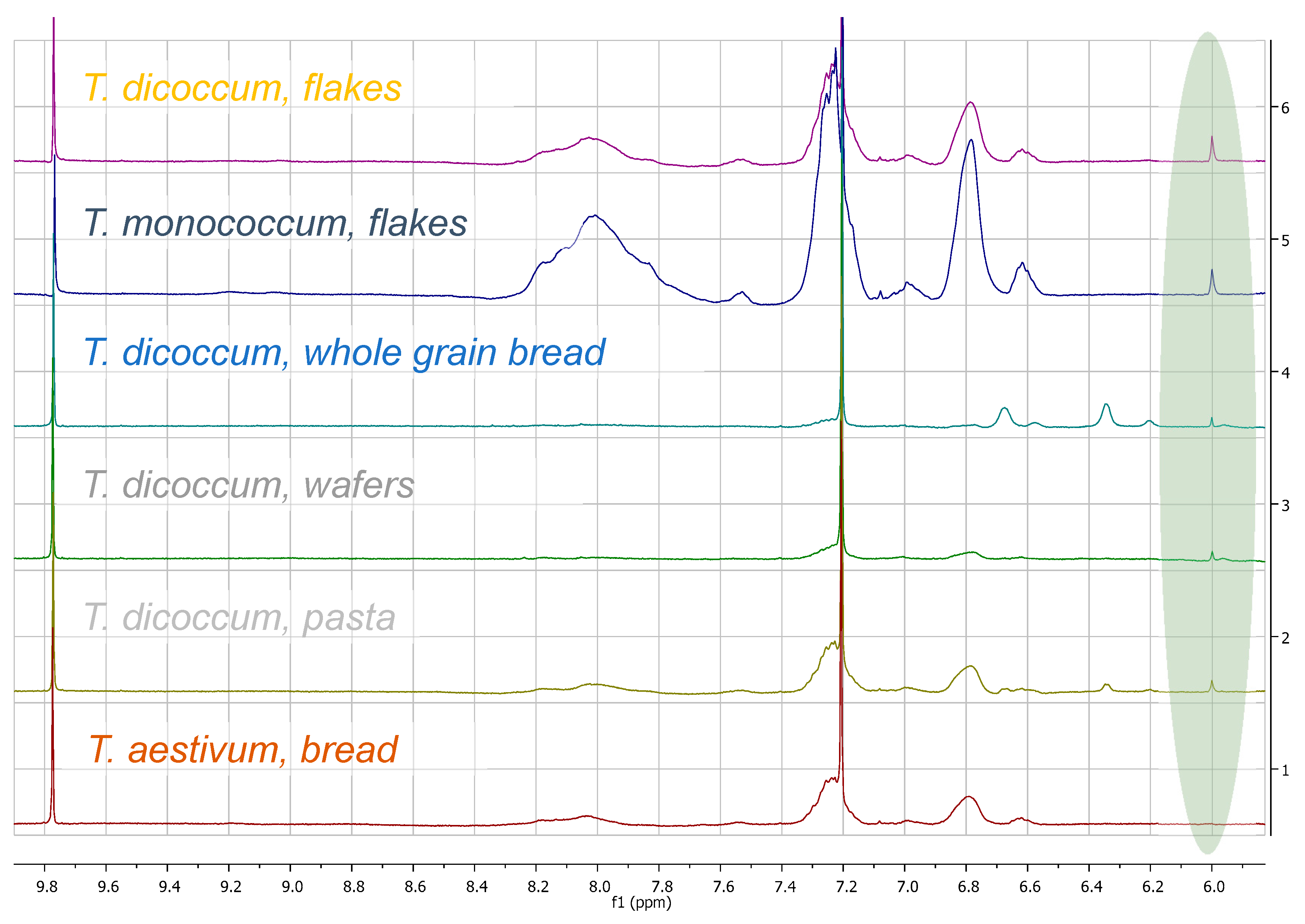
3. Results
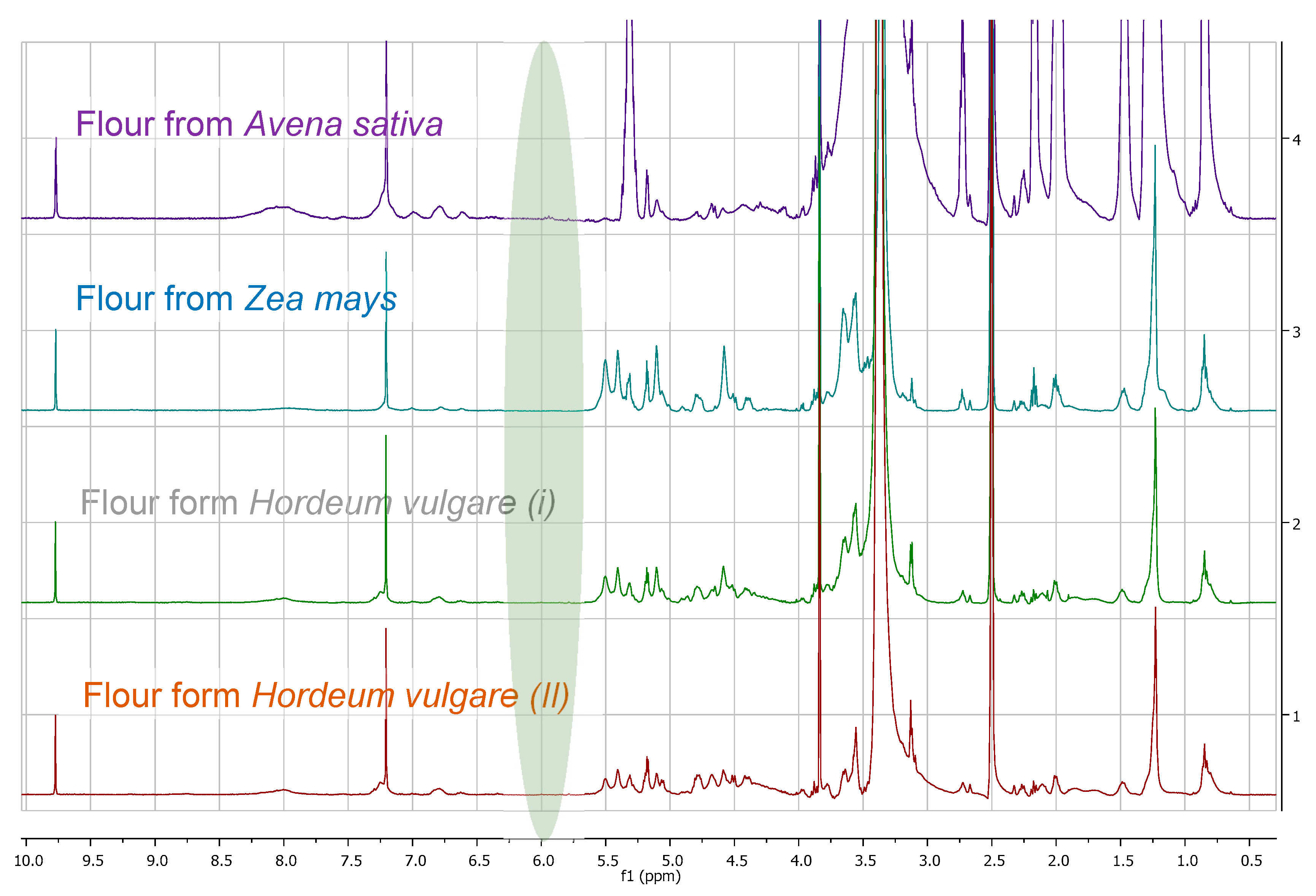
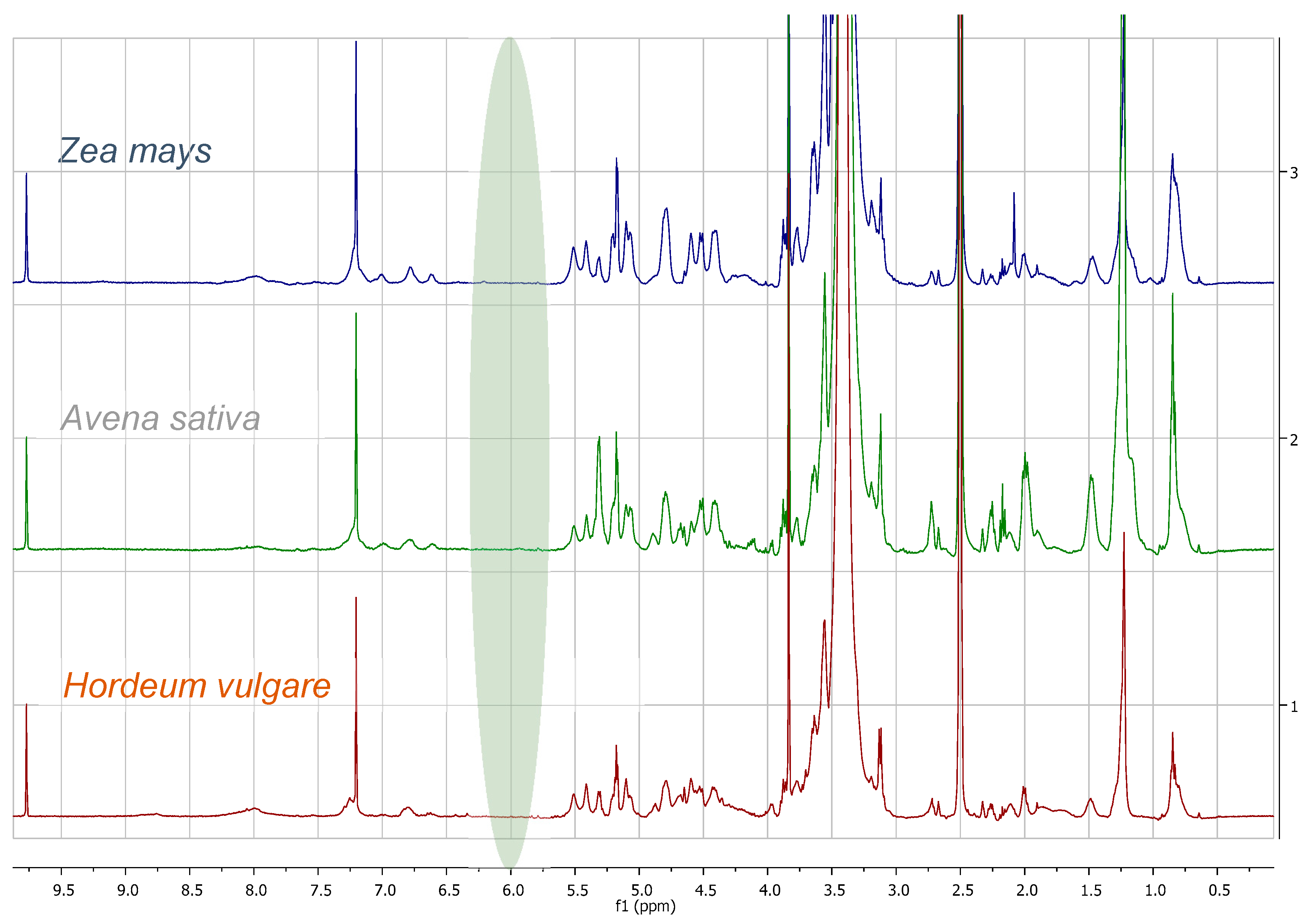
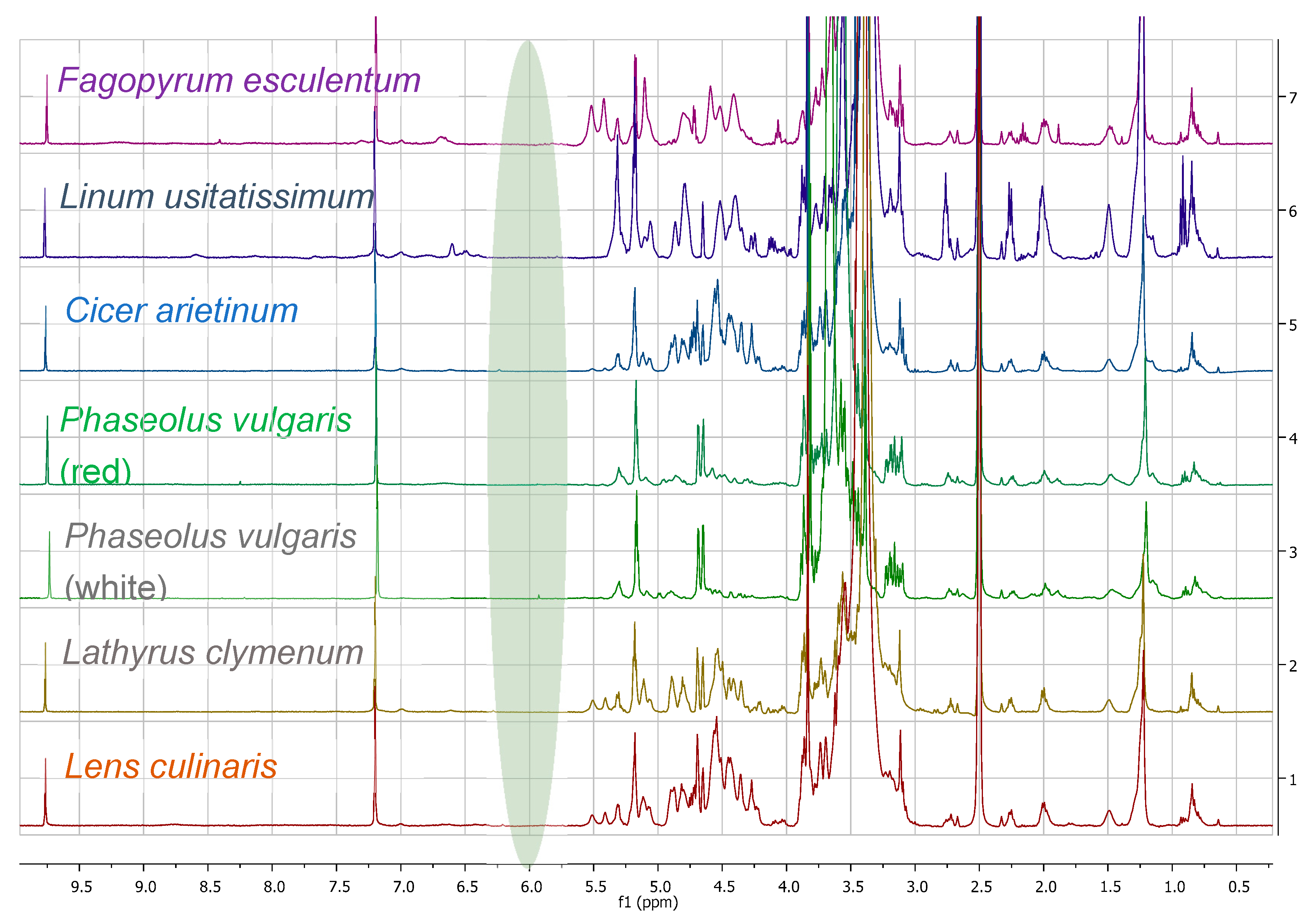
3.1. Comparison among Flour Extracts
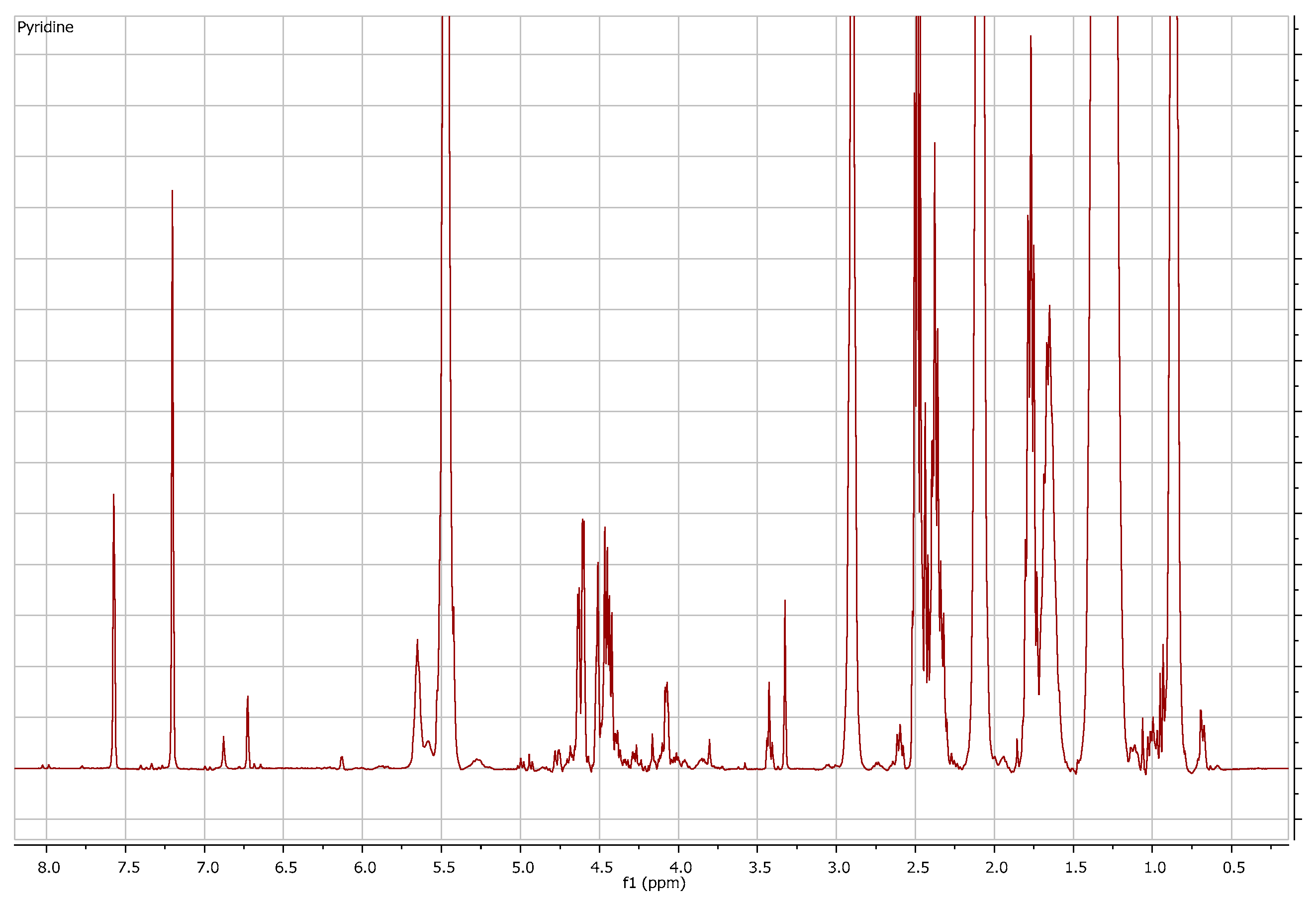
3.2. Structure Elucidation Results
3.3. Quantitation Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Product | Triticum spp. | Origin | ARs Content (mg/kg) | Reference |
|---|---|---|---|---|
| whole-wheat flour | Triticum spp. | Finland | 759 | [6] |
| white wheat flour | 47 | |||
| white wheat flour, organic | 44 | |||
| wheat bran | 3225 | |||
| white wheat bread | nd | |||
| pasta | 48 | |||
| T. durum | France, Germany, and United Kingdom | 687 | [4] | |
| T. aestivum | 909 | |||
| T. spelta | 819 | |||
| T. timophevi | 1480 | |||
| T. compactum | 1090 | |||
| T. ispahanicum | 982 | |||
| T. polonicum | 951 | |||
| T. paleocolchicum | 916 | |||
| T. carthlicum | 876 | |||
| T. sphaerococcum | 862 | |||
| T. dicoccum | 819 | |||
| T. dicoccoides | 787 | |||
| T. orientale | 691 | |||
| T. araraticum | 599 | |||
| T. turanicum | 200 | |||
| Commercial wheat bran | Triticum spp. | Uppsala, Sweden | 2672 | |
| Commercial wheat bran based cereal | 1784 | |||
| Commercial wheat breakfast biscuits | 558 | |||
| Commercial whole grain wheat flour | 550 | |||
| Commercial crusty whole grain wheat bread | 222 | |||
| Commercial wholemeal wheat crackers | 179 | |||
| Commercial wholemeal wheat bread | 142 | |||
| Commercial wheat crispbread | 58 | |||
| Commercial digestive biscuits | 57 | |||
| Commercial white wheat flour | nd | |||
| Commercial white wheat bread | nd | |||
| refined wheat | Triticum spp. | US | 20.9 | |
| semolina | T. durum | US | 71.3 | [17] |
| whole grain | T. durum | US | 444.2 | |
| whole grain | T. monococcum | Germany | 391 | |
| whole grain | T. turanicum | Germany | 255.5 | |
| refined wheat flakes + bran | Triticum spp. | France | 100.7 | |
| white wheat bread | US | 19.8 | ||
| white wheat bread | France | 28 | ||
| refined wheat tortilla | US | 13.6 | ||
| refined wheat crackers | US | 16.8 | ||
| refined wheat dry toast | Italy | 34.2 | ||
| WG wheat bread | US | 277.5 | ||
| WG wheat roll | US | 467 | ||
| WG wheat dry toast | Italy | 193 | ||
| WG wheat dry toast | France | 192.8 | ||
| refined wheat pasta | US | 44 | ||
| refined wheat pasta (raw) | US | 53.5 | ||
| refined wheat pasta (cooked) | US | 58 | ||
| refined wheat pasta | Italy | 49.5 | ||
| WG wheat pasta | Italy | 201.2 | ||
| WG wheat pasta | US | 237.5 | ||
| WG wheat pasta 4 (raw) | US | 348.9 | ||
| WG wheat pasta 4 (cooked) | US | 380.3 | ||
| WG wheat pasta | Netherlands | 383.1 | ||
| WG wheat pasta | Italy | 214.2 | ||
| Kernel | T. durum | Austria | 251–475 | [10] |
| France | 447–467 | |||
| Kazakstan | 488–618 | |||
| Russia | 483 | |||
| Spain | 401–526 | |||
| Sweden | 407–490 | |||
| Commercial pasta | Sweden | 215–270 | ||
| Kernel | T. aestivum spring | Germany | 663–862 | [18] |
| T. aestivum winter | 591–1077 | |||
| T. durum spring | Austria | 430–797 | ||
| T. durum winter | 554–675 | |||
| Whole Grain | T. aestivum winter | Sweden | 432–634 | [5] |
| T. aestivum spring | 227–353 | |||
| wheat bran | T. aestivum | 2211 ± 605 | ||
| white wheat flour | T. aestivum | nd | ||
| Commercial white bread | Triticum spp. | Latvia | 24.7–26.8 | [19] |
| Commercial white bread with seeds | 26.4–27.7 | |||
| Commercial white bread | Finland | 24.9–30.8 | ||
| whole-grain wheat | T. aestivum | Poland | 586–943 | [7] |
| whole-grain wheat ground | 565–879 | |||
| wheat bran | 2388–3186 | |||
| Commercial whole-wheat flour | Triticum spp. | 258–269 | ||
| Commercial breakfast wheat cereals | 131–671 | |||
| Commercial soft wheat bread | 0–23 | |||
| bread | T. spelta | 390 | ||
| whole-grain bread | Triticum spp. | 140–497 | ||
| Wheat Grain | T. spelta | Hungary | 427–605 | [8] |
| T. durum | 327–399 | |||
| T.monococcum | 399–595 | |||
| T.dicoccum | 444–581 | |||
| T. aestivum spring | 341–416 | |||
| T. aestivum winter | 340–410 | |||
| Grains | T. durum | Hungary | 370 ± 100–452 ± 138 | [16] |
| T. aestivum spring | 494 ± 79–536 ± 111 | |||
| T. aestivum winter | 500 ± 114–655 ± 142 | |||
| Grains | T. aestivum winter | Sweden | 600 | [1] |
| Bran | T. aestivum | China | 697–1732 | [13] |
| White flour | T. spelta | China | 31.8 | [15] |
| organic white wheat flour | Triticum spp. | China | 26.3 | |
| white wheat flour | China | 29.9–34.7 | ||
| white wheat flour | Sweden | 13.5–27.6 | ||
| semolina | China | 46.6 | ||
| brown wheat flour | China | 98.1- 215.6 | ||
| Whole grain wheat flour | China | 285.4–660.3 | ||
| organic whole grain wheat flour | China | 488.9 | ||
| Whole grain wheat flour | India | 138.4–345.5 | ||
| Whole grain white wheat | China | 555.5 | ||
| grains | T. spelta | China | 455.3 | |
| flour | T. spelta | Sweden | 507.3 | |
| Whole grain wheat pasta | China | 40.8 | ||
| Whole grain wheat pasta | Triticum spp. | Italy | 313.7–340.5 | |
| Whole grain wheat pasta | United Kingdom | 422.8–608.5 |
References
- Gohil, S.; Pettersson, D.; Salomonssona, A. Analysis of Alkyl- and Alkenylresorcinols in Triticale. J. Sci. Food Agric. 1988, 45, 43–52. [Google Scholar] [CrossRef]
- Kozubek, A.; Tyman, J.H.P. Resorcinolic lipids, the natural non-isoprenoid phenolic amphiphiles and their biological activity. Chem. Rev. 1999, 99, 1–26. [Google Scholar] [CrossRef]
- Landberg, R.; Marklund, M.; Kamal-Eldin, A.; Aman, P. An update on alkylresorcinols-Occurrence, bioavailability, bioactivity and utility as biomarkers. J. Funct. Foods 2014, 7, 77–89. [Google Scholar] [CrossRef]
- Ross, A.B.; Shepherd, M.J.; Schüpphaus, M.; Sinclair, V.; Alfaro, B.; Kamal-Eldin, A.; Aman, P. Alkylresorcinols in Cereals and Cereal Products. J. Agric. Food Chem. 2003, 51, 4111–4118. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ross, A.B.; Aman, P.; Kamal-Eldin, A. Alkylresorcinols as Markers of Whole Grain Wheat and Rye in Cereal Products. J. Agric. Food Chem. 2004, 52, 8242–8246. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Pihlava, J.M.; Hellström, J. Contents of phenolic acids, alkyl- and alkenylresorcinols, and avenanthramides in commercial grain products. J. Agric. Food Chem. 2005, 53, 8290–8295. [Google Scholar] [CrossRef] [PubMed]
- Kulawinek, M.; Jaromin, A.; Kozubek, A.; Zarnowski, R. Alkylresorcinols in Selected Polish Rye and Wheat Cereals and Whole-Grain Cereal Products. J. Agric. Food Chem. 2008, 56, 7236–7242. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Kamal-Eldin, A.; Fras, A.; Boros, D.; Aman, P. Alkylresorcinols in Wheat Varieties in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9722–9725. [Google Scholar] [CrossRef]
- Nyström, L.; Lampi, A.M.; Andersson, A.A. Phytochemicals and dietary fiber components in rye varieties in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9758–9766. [Google Scholar] [CrossRef]
- Landberg, R.; Kamal-Eldin, A.; Andersson, R.; Aman, P. Alkylresorcinol Content and Homologue Composition in Durum Wheat (Triticum durum) Kernels and Pasta Products. J. Agric. Food Chem. 2006, 54, 3012–3014. [Google Scholar] [CrossRef]
- Landberg, R.; Linko, A.M.; Kamal-Eldin, A.; Vessby, B.; Adlercreutz, H.; Aman, P. Human plasma kinetics and relative bioavailability of alkylresorcinols after intake of rye bran. J. Nutr. 2006, 136, 2760–2765. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Soroka, D.N.; Sang, S. Synthesis and inhibitory activities against colon cancer cell growth and proteasome of alkylresorcinols. J. Agric. Food Chem. 2012, 60, 8624–8631. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hao, Y.; Wang, Z.; Ni, F.; Wang, Y.; Gong, L.; Sun, B.; Wang, J. Identification, quantification and anti-inflammatory activity of 5-n-alkylresorcinols from 21 different wheat varieties. J. Agric. Food Chem. 2018, 66, 9241–9247. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other than those Referring to the Reduction of Disease Risk and to Children’s Develop-Ment and HealthText with EEA Relevance; European Commission: Brussels, Belgium, 2012. [Google Scholar]
- Ross, A.B.; Kochhar, S. Rapid and Sensitive Analysis of Alkylresorcinols from Cereal Grains and Products Using HPLC—Coularray-Based Electrochemical Detection. J. Agric. Food Chem. 2009, 57, 5187–5193. [Google Scholar] [CrossRef]
- Landberg, R.; Andersson, A.A.M.; Åman, P.; Kamal-Eldin, A. Comparison of GC and colorimetry for the determination of alkylresorcinol homologues in cereal grains and products. Food Chem. 2009, 113, 1363–1369. [Google Scholar] [CrossRef]
- Ross, A.B. Analysis of Alkylresorcinols in Cereal Grains and Products Using Ultrahigh-Pressure Liquid Chromatography with Fluorescence, Ultraviolet, and CoulArray Electrochemical Detection. J. Agric. Food Chem. 2012, 60, 8954–8962. [Google Scholar] [CrossRef]
- Knödler, M.; Most, M.; Schieber, A.; Carle, R. A novel approach to authenticity control of whole grain durum wheat (Triticum durum Desf.) flour and pasta, based on analysis of alkylresorcinol composition. Food Chem. 2010, 118, 177–181. [Google Scholar] [CrossRef]
- Meija, L.; Samaletdin, A.; Koskela, A.; Lejnieks, A.; Lietuvietis, V.; Adlercreutz, H. Alkylresorcinols in Latvian and Finnish breads. Integr. J. Food Sci. Nutr. 2013, 64, 117–121. [Google Scholar] [CrossRef]
- Sampietro, D.A.; Jimenez, C.M.; Belizán, M.M.; Vattuone, M.A.; Catalán, C.A.N. Development and validation of a micromethod for fast quantification of 5-n-alkylresorcinols in grains and whole grain products. Food Chem. 2013, 141, 3546–3551. [Google Scholar] [CrossRef]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Direct measurement of oleocanthal and oleacein levels in olive oil by quantitative 1H NMR. Establishment of a new index for the characterization of extra virgin olive oils. J. Agric. Food Chem. 2012, 60, 11696–11703. [Google Scholar] [CrossRef]
- Manoukian, P.; Melliou, E.; Liouni, M.; Magiatis, P. Identification and quantitation of benzoxazinoids in wheat malt beer by qNMR and GC–MS. LWT Food Sci. Technol. 2016, 65, 1133–1137. [Google Scholar] [CrossRef]
- Nikolantonaki, M.; Magiatis, P.; Waterhouse, A.L. Direct analysis of free and sulfite-bound carbonyl compounds in wine by two-dimensional quantitative proton and carbon nuclear magnetic resonance spectroscopy. Anal. Chem. 2015, 87, 10799–10806. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Pastor, I.; Luque-Muñoz, A.; Rivas, F.; Medina-O’Donnell, M.; Martinez, A.; Gonzalez-Maldonado, R.; Haidour, A.; Parra, A. Quantitative NMR analysis of L -Dopa in seeds from two varieties of Mucuna pruriens. Phytochem. Anal. 2019, 30, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Inagaki, R.; Sugimoto, N.; Akiyama, H.; Nagatsu, A. Application of a quantitative 1H-NMR (1H-qNMR) method for the determination of geniposidic acid and acteoside in Plantaginis semen. J. Nat. Med. 2017, 71, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, S.S.; Girreser, U.; Zidorn, C. Quantification of the total amount of black cohosh cycloartanoids by integration of one specific 1H NMR signal. J. Pharm. Biomed. Anal. 2018, 155, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, K.; Akihisa, T.; Tokuda, H.; Ukiya, M.; Higashihara, H.; Mukainaka, T.; Iizuka, M.; Hayashi, Y.; Kimura, Y.; Nishino, H. Sterol ferulates, sterols, and 5-alk(en)ylresorcinols from wheat, rye, and corn bran oils and their inhibitory effects on Epstein−Barr virus activation. J. Agric. Food Chem. 2003, 51, 6683–6688. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-Q.; Yang, C.-G.; Yang, L.-M.; Yang, L.-J. Ultrasound-assisted Wittig reaction and synthesis of 5-alkyl- and 5-alkenyl-resorcinols. J. Chem. Res. 2009, 2009, 183–185. [Google Scholar] [CrossRef]
- Pauli, G.F.; Gödecke, T.; Jaki, B.U.; Lankin, D.C. Quantitative 1H NMR. Development and potential of an analytical method: An update. J. Nat. Prod. 2012, 75, 834–851. [Google Scholar] [CrossRef]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR spectroscopy. Trends Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]
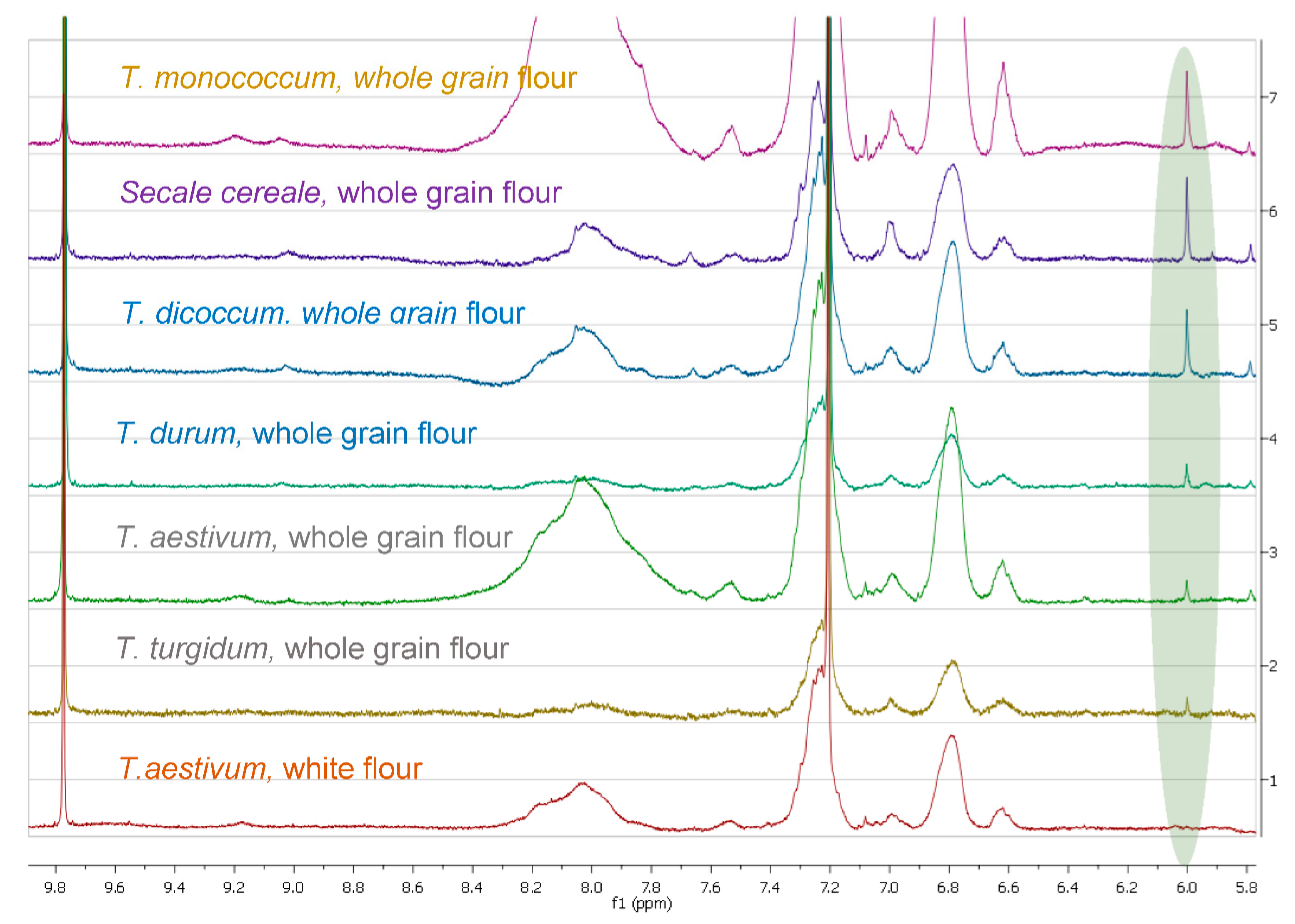
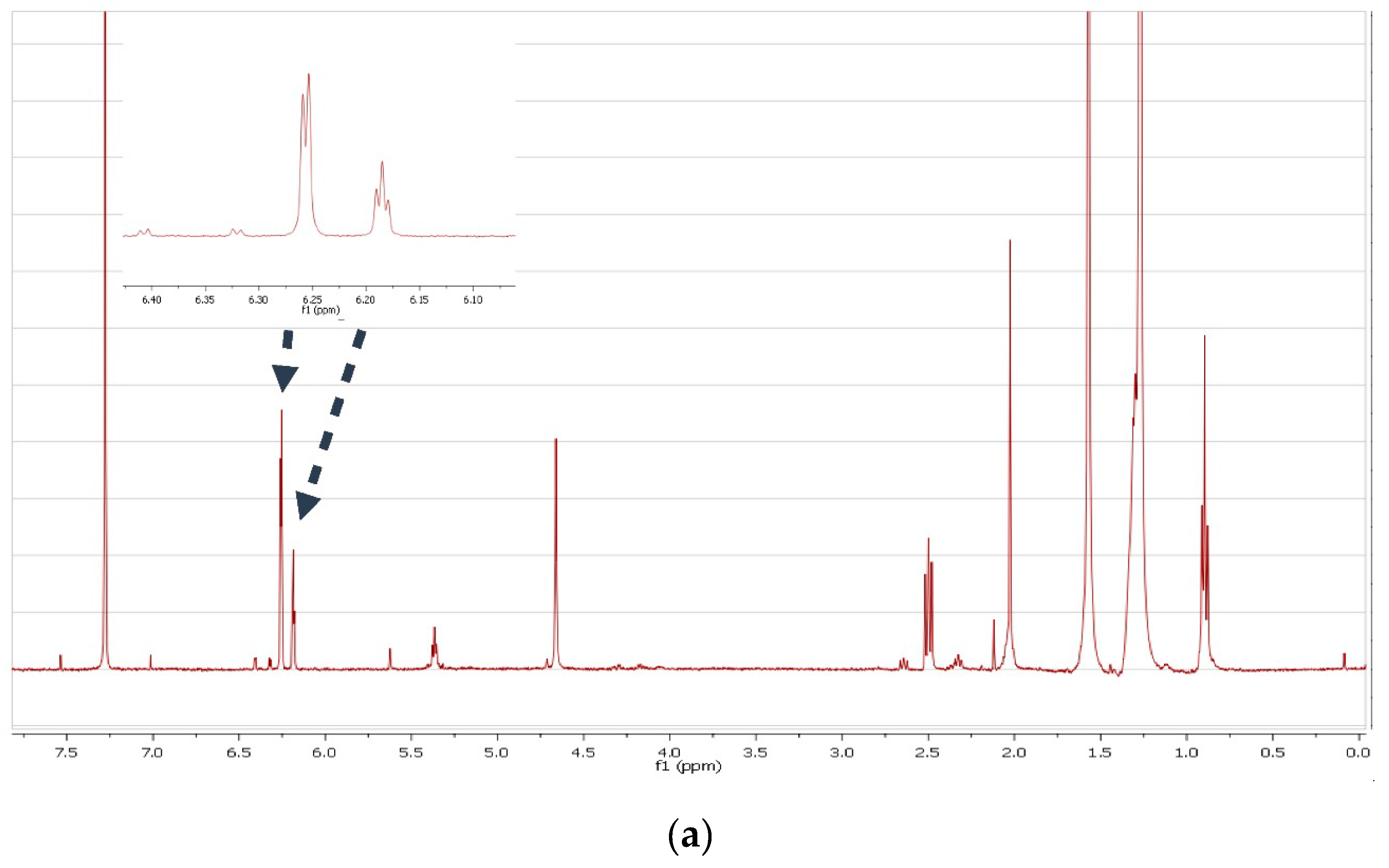
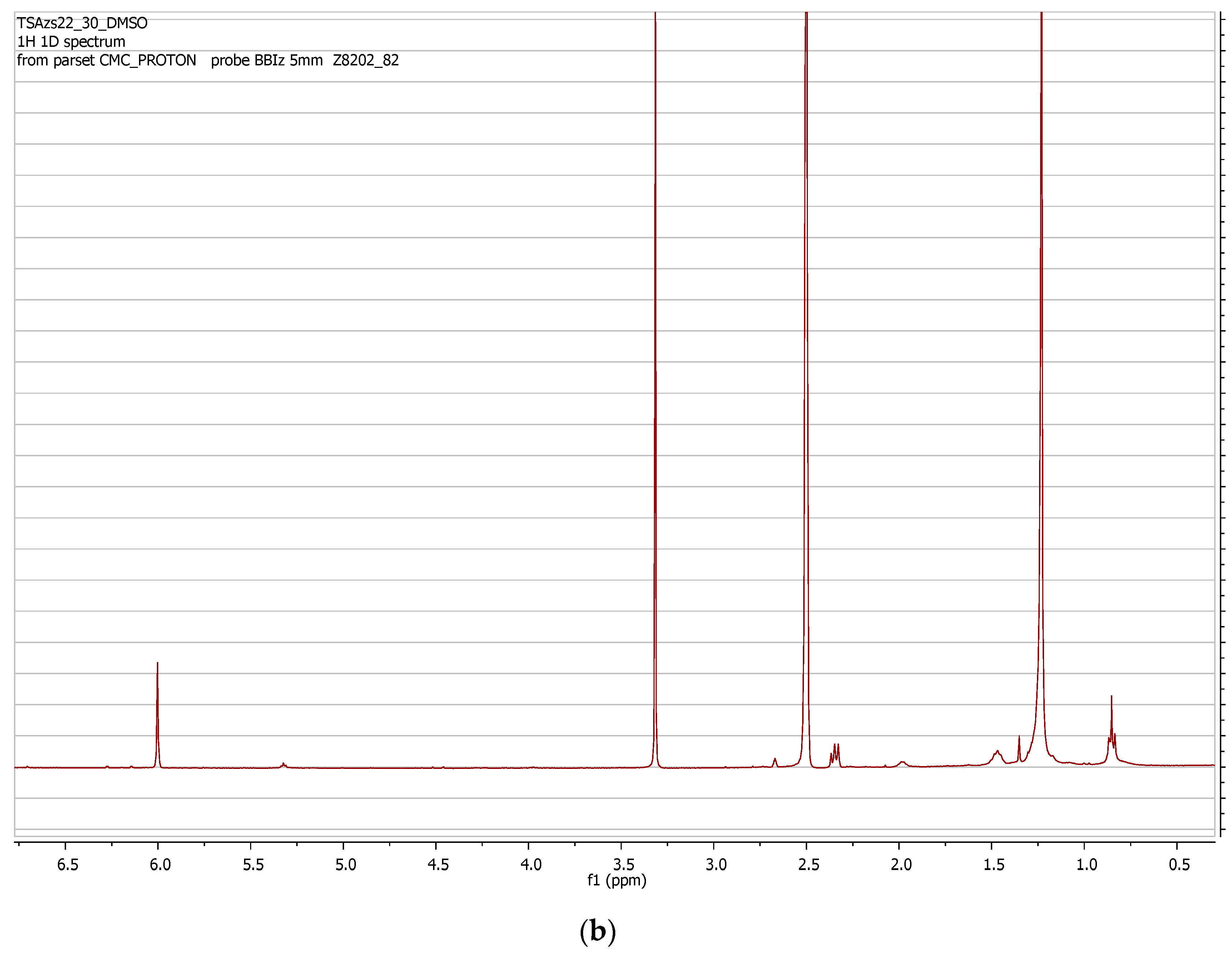
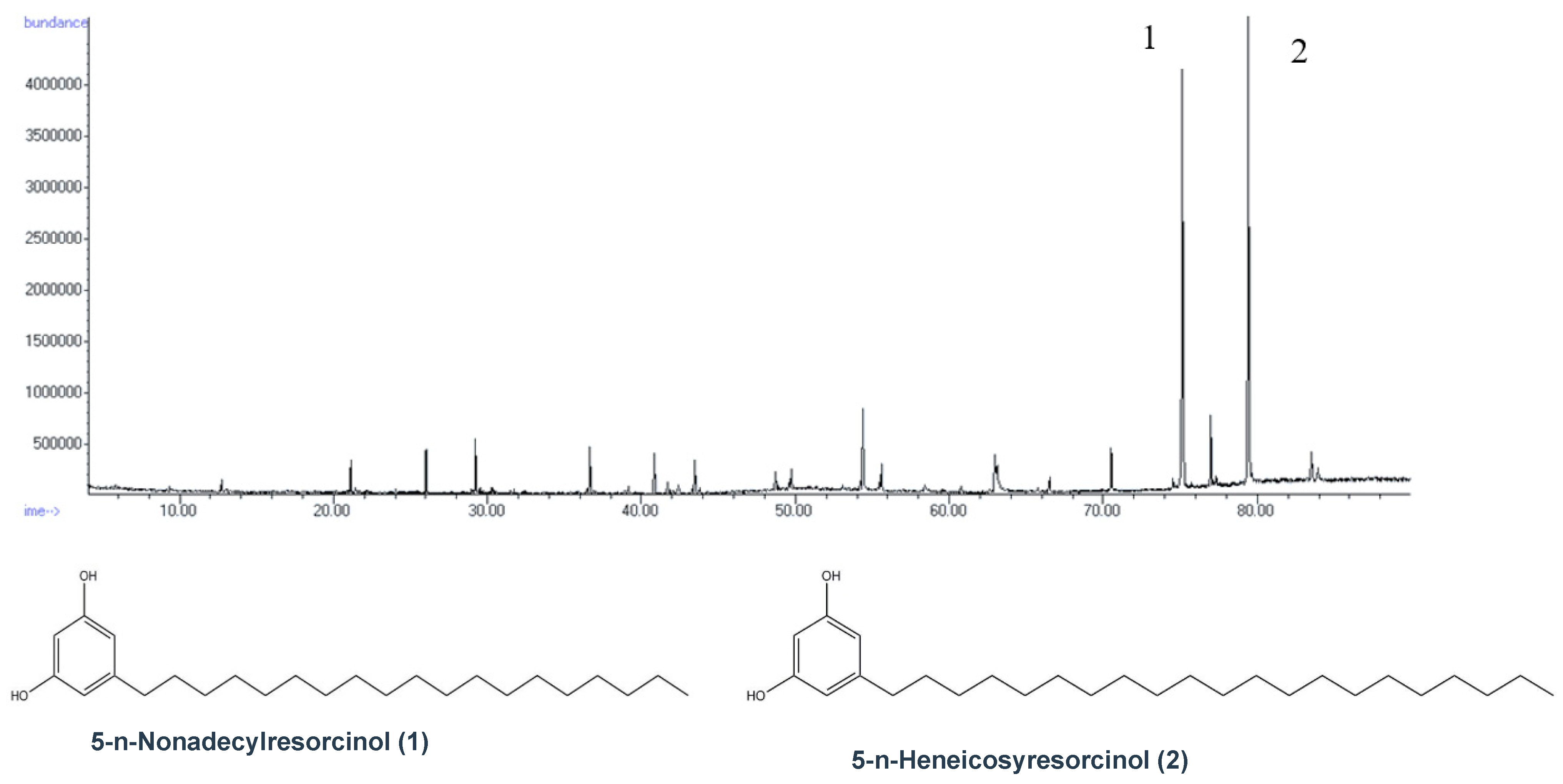
| Sample | ARS mg/Kg | ARS mmol/Kg |
|---|---|---|
| Flours | ||
| T. monococcum, whole-grain flour | 638 ± 32 | 1.64 ± 0.08 |
| Secale cereale, whole-grain flour | 574 ± 23 | 1.47 ± 0.06 |
| T. dicoccum, whole-grain flour | 510 ± 30 | 1.31 ± 0.07 |
| T. durum, whole-grain flour | 191 ± 6 | 0.49 ± 0.02 |
| T. aestivum, whole-grain flour | 121 ± 5 | 0.33 ± 0.01 |
| T. turgidum, whole-grain flour | 63 ± 3 | 0.16 ± 0.01 |
| T. aestivum, white-flour | ND | ND |
| Wheat grains | ||
| T. dicoccum | 1148 ± 58 | 2.95 ± 0.15 |
| T. monococcum | 925 ± 74 | 2.37 ± 0.19 |
| T. aestivum | 255 ± 23 | 0.65 ± 0.06 |
| T. durum (cv.deveta) | 255 ± 12 | 0.65 ± 0.03 |
| T. durum (cv.dourouki) | 191 ± 4 | 0.49 ± 0.01 |
| Processed products | ||
| T. dicoccum Bread from whole-grain flour | 455 ± 32 | 1.17 ± 0.08 |
| T. dicoccum Flakes | 1090 ± 54 | 2.79 ± 0.14 |
| T. dicoccum Wafers | 351 ± 14 | 0.9 ± 0.04 |
| T. dicoccum Pasta | 330 ± 10 | 0.85 ± 0.03 |
| T. monococcum Flakes | 1084 ± 97 | 2.78 ± 0.25 |
| T. aestivum Bread from white flour | ND | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsirivakou, A.; Melliou, E.; Magiatis, P. A Method for the Rapid Measurement of Alkylresorcinols in Flour, Bread and Related Products Based on 1H qNMR. Foods 2020, 9, 1025. https://doi.org/10.3390/foods9081025
Tsirivakou A, Melliou E, Magiatis P. A Method for the Rapid Measurement of Alkylresorcinols in Flour, Bread and Related Products Based on 1H qNMR. Foods. 2020; 9(8):1025. https://doi.org/10.3390/foods9081025
Chicago/Turabian StyleTsirivakou, Athina, Eleni Melliou, and Prokopios Magiatis. 2020. "A Method for the Rapid Measurement of Alkylresorcinols in Flour, Bread and Related Products Based on 1H qNMR" Foods 9, no. 8: 1025. https://doi.org/10.3390/foods9081025
APA StyleTsirivakou, A., Melliou, E., & Magiatis, P. (2020). A Method for the Rapid Measurement of Alkylresorcinols in Flour, Bread and Related Products Based on 1H qNMR. Foods, 9(8), 1025. https://doi.org/10.3390/foods9081025






