Impact of Stability of Enriched Oil with Phenolic Extract from Olive Mill Wastewaters
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection
2.3. OMWW Extraction
2.4. Production of Enriched Sunflower Oil
Extraction of Antioxidant Compounds
2.5. Determination of Total Phenol Content and Evaluation of Antioxidant Activity
Identification and Quantification of Phenolic Compounds
2.6. Measurement of Chemical and Physical Properties of Oils
Sunflower Oil Oxidative Stability in Accelerated Storage Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. Water Extract Characterization
3.2. Evaluation of Effect of WE on Sunflower Oil Stability
3.2.1. Qualitative Parameters
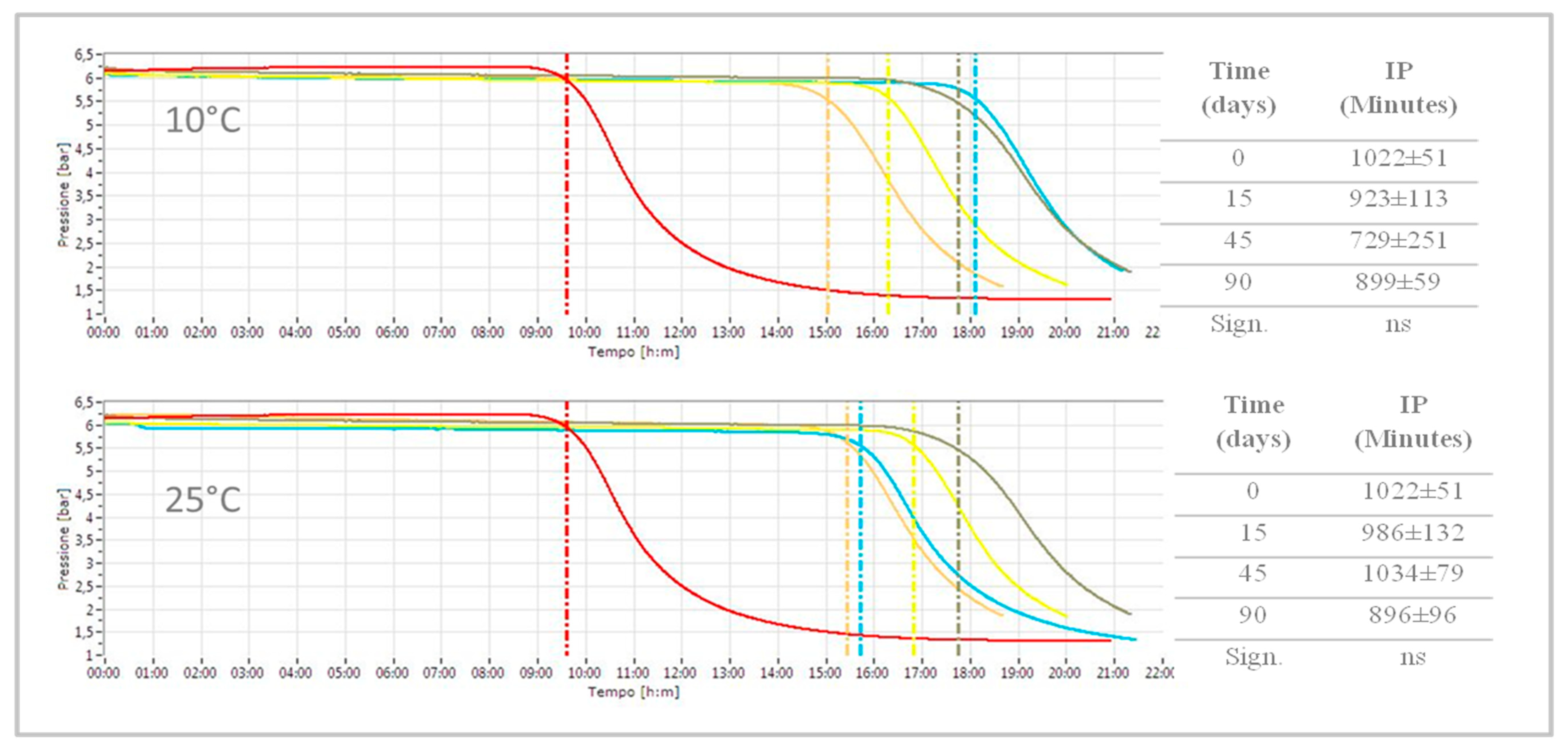
3.2.2. Oxidative Stability
3.2.3. Phenolic Composition and Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yay, A.; Oral, H.V.; Onay, T.T.; Yengun, O. A study on olive mill wastewater management in Turkey: A questionnaire and experimental approach. Resour. Conserv. Recycl. 2012, 60, 64–71. [Google Scholar]
- Espadas-Aldana, G.; Vialle, C.; Belaud, J.P.; Vaca-Garcia, C.; Sablayrolles, C. Analysis and trends for Life Cycle Assessment of olive oil production. Sust. Prod. Consum. 2019, 19, 216–230. [Google Scholar] [CrossRef]
- Cinar, O.; Alma, M.H. Environmental assessment of olive oil production: Olive oil mill wastes and their disposal. Acta Hortic. 2008, 791, 645–649. [Google Scholar] [CrossRef]
- Markou, G.; Georgakakis, D.; Plagou, K.; Salakou, G.; Christopoulou, N. Balanced waste management of 2- and 3-phase olive oil mills in relation to the seed oil extraction plant. Terr. Aquat. Environ. Toxicol. 2010, 4, 109–112. [Google Scholar]
- El Hassani, F.Z.; Fadile, A.; Faouzi, M.; Zinedine, A.; Merzouki, M.; Benlemlih, M. The long term effect of Olive Mill Wastewater (OMW) on organic matter humification in a semi-arid soil. Heliyon 2020, 6, e03181. [Google Scholar] [CrossRef]
- Yangui, A.; Abderrabba, M. Towards a high yield recovery of polyphenols from olive mill wastewater on activated carbon coated with milk proteins: Experimental design and antioxidant activity. Food Chem. 2018, 262, 102–109. [Google Scholar] [CrossRef]
- Dutournié, P.; Jeguirim, M.; Khiari, B.; Goddard, M.L.; Jellali, S. Olive mill wastewater: From a pollutant to green fuel, agricultural water source and biofertilizer. Part 2: Water Recovery. Water 2019, 11, 768. [Google Scholar] [CrossRef]
- Soberón, L.F.; Carelli, A.A.; González, M.T.; Ceci, L.N. Method for phenol recovery from “alperujo”: Numerical optimization and predictive model. Eur. Food Res. Technol. 2019, 245, 1641–1650. [Google Scholar] [CrossRef]
- Caporaso, N.; Formisano, D.; Genovese, A. Use of phenolic compounds from olive mill wastewater as valuable ingredients for functional foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2829–2841. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Sicari, V.; Piscopo, A.; Louadj, L. Antioxidant activity of olive oil mill wastewater obtained from different thermal treatments. Grasas y Aceites 2012, 63, 209–213. [Google Scholar] [CrossRef]
- Xynos, N.; Abatis, D.; Argyropoulou, A.; Polychronopoulos, P.; Aligiannis, N.; Skaltsounis, A.L. Development of a sustainable procedure for the recovery of hydroxytyrosol from table olive processing wastewater using adsorption resin technology and centrifugal partition chromatography. Planta Med. 2015, 81, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- De Bruno, A.; Romeo, R.; Fedele, F.L.; Sicari, A.; Piscopo, A.; Poiana, M. Antioxidant activity shown by olive pomace extracts. J. Environ. Sci. Health 2018, 53 Pt B, 526–533. [Google Scholar] [CrossRef]
- Galanakis, C.M. Emerging technologies for the production of nutraceuticals from agricultural by-products: A viewpoint of opportunities and challenges. Food Bioprod. Process. 2013, 91, 575–579. [Google Scholar] [CrossRef]
- Frascari, D.; Bacca, A.E.M.; Wardenaar, T.; Oertléc, E.; Pinellia, D. Continuous flow adsorption of phenolic compounds fromolive mill wastewater with resin XAD16N: Life cycle assessment, cost–benefit analysis and process optimization. J. Chem. Technol. Biotechnol. 2019, 94, 1968–1981. [Google Scholar] [CrossRef]
- Ferri, F.; Bertin, L.; Scoma, A.; Marchettia, L.; Fava, F. Recovery of low molecular weight phenols through solid-phase extraction. Chem. Eng. J. 2011, 166, 994–1001. [Google Scholar] [CrossRef]
- Hellwig, V.; Gasser, J. Polyphenols from waste streams of food industry: Valorisation of blanch water from marzipan production. Phytochem. Rev. 2020. [Google Scholar] [CrossRef]
- Scoma, A.; Bertin, L.; Zanaroli, G.; Fraraccio, S.; Fava, F. A physicochemical-biotechnological approach for an integrated valorization of olive mill wastewater. Bioresour. Technol. 2011, 102, 10273–10279. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Vavouraki, A.I.; Kornaros, M.E.; Paraskeva, C.A. Purification of olive mill wastewater phenols through membrane filtration and resin adsorption/desorption. J. Hazard. Mater. 2015, 285, 69–76. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; González-Hernández, R.; Martinez-Ferez, A. On the effect of the operating parameters for two-phase olive-oil washing wastewater combined phenolic compounds recovery and reclamation by novel ion exchange resins. Sep. Purif. Technol. 2018, 195, 50–59. [Google Scholar] [CrossRef]
- Bertin, L.; Ferri, F.; Scoma, A.; Marchettia, L.; Fava, F. Recovery of high added value natural polyphenols from actual olive millwastewater through solid phase extraction. Chem. Eng. J. 2011, 171, 1287–1293. [Google Scholar] [CrossRef]
- Papaoikonomou, L.; Labanaris, K.; Kaderides, K.; Goula, A.M. Adsorption–desorption of phenolic compounds from olive mill wastewater using a novel low-cost biosorbent. Environ. Sci. Pollut. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Yu, R.; Li, J.; Wang, N.; Wang, N.; Niu, L.; Zhang, Y. Application of several novel natural antioxidants to inhibit oxidation of tree peony seed oil. Cyta J. Food 2018, 16, 1071–1078. [Google Scholar] [CrossRef]
- Castelo-Branco, V.N.; Santana, I.; Di-Sarli, V.O.; Freitas, S.P.; Torres, A.G. Antioxidant capacity is a surrogate measure of the quality and stability of vegetable oils. Eur. J. Lipid Sci. Technol. 2016, 118, 224–235. [Google Scholar] [CrossRef]
- Gotor, A.A.; Rhazi, L. Effects of refining process on sunflower oil minor components: A review. OCL 2016, 23, D207. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.D.; Alves, V. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar]
- Jiménez-Aspee, F.; Quispe, C.; del Pilar, C.S.M.; Gonzalez, J.F.; Hüneke, E.; Theoduloz, C.; Schmeda-Hirschmann, G. Antioxidant activity and characterization of constituents in copao fruits (Eulychnia acida Phil., Cactaceae) by HPLC–DAD–MS/MSn. Food Res. Int. 2014, 62, 286–298. [Google Scholar] [CrossRef]
- Suàrez, M.; Valls, R.M.; Motilva, M.P.; Màcia, A.; Fernàndez, S.; Giralt, M.; Solà, R.; Motilva, M.J. Study of stability during storage a phenol-enriched olive oil. Eur. J. Lipid Sci. Technol. 2011, 113, 894–903. [Google Scholar] [CrossRef]
- Baiano, A.; Gambacorta, G.; Terracone, C.; Previtali, M.A.; Lamacchia, C.; La Notte, E. Changes in phenolic content and antioxidant activity of Italian Extra-Virgin Olive Oils during storage. J. Food Sci. 2009, 4, 177–183. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Evans, C.R. Antioxidant activity applying an improved ABTS radical cation decolorazation assay. Free Radic Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Romeo, R.; De Bruno, A.; Imeneo, V.; Piscopo, A.; Poiana, M. Evaluation of enrichment with antioxidants from olive oil mill wastes in hydrophilic model system. J. Food Proc. Pres. 2019, 43, e14211. [Google Scholar] [CrossRef]
- Pizarro, M.L.; Becerra, M.; Sayago, A.; Beltrán, M.; Beltrán, R. Comparison of different extraction methods to determine phenolic compounds in virgin olive oil. Food Anal. Methods 2013, 6, 123–132. [Google Scholar] [CrossRef]
- AOCS. Method Ca 5a 40. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 6th ed.; AOCS Press: Champaign, IL, USA, 2017. [Google Scholar]
- AOCS. Method Cd 8–53. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 6th ed.; AOCS Press: Champaign, IL, USA, 2017. [Google Scholar]
- AOCS. Method Ch 5-91. In Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS Press: Champaign, IL, USA, 1989. [Google Scholar]
- AOCS. Method Cd 12c-16. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 6th ed.; AOCS Press: Champaign, IL, USA, 2017. [Google Scholar]
- Senol, A.; Hasdemir, İ.M.; Hasdemir, B.; Kurdaş, İ. Adsorptive removal of biophenols from olive mill wastewaters (OMW) by activated carbon: Mass transfer, equilibrium and kinetic studies. Asia Pac. J. Chem. Eng. 2017, 12, 128–146. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Yuan, J.; Zhang, C. Adsorption characteristics of adsorbent resins and antioxidant capacity for enrichment of phenolics from two-phase olive waste. J. Chrom. B 2017, 1040, 38–46. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Jia, Z.; Scarlett, C.J.; Sheng, Z. Adsorption/desorption characteristics and enrichment of quercetin, luteolin and apigenin from Flos populi using macroporous resin. Rev. Bras. Farmacogn. 2019, 29, 69–76. [Google Scholar] [CrossRef]
- Lins, P.G.; Pugine, S.M.P.; Scatolini, A.M.; de Melo, M.P. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon 2018, 4, e00805. [Google Scholar] [CrossRef]
- Abramovič, H.; Grobin, B.; Ulrih, N.P.; Cigic, B. Relevance and standardization of in vitro antioxidant assays: ABTS, DPPH, and Folin–Ciocalteu. J. Chem. 2018. [Google Scholar] [CrossRef]
- Pal, U.S.; Patra, R.K.; Sahoo, N.R.; Bakhara, C.K.; Panda, M.K. Effect of refining on quality and composition of sunflower oil. J. Food Sci. Technol. 2015, 52, 4613–4618. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Kroh, L.W.; Mörsel, J.T. Radical scavenging activity of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.), and niger (Guizotia abyssinica Cass.) crude seed oils and oil fractions. J. Agric. Food Chem. 2003, 51, 6961–6969. [Google Scholar] [CrossRef]
- Fki, I.; Allouche, N.; Sayadi, S. The use of polyphenolic extrcat, purified hydroxytyrosol and 3, 4-dihidroxyphenyl acetic acid from olive mill wastewater for the stabilization of refined oils: A potential alternative to synthetic antioxidants. Food Chem. 2005, 93, 197–204. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; Lembo, G.; Aretini, A.; Nag, A. Studies on oxidative stabilisation of lard by natural antioxidants recovered from olive-oil mill wastewater. Food Chem. 2007, 100, 998–1004. [Google Scholar] [CrossRef]
- Sayyari, Z.; Farahmandfar, R. Stabilization of sunflower oil with pussy willow (Salix aegyptiaca) extract and essential oil. Food Sci. Nutr. 2017, 5, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Koprivnjak, O.; Škevin, D.; Valić, S.; Majetić, V.; Petričević, S.; Ljubenkov, I. The antioxidant capacity and oxidative stability of virgin olive oil enriched with phospholipids. Food Chem. 2008, 111, 121–126. [Google Scholar] [CrossRef]
- Lafka, T.I.; Lazou, A.E.; Sinanoglou, V.J.; Lazos, E.S. Phenolic extracts from wild olive leaves and their potential as edible oils antioxidants. Foods 2013, 2, 18–31. [Google Scholar] [CrossRef]
- Suarez, M.; Romero, M.P.; Motilva, M.J. Development of a phenol-enriched olive oil with phenolic compounds from olive cake. J. Agric. Food Chem. 2010, 58, 10396–10403. [Google Scholar] [CrossRef]
- Judde, A.; Villeneuve, P.; Rossignol-Castera, A.; Le Guillou, A. Antioxidant effect of soy lecithins on vegetable oil stability and their synergism with tocopherols. J. Am. Oil Chem. Soc. 2003, 80, 1209–1215. [Google Scholar] [CrossRef]
- de Medina, V.S.; Priego-Capote, F.; de Castro, M.D.L. Characterization of refined edible oils enriched with phenolic extracts from olive leaves and pomace. J. Agric. Food Chem. 2012, 60, 5866–5873. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
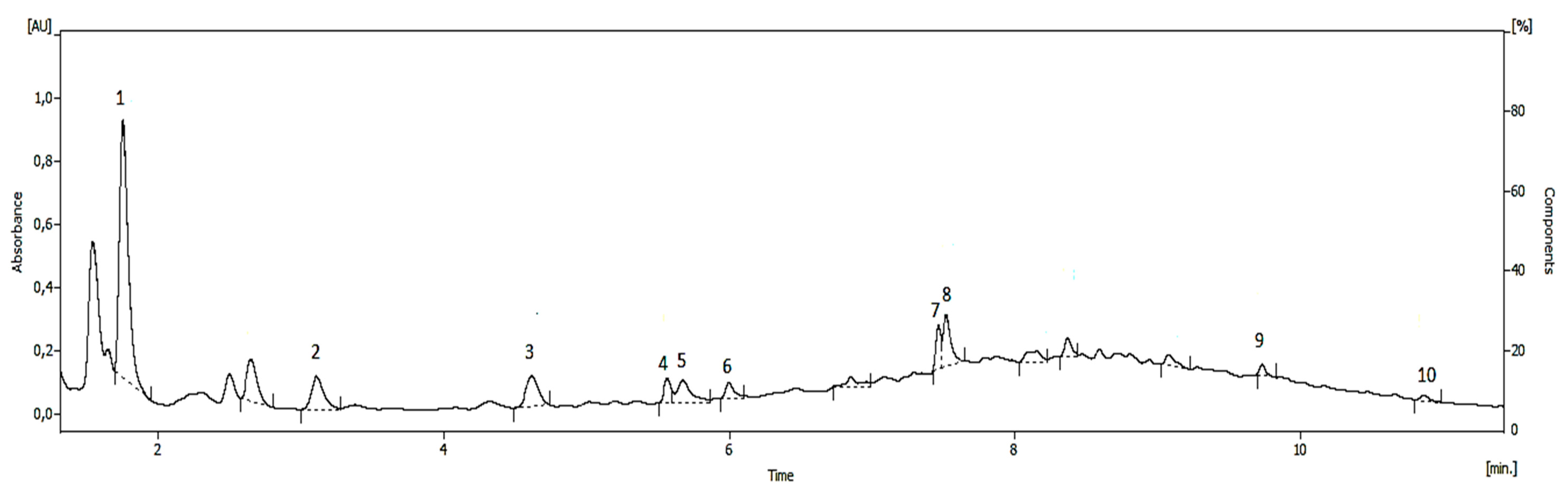
| Hydroxytyrosol | 834.51 ± 0.71 |
| Tyrosol | 147.55 ± 0.70 |
| Chlorogenic Acid | 16.06 ± 0.70 |
| Vanillic Acid | 40.25 ± 0.17 |
| Caffeic Acid | 20.53 ± 0.47 |
| P-Cumaric Acid | 61.06 ± 0.71 |
| Oleuropein | 65.09 ± 0.67 |
| Apigenin | 74.62 ± 0.71 |
| Verbascoside | 876.91 ± 0.91 |
| Luteolin | 14.11 ± 0.89 |
| Total Polyphenol Content | 788.96 ± 1.41 |
| ABTS | 2569.19 ± 399.90 |
| DPPH | 114.37 ± 151.87 |
| Free acidity (Oleic acid %) | 0.05 ± 0.00 |
| Peroxide value (mEq O2 kg−1) | 6.10 ± 0.15 |
| Moisture (%) | 0.31 ± 0.01 |
| Induction Period (minutes) | 576 ± 0.01 |
| K232 | 2.45 ± 0.09 |
| K270 | 1.24 ± 0.08 |
| Temperature | Time (days) | Free Acidity (Oleic acid %) | Peroxide Value (mEq O2 kg−1) | Moisture (%) | K232 | K270 |
|---|---|---|---|---|---|---|
| 0 | 0.28 ± 0.02 b | 3.07 ± 0.03 c | 1.08 ± 0.14 | 2.49 ± 0.13 | 1.40 ± 0.01 | |
| 15 | 0.28 ± 0.03 b | 2.95 ± 0.13 c | 0.95 ± 0.03 | 2.57 ± 0.35 | 1.33 ± 0.20 | |
| 10 °C | 45 | 0.23 ± 0.03 c | 5.51 ± 0.21 a | 1.0 ± 0.9 | 2.55 ± 0.21 | 1.50 ± 0.04 |
| 90 | 0.32 ±0.02 a | 3.85 ± 0.16 b | 1.0 ± 0.09 | 2.55 ± 0.21 | 1.50 ± 0.04 | |
| Significance | ** | ** | ns | ns | ns | |
| 0 | 0.28 ± 0.02 b | 3.07 ± 0.03 d | 1.08 ± 0.14 | 2.49 ± 0.13 | 1.40 ± 0.01 | |
| 15 | 0.28 ± 0.00 b | 4.32 ± 0.01 b | 1.04 ± 0.16 | 2.84 ± 0.17 | 1.42 ± 0.03 | |
| 25 °C | 45 | 0.23 ± 0.00 c | 5.47 ± 0.18 a | 1.80 ± 0.53 | 2.86 ± 0.39 | 1.40 ± 0.03 |
| 90 | 0.35 ± 0.03 a | 3.78 ± 0.10 c | 0.77 ± 0.54 | 2.55 ± 0.21 | 1.37 ± 0.05 | |
| Significance | ** | ** | ns | ns | ns |
| 10 °C | 0 | 45 | 90 | Significance |
| Hydroxytyrosol | 39.65 ± 0.6 a | 16.25 ± 0.47 b | 15.06 ± 1.95 b | ** |
| Tyrosol | 36.15 ± 0.16 a | 24.78 ± 0.5 b | 14.44 ± 1.23 c | ** |
| Vanillic acid | 1.25 ± 0.01 a | 0.70 ± 0.02 c | 0.70 ± 0.01 b | ** |
| Caffeic acid | 20.79 ± 0.59 a | 13.66 ± 0.02 b | 13.66 ± 0.00 b | ** |
| Verbascoside | 9.11 ± 0.12 a | 8.74 ± 0.04 b | 8.92 ± 0.06 b | ** |
| Luteolin | 18.19 ± 0.17 a | 12.19 ± 0.00 b | 12.24 ± 0.04 b | ** |
| Apigenin | 11.05 ± 0.23 a | 4.59 ± 0.03 b | 4.69 ± 0.03 b | ** |
| 25 °C | 0 | 45 | 90 | Significance |
| Hydroxytyrosol | 39.65 ± 0.6 a | 15.77 ± 0.35 b | 15.88 ± 1.24 b | ** |
| Tyrosol | 36.15 ± 0.16 a | 25.54 ± 0.32 b | 15.47 ± 1.29 c | ** |
| Vanillic acid | 1.25 ± 0.01 a | 0.79 ± 0.04 c | 0.98 ± 0.04 b | ** |
| Caffeic acid | 20.79 ± 0.59 a | 13.72 ± 0.02 b | 13.79 ± 0.00 b | ** |
| Verbascoside | 9.11 ± 0.12 a | 8.73 ± 0.00 b | 8.87 ± 0.02 b | ** |
| Luteolin | 18.19 ± 0.17 a | 12.19 ± 0.00 b | 12.18 ± 0.02 b | ** |
| Apigenin | 11.06 ± 0.23 a | 4.61 ± 0.04 b | 4.74 ± 0.03 b | ** |
| Temperature | Time (days) | TPC | ABTS | DPPH | ORAC |
|---|---|---|---|---|---|
| 0 | 37 ± 1 a | 1536.18 ± 1.55 a | 74.67 ± 2.79 b | 157.39 ± 0.86 a | |
| 15 | 26 ± 5 b | 1283.06 ± 4.93 b | 80.82 ± 2.50 a | 143.82 ± 0.88 c | |
| 10 °C | 45 | 23 ± 2 b | 1203.61 ± 7.69 c | 56.63 ± 1.66 d | 127.17 ± 0.91 d |
| 90 | 23 ± 3 b | 1111.41 ± 9.78 d | 68.29 ± 0.88 c | 151.58 ± 1.22 b | |
| Significance | ** | ** | ** | ** | |
| 0 | 37 ± 1 a | 1536.18 ± 1.55 a | 74.67 ± 2.79 a | 157.39 ± 0.86 a | |
| 15 | 25 ± 3 b | 1285.05 ± 5.09 b | 68.29 ± 3.45 a | 153.21 ± 0.85 b | |
| 25 °C | 45 | 25 ± 2 b | 1240.37 ± 11.13 b | 55.19 ± 2.76 b | 128.90 ± 0.80 c |
| 90 | 24 ± 2 b | 1204.21 ± 83.51 b | 69.58 ± 1.27 b | 152.15 ± 1.53 b | |
| Significance | ** | ** | ** | ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeo, R.; De Bruno, A.; Imeneo, V.; Piscopo, A.; Poiana, M. Impact of Stability of Enriched Oil with Phenolic Extract from Olive Mill Wastewaters. Foods 2020, 9, 856. https://doi.org/10.3390/foods9070856
Romeo R, De Bruno A, Imeneo V, Piscopo A, Poiana M. Impact of Stability of Enriched Oil with Phenolic Extract from Olive Mill Wastewaters. Foods. 2020; 9(7):856. https://doi.org/10.3390/foods9070856
Chicago/Turabian StyleRomeo, Rosa, Alessandra De Bruno, Valeria Imeneo, Amalia Piscopo, and Marco Poiana. 2020. "Impact of Stability of Enriched Oil with Phenolic Extract from Olive Mill Wastewaters" Foods 9, no. 7: 856. https://doi.org/10.3390/foods9070856
APA StyleRomeo, R., De Bruno, A., Imeneo, V., Piscopo, A., & Poiana, M. (2020). Impact of Stability of Enriched Oil with Phenolic Extract from Olive Mill Wastewaters. Foods, 9(7), 856. https://doi.org/10.3390/foods9070856









