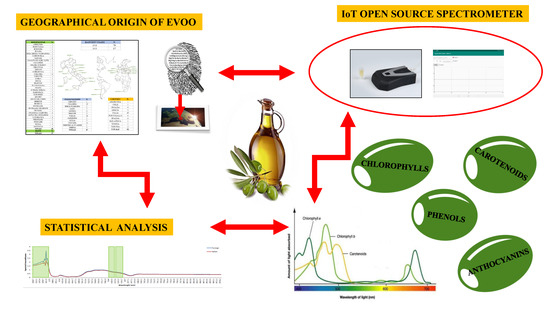
An Artificial Intelligence Approach for Italian EVOO Origin Traceability through an Open Source IoT Spectrometer
Abstract
1. Introduction
2. Materials and Methods
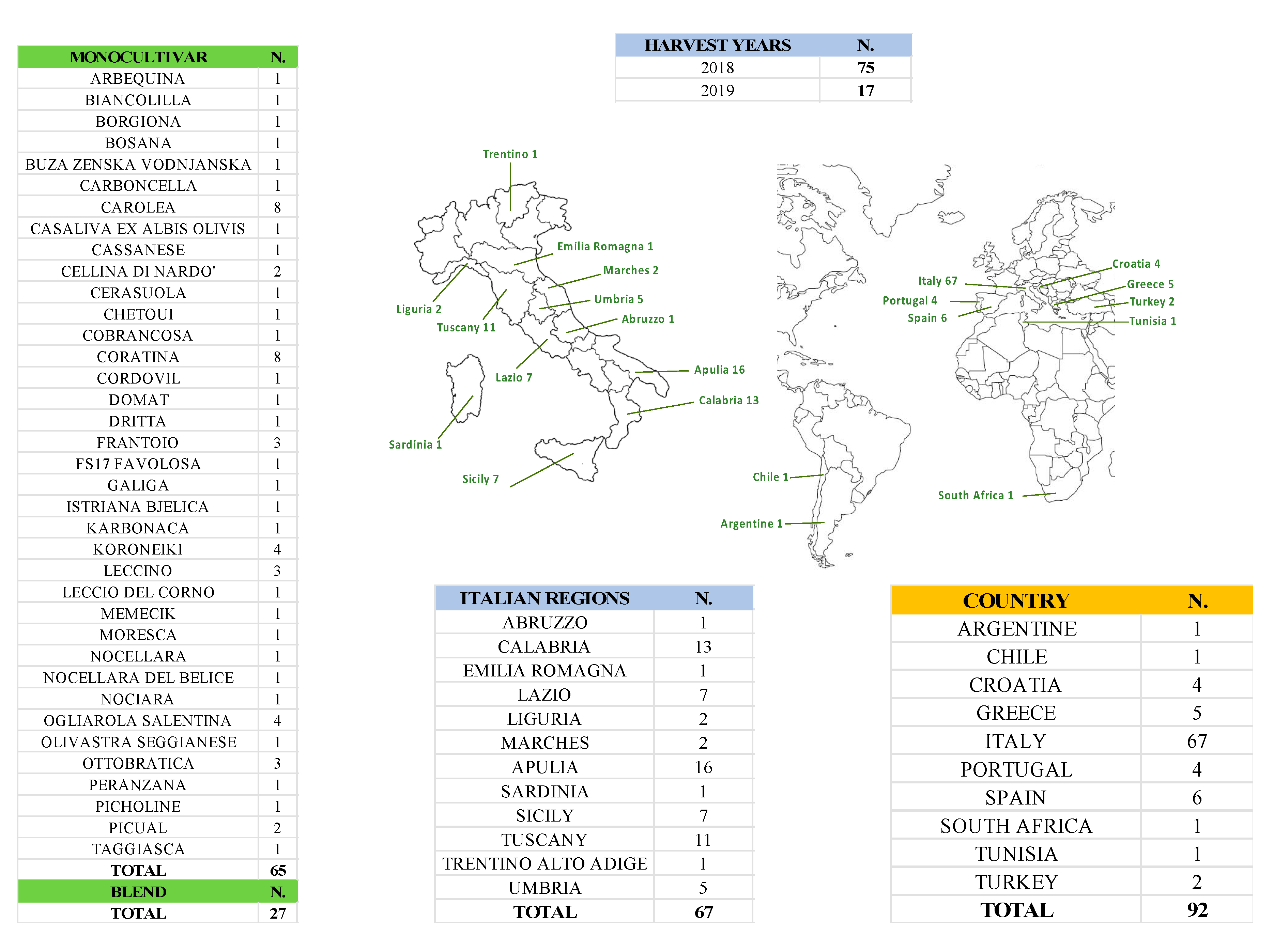
2.1. EVOO Samples
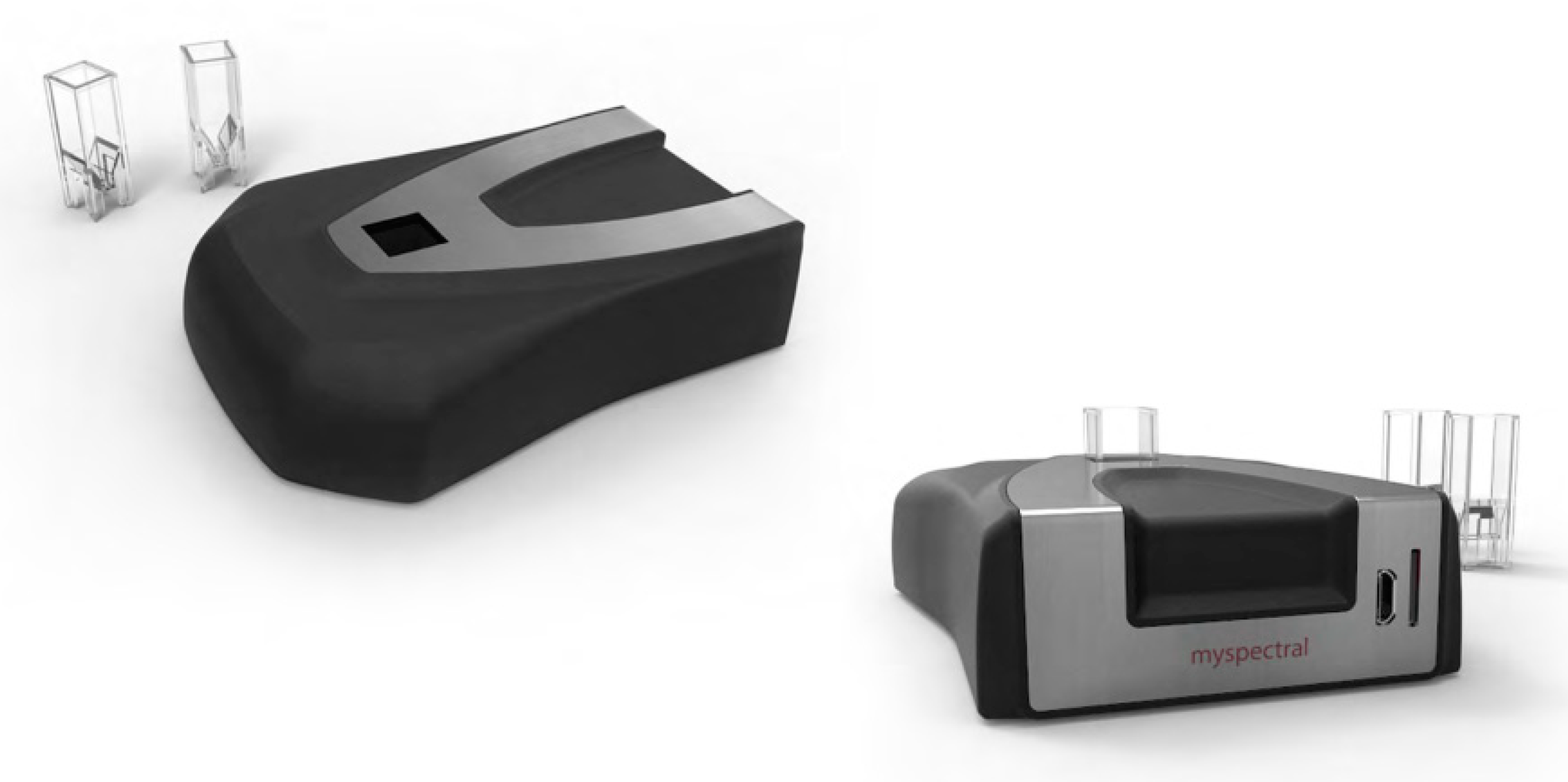
2.2. The Open Source IoT Spectrometer
2.3. Statistical Analysis
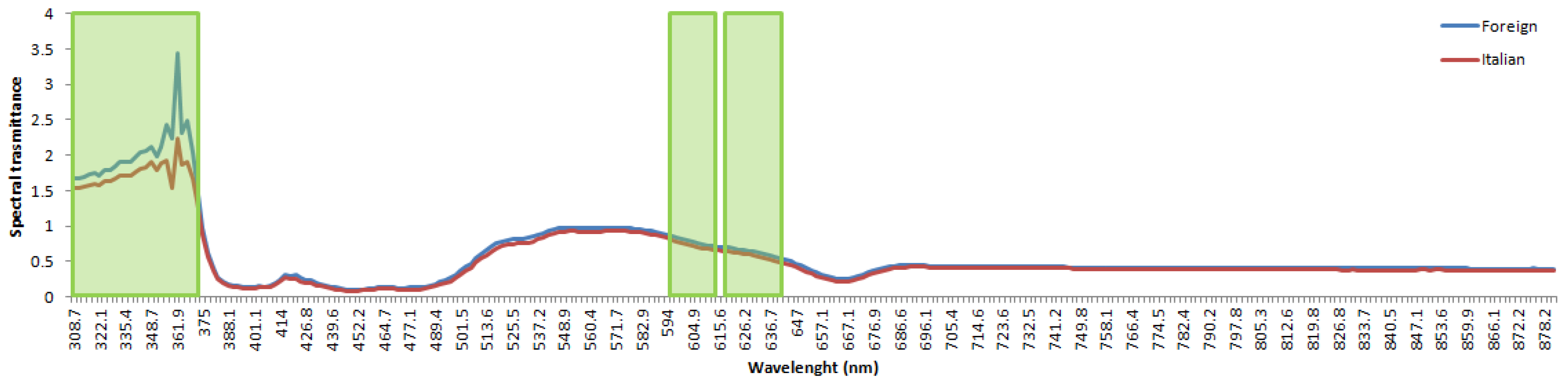
3. Results and Discussion
3.1. Artificial Intelligence Modeling Based on VIS-NIR Spectra
3.2. Feature Importance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benito, M.; Oria, R.; Sánchez-Gimeno, A.C. Characterization of the olive oil from three potentially interesting varieties from Aragon (Spain). Food Sci. Technol. Int. 2010, 16, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, M.; Bucci, R.; Magrì, A.D.; Magrì, A.L.; Marini, F. Tracing the origin of extra virgin olive oils by infrared spectroscopy and chemometrics: A case study. Anal. Chim. Acta 2012, 717, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Chiavaro, E.; Cerretani, L.; Di Matteo, A.; Barnaba, C.; Bendini, A.; Iacumin, P. Application of a multidisciplinary approach for the evaluation of traceability of extra virgin olive oil. Eur. J. Lipid Sci. Technol. 2011, 113, 1509–1519. [Google Scholar] [CrossRef]
- Violino, S.; Pallottino, F.; Sperandio, G.; Figorilli, S.; Antonucci, F.; Ioannoni, V.; Fappiano, D.; Costa, C. Are the innovative electronic labels for extra virgin olive oil sustainable, traceable, and accepted by consumers? Foods 2019, 8, 529. [Google Scholar] [CrossRef]
- Girelli, C.R.; Del Coco, L.; Zelasco, S.; Salimonti, A.; Conforti, F.L.; Biagianti, A.; Barbini, D.; Fanizzi, F.P. Traceability of “Tuscan PGI” extra virgin olive oils by 1H NMR metabolic profiles collection and analysis. Metabolites 2018, 8, 60. [Google Scholar] [CrossRef]
- Cosio, M.S.; Ballabio, D.; Benedetti, S.; Gigliotti, C. Geographical origin and authentication of extra virgin olive oils by an electronic nose in combination with artificial neural networks. Anal. Chim. Acta 2006, 567, 202–210. [Google Scholar] [CrossRef]
- Ferronato, M. Il Mercato dell’Olio di Oliva Made in Italy Tra Vulnerabilità ed Eccellenza. Bachelor’s Thesis, Università Ca’Foscari, Venezia, Italy, 2016. [Google Scholar]
- Cappelli, L.; D’ascenzo, F.; Ruggieri, R.; Rossetti, F.; Scalingi, A. The attitude of consumers towards “Made in Italy” products. An empirical analysis among Italian customers. Manag. Mark. Chall. Knowl. Soc. 2019, 14, 31–47. [Google Scholar] [CrossRef]
- Violino, S.; Antonucci, F.; Pallottino, F.; Cecchini, C.; Figorilli, S.; Costa, C. Food traceability: A term map analysis basic review. Eur. Food Res. Technol. 2019, 1–11. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 178/2002 of the European parliament and of the council. Laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off. J. Eur. Union 2002, 31, 1–24. [Google Scholar]
- Violino, S.; Pallottino, F.; Sperandio, G.; Figorilli, S.; Ortenzi, L.; Tocci, F.; Vasta, S.; Imperi, G.; Costa, C. A full technological traceability system for extra virgin olive oil. Foods 2020, 9, 624. [Google Scholar] [CrossRef]
- Antonucci, F.; Figorilli, S.; Costa, C.; Pallottino, F.; Raso, L.; Menesatti, P. A review on blockchain applications in the agri-food sector. J. Sci. Food Agric. 2019, 99, 6129–6138. [Google Scholar] [CrossRef] [PubMed]
- Espiñeira, M.; Santaclara, F.J. What is food traceability. In Advances in Food Traceability Techniques and Technologies; Woodhead Publishing: Cambridge, UK, 2016; pp. 3–8. [Google Scholar]
- Perri, E.; Benincasa, C. Olive oil traceability. In Olive Germplasm: The Olive Cultivation, Table and Olive Oil Industry in Italy; Muzzalupo, I., Ed.; IntechOpen: London, UK, 2012; pp. 265–286. [Google Scholar]
- Ou, G.; Hu, R.; Zhang, L.; Li, P.; Luo, X.; Zhang, Z. Advanced detection methods for traceability of origin and authenticity of olive oils. Anal. Methods 2015, 7, 5731–5739. [Google Scholar] [CrossRef]
- Aparicio, R.; García-González, D.L. Olive oil characterization and traceability. In Handbook of Olive Oil; Springer: Boston, MA, USA, 2013; pp. 431–478. [Google Scholar]
- Benincasa, C.; Lewis, J.; Perri, E.; Sindona, G.; Tagarelli, A. Determination of trace element in Italian virgin olive oils and their characterization according to geographical origin by statistical analysis. Anal. Chim. Acta 2007, 585, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Portarena, S.; Farinelli, D.; Lauteri, M.; Famiani, F.; Esti, M.; Brugnoli, E. Stable isotope and fatty acid compositions of monovarietal olive oils: Implications of ripening stage and climate effects as determinants in traceability studies. Food Control 2015, 57, 129–135. [Google Scholar] [CrossRef]
- Benincasa, C.; Romano, E.; Pellegrino, M.; Perri, E. Characterization of phenolic profiles of Italian single cultivar olive leaves (Olea europaea L.) by mass spectrometry. Mass Spectrom. Purif. Tech. 2018, 4, 2. [Google Scholar] [CrossRef]
- Mohamed, M.B.; Guasmi, F.; Alia, S.B.; Radhouani, F.; Faghim, J.; Triki, T.; Grati, K.N.; Baffi, C.; Lucini, L.; Benincasa, C. The LC-MS/MS characterization of phenolic compounds in leaves allows classifying olive cultivars grown in South Tunisia. Biochem. Syst. Ecol. 2018, 78, 84–90. [Google Scholar] [CrossRef]
- Rotondo, A.; Mannina, L.; Salvo, A. Multiple Assignment Recovered Analysis (MARA) NMR for a direct food labeling: The case study of olive oils. Food Anal. Methods 2019, 12, 1238–1245. [Google Scholar] [CrossRef]
- Hirri, A.; Gammouh, M.; Gorfti, A.; Kzaiber, F.; Bassbasi, M.; Souhassou, S.; Balouki, A.; Oussama, A. The use of Fourier transform mid infrared (FT–MIR) spectroscopy for detection and estimation of extra virgin olive oil adulteration with old olive oil. Sky J. Food Sci. 2015, 4, 60–66. [Google Scholar]
- Merás, I.D.; Manzano, J.D.; Rodríguez, D.A.; de la Peña, A.M. Detection and quantification of extra virgin olive oil adulteration by means of autofluorescence excitation-emission profiles combined with multi-way classification. Talanta 2018, 178, 751–762. [Google Scholar]
- Kumar, S.; Kahlon, T.; Chaudhary, S. A rapid screening for adulterants in olive oil using DNA barcodes. Food Chem. 2011, 127, 1335–1341. [Google Scholar] [CrossRef]
- Tengstrand, E.; Rosén, J.; Hellenäs, K.E.; Aberg, K.M. A concept study on non-targeted screening for chemical contaminants in food using liquid chromatography-mass spectrometry in combination with a metabolomics approach. Anal. Bioanal. Chem. 2013, 405, 1237–1243. [Google Scholar] [CrossRef]
- Knolhoff, A.M.; Croley, T.R. Non-targeted screening approaches for contaminants and adulterants in food using liquid chromatography hyphenated to high resolution mass spectrometry. J. Chromatogr. A 2016, 1428, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Vlek, C.; Steg, L. Human behavior and environmental sustainability: Problems, driving forces, and research topics. J. Soc. Issues 2007, 63, 1–19. [Google Scholar] [CrossRef]
- Mossoba, M.M.; Azizian, H.; Fardin-Kia, A.R.; Karunathilaka, S.R.; Kramer, J.K.G. First application of newly developed FT-NIR spectroscopic methodology to predict authenticity of extra virgin olive oil retail products in the USA. Lipids 2017, 52, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Carvelo, A.M.; Osorio, M.T.; Koidis, A.; González-Casado, A.; Cuadros-Rodríguez, L. Chemometric classification and quantification of olive oil in blends with any edible vegetable oils using FTIR-ATR and Raman spectroscopy. LWT Food Sci. Technol. 2017, 86, 174–184. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Perspective of vibrational spectroscopy analytical methods in on-field/official control of olives and virgin olive oil. Eur. J. Lipid Sci. Technol. 2017, 119. [Google Scholar] [CrossRef]
- Karunathilaka, S.R.; Kia, A.R.F.; Srigley, C.; Chung, J.K.; Mossoba, M.M. Nontargeted, rapid screening of extra virgin olive oil products for authenticity using near-infrared spectroscopy in combination with conformity index and multivariate statistical analyses. J. Food Sci. 2016, 81, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, J.A.; García, J.F. Sorting olive oil based on alpha-tocopherol and total tocopherol content using Near-Infra-Red Spectroscopy (NIRS) analysis. J. Food Eng. 2017, 202, 79–88. [Google Scholar] [CrossRef]
- Costa, A.F.; Coelho, M.J.; Gambarra, F.F.; Bezerra, S.R.; Harrop, R.K.; Ugulino, M.C. NIR spectrometric determination of quality parameters in vegetable oils using PLS and variable selection. Food Res. Int. 2008, 41, 341–348. [Google Scholar]
- Eskin, M.G.; Torabfam, M.; Psillakis, E.; Cincinelli, A.; Kurt, H.; Yüce, M. Real-time water quality monitoring of an artificial lake using a portable, affordable, simple, arduino-based open source sensor. Environ. Eng. 2019, 6, 7–14. [Google Scholar] [CrossRef]
- Beghi, R.; Buratti, S.; Giovenzana, V.; Benedetti, S.; Guidetti, R. Electronic nose and visible-near infrared spectroscopy in fruit and vegetable monitoring. Rev. Anal. Chem. 2017, 36, 36. [Google Scholar] [CrossRef]
- Galtier, O.; Dupuy, N.; Le Dreau, Y.; Ollivier, D.; Pinatec, C.; Kister, J.; Artaud, J. Geographic origins and compositions of virgin olive oils determinated by chemometric analysis of NIR spectra. Anal. Chem. Acta 2006, 595, 136–144. [Google Scholar] [CrossRef]
- Azizian, H.; Mossoba, M.M.; Fardin-Kia, A.R.; Delmonte, P.; Karunathilaka, S.R.; Kramer, J.K.G. Novel, rapid identification, and quantification of adulterants in extra virgin olive oil using near-infrared spectroscopy and chemometrics. Lipids 2015, 50, 705–718. [Google Scholar] [CrossRef]
- Langsrud, O. 50–50 Multivariate analysis of variance for collinear responses. J. R. Stat. Soc. Ser. D 2002, 51, 305–317. [Google Scholar] [CrossRef]
- Infantino, A.; Zaccardelli, M.; Costa, C.; Ozkilinc, H.; Habibi, A.; Peever, T. A new disease of grasspea (Lathyrus sativus) caused by Ascochyta lentis var. lathyri. Int. J. Plant Pathol. 2016, 541–548. [Google Scholar]
- MacKay, D.J.C. Bayesian interpolation. Neural Comput. 1992, 4, 415–447. [Google Scholar] [CrossRef]
- Foresee, F.D.; Martin, T.H. Gauss-Newton approximation to Bayesian learning. In Proceedings of the International Joint Conference on Neural Networks IEEE, Houston, TX, USA, 12 June 1997; Volume 3, pp. 1930–1935. [Google Scholar]
- Kennard, R.W.; Stone, L.A. Computer aided design of experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- Banko, M.; Brill, E. Scaling to very large corpora for natural language disambiguation. In Proceedings of the 39th Annual Meeting on Association for Computational Linguistics, Toulouse, France, 6–11 July 2001; pp. 26–33. [Google Scholar]
- Bucci, R.; Magrí, A.D.; Magrí, A.L.; Marini, D.; Marini, F. Chemical authentication of extra virgin olive oil varieties by supervised chemometric procedures. J. Agric. Food Chem. 2002, 50, 413–418. [Google Scholar] [CrossRef]
- Portarena, S.; Baldacchini, C.; Brugnoli, E. Geographical discrimination of extra-virgin olive oils from the Italian coasts by combining stable isotope data and carotenoid content within a multivariate analysis. Food Chem. 2017, 215, 1–6. [Google Scholar] [CrossRef]
- Giuliani, A.; Cerretani, L.; Cichelli, A. Chlorophylls in olive and in olive oil: Chemistry and occurrences, critical. Food Sci. Nutr. 2011, 51, 678–690. [Google Scholar] [CrossRef]
- El Sohaimy, A.A.S.; El-Sheikh, H.M.; Refaay, M.T.; Zaytoun, A.M. Effect of harvesting in different ripening stages on olive (Olea europaea) oil quality. Am. J. Food Technol. 2016, 11, 1–11. [Google Scholar] [CrossRef][Green Version]
- Aprile, A.; Negro, C.; Sabella, E.; Luvisi, A.; Nicolì, F.; Nutricati, E.; Vergine, M.; Miceli, A.; Blando, F.; De Bellis, L. Antioxidant activity and anthocyanin contents in olives (cv. Cellina di Nardò) during ripening and after fermentation. Antioxidants 2019, 8, 138. [Google Scholar] [CrossRef]
- Mínguez-Mosquera, M.I.; Gandul-Rojas, B.; Garrido-Fernández, J.; Gallardo-Guerrero, L. Pigments present in virgin olive oil. J. Am. Oil Chem. Soc. 1990, 67, 192–196. [Google Scholar] [CrossRef]
- Criado, M.N.; Motilva, M.J.; Goni, M.; Romero, M.P. Comparative study of the effect of the maturation process of olive fruit on the chlorophyll and carotenoid fractions of drupes and virgin olive oils of Arbequina variety in Spain. Food Chem. 2007, 100, 748–755. [Google Scholar] [CrossRef]
- Ramírez-Anaya, J.; Samaniego-Sánchez, C.; Castañeda-Saucedo, M.C.; Villalón-Mir, M.; López-García de la Serrana, H. Phenols and the antioxidant capacity of Mediterranean vegetables prepared with extra virgin olive oil using different domestic cooking techniques. Food Chem. 2015, 188, 430–438. [Google Scholar] [CrossRef]
- Roca, M.; Minguez-Mosquera, M.I. Change in the natural ratio between chlorophylls and carotenoids in olive fruit during processing for virgin olive oil. J. Am. Oil Chem. Soc. 2001, 78, 133–138. [Google Scholar] [CrossRef]
- Schwartz, S.J.; Woo, S.L.; Von Elbe, J.H. High-performance liquid chromatography of chlorophylls and their derivatives in fresh and processed spinach. J. Agric. Food Chem. 1981, 29, 533–535. [Google Scholar] [CrossRef]
- Aitzetmüller, K. Chlorophyll-abbauprodukte in pflanzlichen ölen. Eur. J. Lipid Sci. Technol. 1989, 91, 99–105. (In German) [Google Scholar] [CrossRef]
- Gertz, C.; Fiebig, H.J. Pyropheophytin a—Determination of thermal degradation products of chlorophyll a in virgin olive oil. Eur. J. Lipid Sci. Technol. 2006, 108, 1062–1065. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Hasrini, R.F.; Octavianti, F. Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry. J. Med. Plant Res. 2011, 5, 7119–7131. [Google Scholar]
- Harwood, J.; Aparicio, R. (Eds.) Handbook of Olive Oil: Analysis and Properties; Springer: New York, NY, USA, 2000. [Google Scholar]
- Psomiadou, E.; Tsimidou, M. Pigments in Greek virgin olive oils: Occurrence and levels. J. Sci. Food Agric. 2001, 81, 640–647. [Google Scholar] [CrossRef]
- Moyano, M.J.; Melendez, A.J.; Alba, J.; Heredia, F.J. A comprehensive study on the colour of virgin olive oils and its relationship with their chlorophylls and carotenoids indexes (I): CIEXYZ non-uniform colour space. Food Res. Int. 2008, 41, 505–512. [Google Scholar] [CrossRef]
- Giuffrida, D.; Salvo, F.; Salvo, A.; Cossignani, L.; Dugo, G. Pigments profile in monovarietal virgin olive oils from various Italian olive varieties. Food Chem. 2011, 124, 1119–1123. [Google Scholar] [CrossRef]
- Domenici, V.; Ancora, D.; Cifelli, M.; Serani, A.; Veracini, C.A.; Zandomeneghi, M. Extraction of pigment information from near-UV vis absorption spectra of extra virgin olive oils. J. Agric. Food Chem. 2014, 62, 9317–9325. [Google Scholar] [CrossRef]
- Fuentes, E.; Baez, M.E.; Bravo, M.; Cid, C.; Labra, F. Determination of total phenolic content in olive oil samples by UV-visible spectrometry and multivariate calibration. Food Anal. Methods 2012, 5, 1311–1319. [Google Scholar] [CrossRef]
- Murphy, K.J.; Walker, K.M.; Dyer, K.A.; Bryan, J. Estimation of daily intake of flavonoids and major food sources in middle-aged Australian men and women. Nutr. Res. 2019, 61, 64–81. [Google Scholar] [CrossRef]
- Torres, A.; Espínola, F.; Moya, M.; Alcalá, S.; Vidal, A.M.; Castro, E. Assessment of phenolic compounds in virgin olive oil by response surface methodology with particular focus on flavonoids and lignans. LWT 2018, 90, 22–30. [Google Scholar] [CrossRef]
- Qiao, Y.Q.; Jiang, P.F.; Gao, Y.Z. Lutein prevents osteoarthritis through Nrf2 activation and down regulation of inflammation. Arch. Med. Sci. AMS 2018, 14, 617–624. [Google Scholar] [CrossRef]


| Source | DF | exVarSS | nPC | nBu | exVarPC | exVarBU | p-Value |
|---|---|---|---|---|---|---|---|
| Italian vs. Foreign | 1 | 0.04276 | 2 | 42 | 0.832 | 1 | 0.005056 |
| Error | 90 | 0.95724 |
| Training (60%) | |
| Number of Cases | 55 |
| Number of hidden layers | 1 |
| Number of nodes | 10 |
| Training time | 1:26:02 |
| Number of trials | 976 |
| % bad predictions | 0.0 |
| Testing (40%) | |
| Number of cases | 37 |
| % bad predictions (N) | 13.51 (5) |
| Italian | Foreign | Total | |
|---|---|---|---|
| Italian | 25 | 2 | 24 |
| Foreign | 3 | 7 | 8 |
| Origin | Cultivar | Commercial |
|---|---|---|
| Italy | Coratina | Yes |
| Italy | Taggiasca | Yes |
| Greece | Koroneiki | Yes |
| Argentina | Coratina | Yes |
| Croatia | Karbonaka | Yes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Violino, S.; Ortenzi, L.; Antonucci, F.; Pallottino, F.; Benincasa, C.; Figorilli, S.; Costa, C. An Artificial Intelligence Approach for Italian EVOO Origin Traceability through an Open Source IoT Spectrometer. Foods 2020, 9, 834. https://doi.org/10.3390/foods9060834
Violino S, Ortenzi L, Antonucci F, Pallottino F, Benincasa C, Figorilli S, Costa C. An Artificial Intelligence Approach for Italian EVOO Origin Traceability through an Open Source IoT Spectrometer. Foods. 2020; 9(6):834. https://doi.org/10.3390/foods9060834
Chicago/Turabian StyleViolino, Simona, Luciano Ortenzi, Francesca Antonucci, Federico Pallottino, Cinzia Benincasa, Simone Figorilli, and Corrado Costa. 2020. "An Artificial Intelligence Approach for Italian EVOO Origin Traceability through an Open Source IoT Spectrometer" Foods 9, no. 6: 834. https://doi.org/10.3390/foods9060834
APA StyleViolino, S., Ortenzi, L., Antonucci, F., Pallottino, F., Benincasa, C., Figorilli, S., & Costa, C. (2020). An Artificial Intelligence Approach for Italian EVOO Origin Traceability through an Open Source IoT Spectrometer. Foods, 9(6), 834. https://doi.org/10.3390/foods9060834