Essential Oil Based PVP-CMC-BC-GG Functional Hydrogel Sachet for ‘Cheese’: Its Shelf Life Confirmed with Anthocyanin (Isolated from Red Cabbage) Bio Stickers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Essential Oil-Based PVP-CMC-BC-GG Films
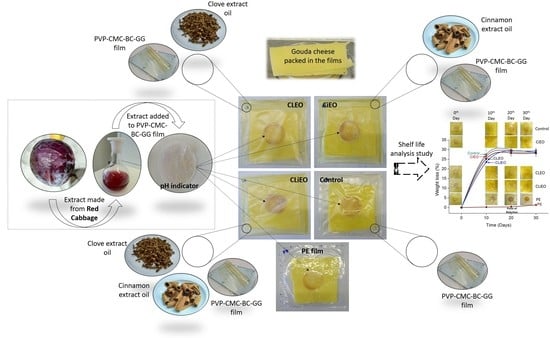
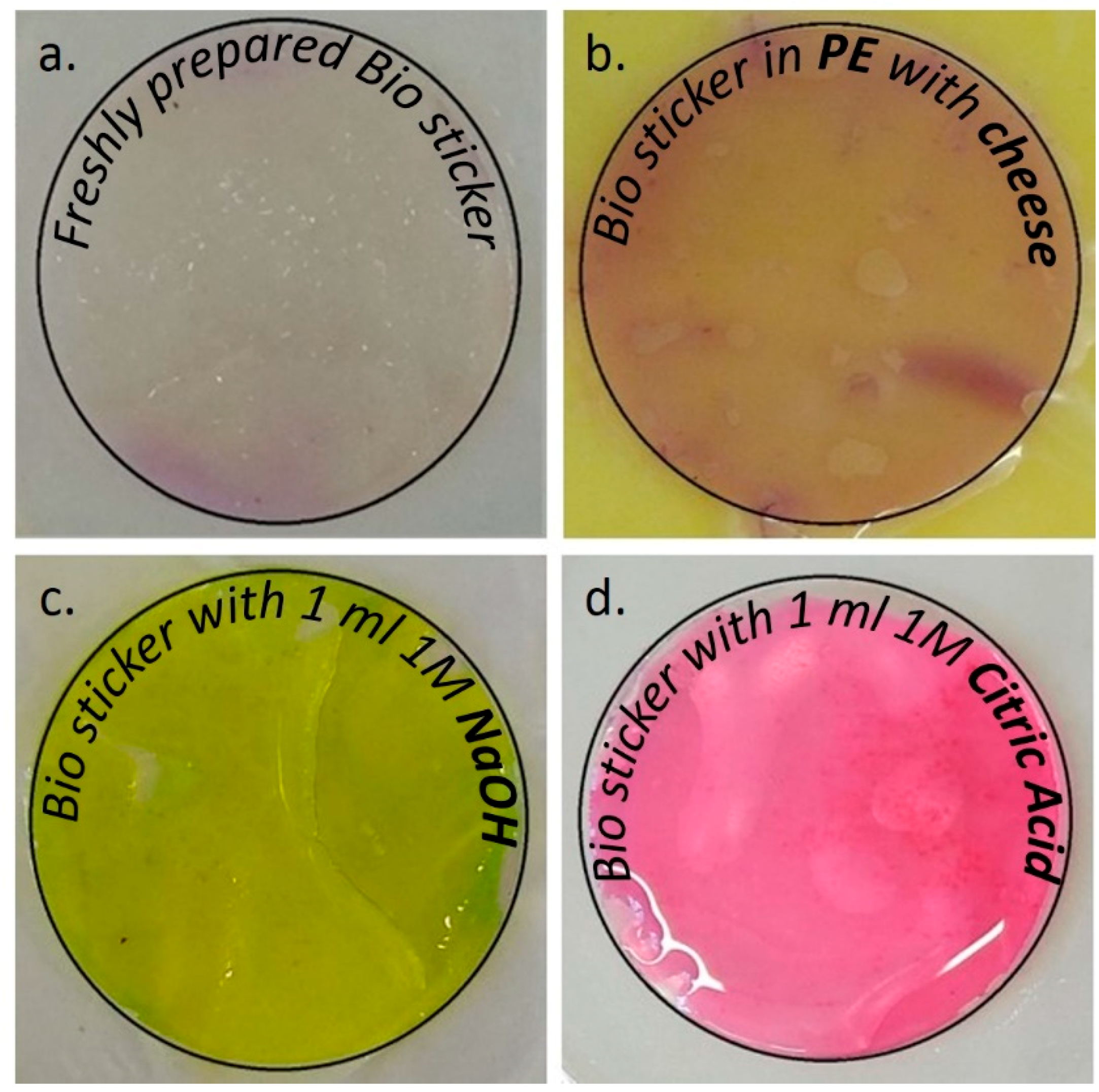
2.3. Preparation of Bio Stickers (Acting as a pH Indicator)
2.4. Antimicrobial Activity
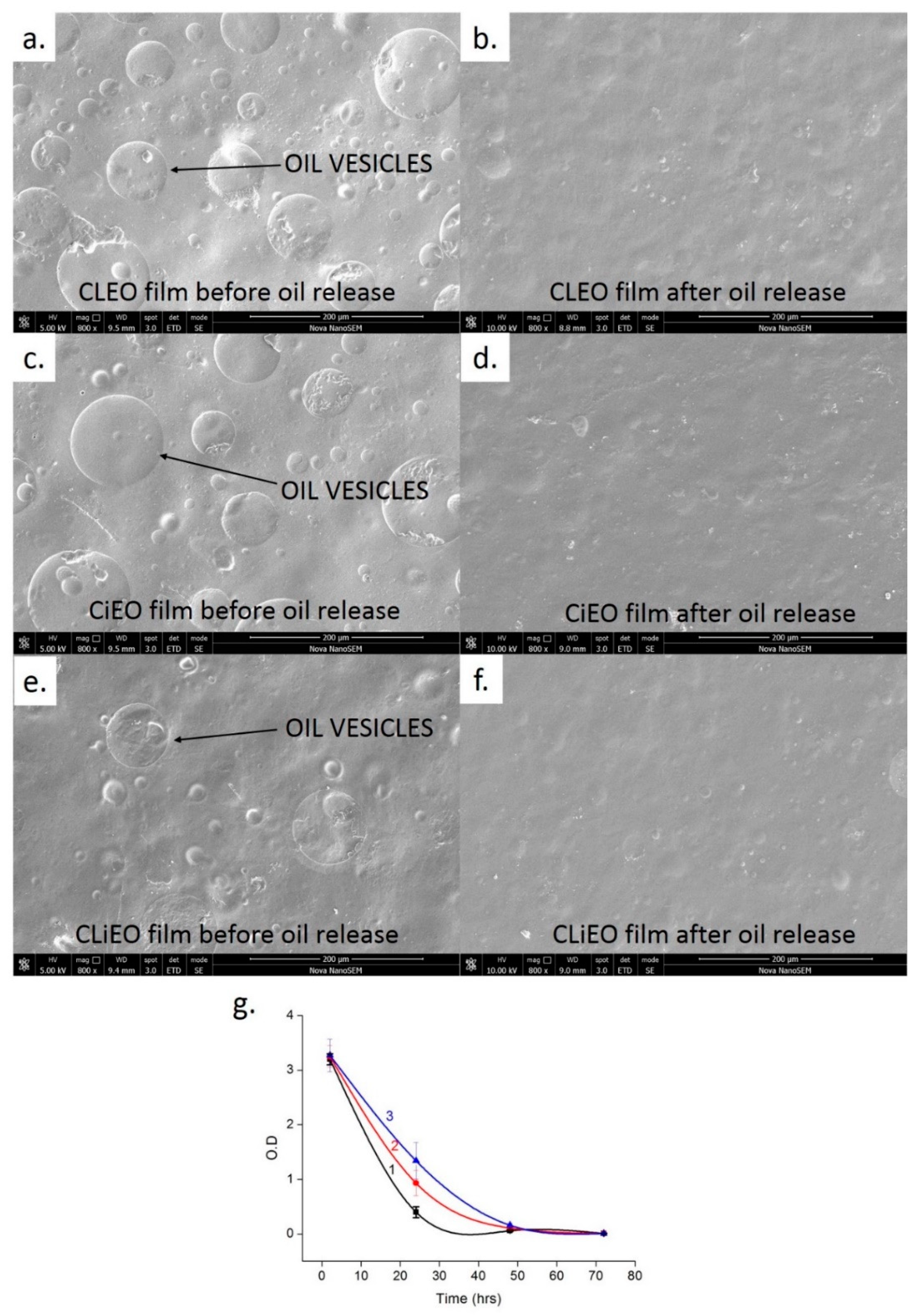
2.5. Migration Test
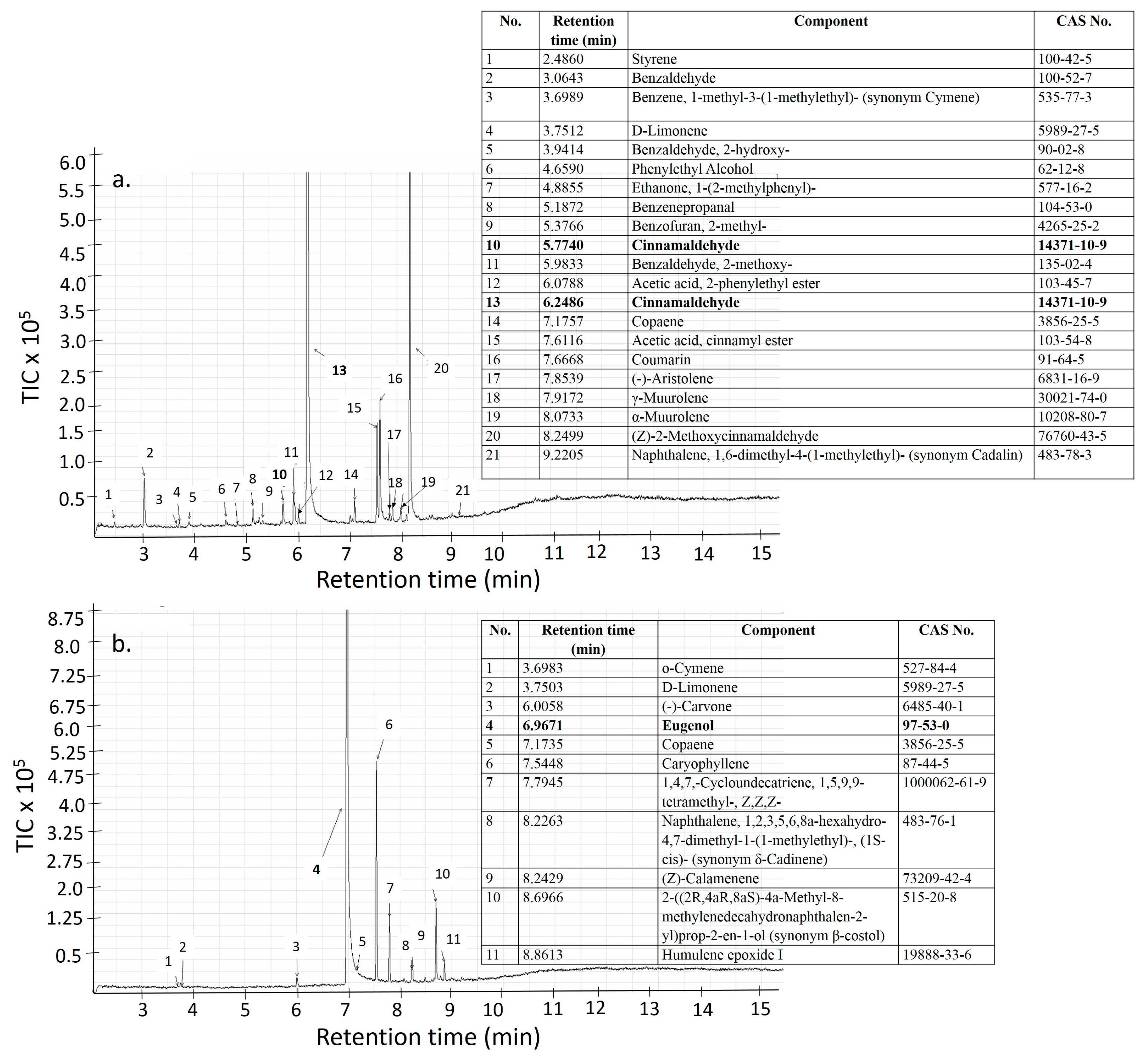
2.6. Characterization of the EO Based PVP-CMC-BC-GG Based Films
2.6.1. Scanning Electron Microscopy
2.6.2. FTIR Investigations
2.6.3. XRD Analysis
2.6.4. Color Assay
2.6.5. Mechanical Properties
2.6.6. Oxygen Permeability
2.6.7. Contact Angle Measurement
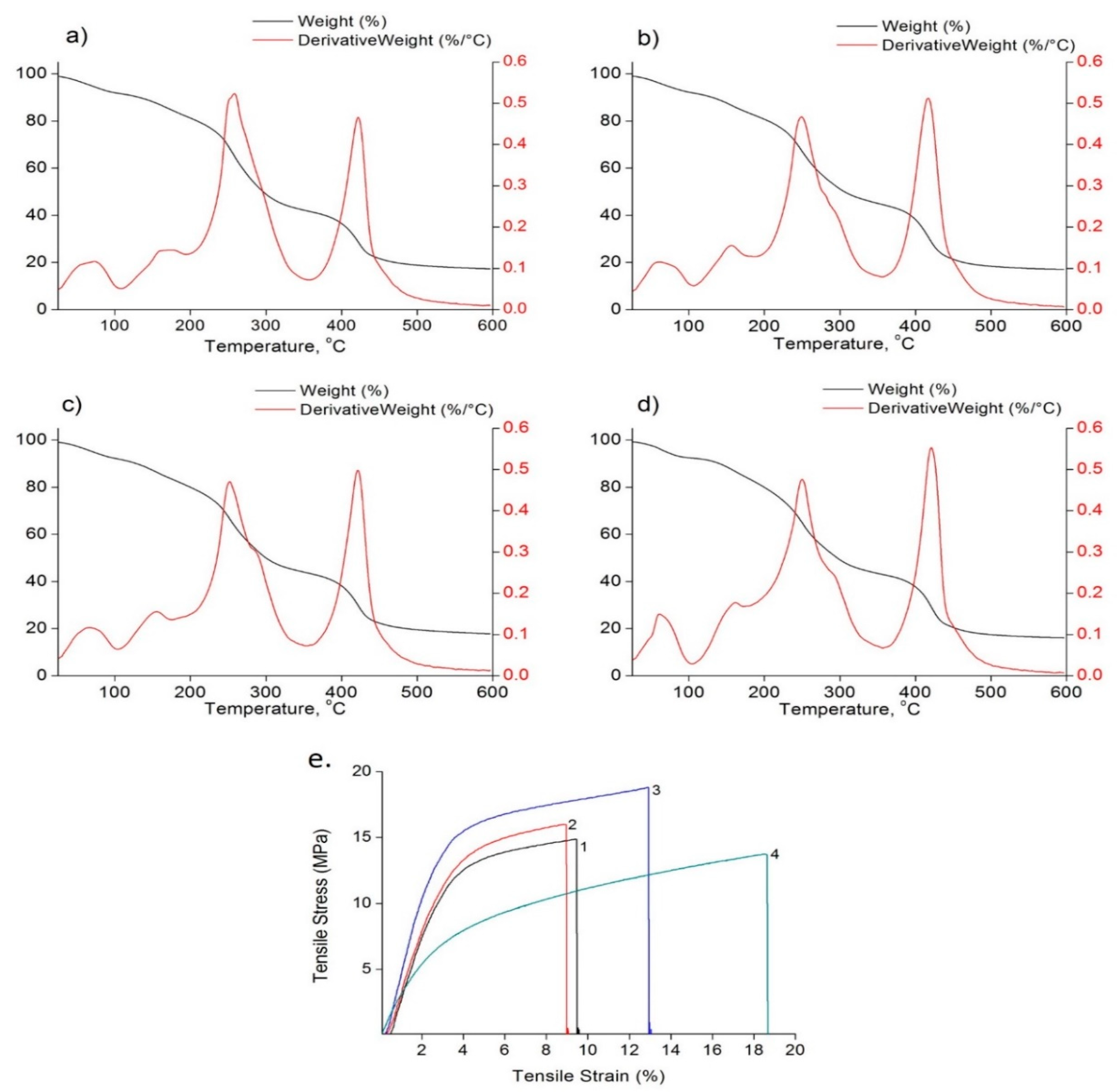
2.6.8. Thermogravimetric Analysis (TGA)
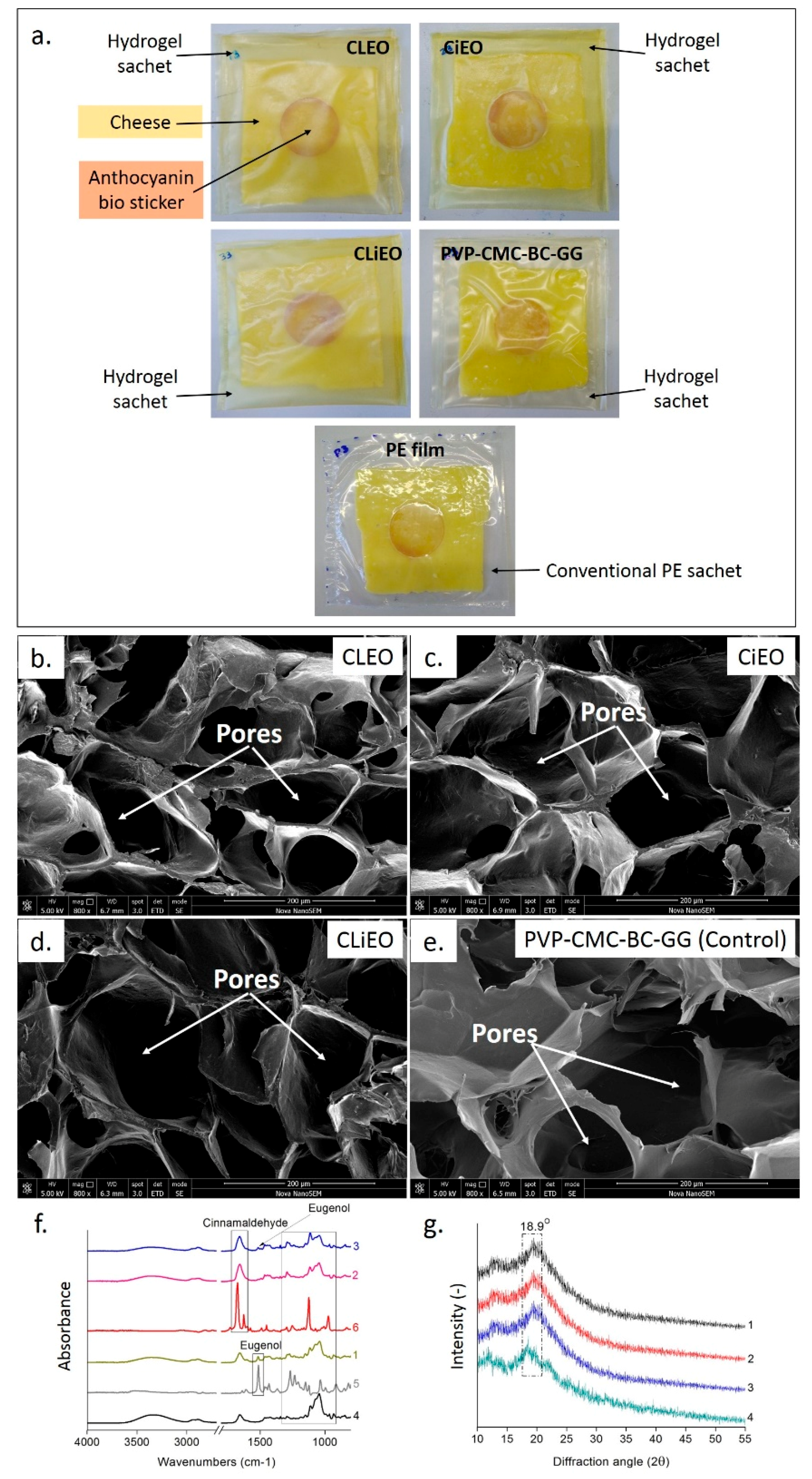
2.7. Proof-of-Concept Application
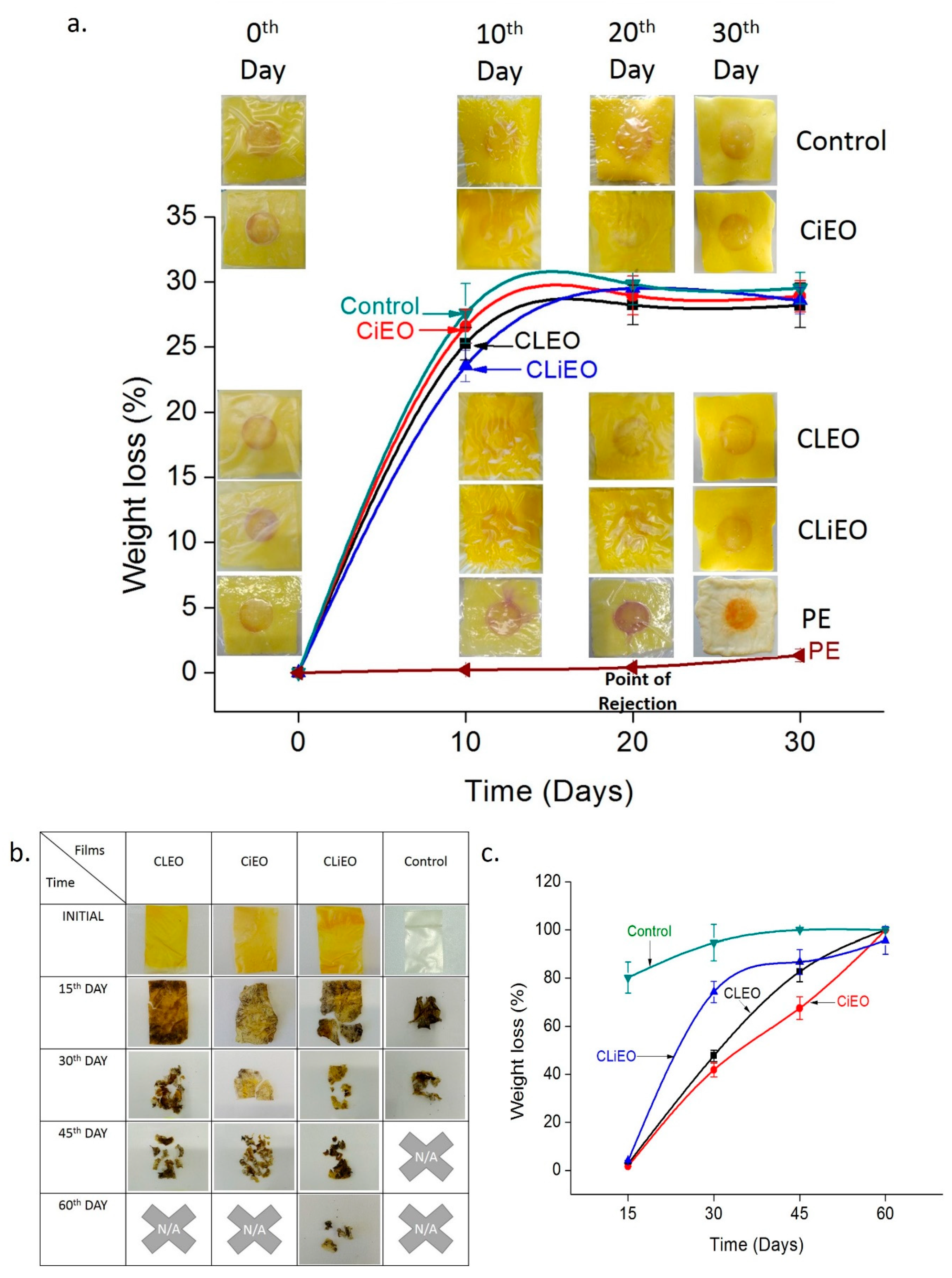
2.8. Biodegradation Studies
2.9. Experimental Design and Statistical Evaluation
3. Results and Discussions
3.1. Structural and Morphological Analysis
3.2. Antimicrobial Properties
3.3. Migration Rate of EOs
3.4. Color Assay
3.5. Physical Qualities
3.6. Thermo-Mechanical Evaluations
3.7. Active and Intelligent Packaging
3.8. Biodegradation Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, T.; Qian, Y.; Wei, J.; Zhou, C. Polymeric Antimicrobial Food Packaging and Its Applications. Polymers 2019, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Saha, N.; Brodnjak, U.V.; Sáha, P. Bacterial cellulose and guar gum based modified PVP-CMC hydrogel films: Characterized for packaging fresh berries. Food Packag. Shelf Life 2019, 22, 100402. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Lu, R.; Xu, J.; Hu, K.; Liu, Y. Preparation of Chitosan/Corn Starch/Cinnamaldehyde Films for Strawberry Preservation. Foods 2019, 8, 423. [Google Scholar] [CrossRef] [PubMed]
- Quesada, J.; Sendra, E.; Navarro, C.; Sayas-Barberá, E. Antimicrobial Active Packaging including Chitosan Films with Thymus vulgaris L. Essential Oil for Ready-to-Eat Meat. Foods 2016, 5, 57. [Google Scholar] [CrossRef]
- Saeed, F.; Afzaal, M.; Tufail, T.; Ahmad, A. Use of Natural Antimicrobial Agents: A Safe Preservation Approach. Act. Antimicrob. Food Packag. 2019. [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Hoque, M.M.; Bari, M.L.; Juneja, V.K.; Kawamoto, S. Antimicrobial activity of cloves and cinnamon extracts against food borne pathogens and spoilage bacteria, and inactivation of Listeria monocytogenes in ground chicken meat with their essential oils. Rep. Natl. Food Res. Inst. Jpn. 2008, 72, 9–21. [Google Scholar]
- Peralta, J.; Bitencourt-Cervi, C.M.; Maciel, V.B.V.; Yoshida, C.M.P.; Carvalho, R.A. Aqueous hibiscus extract as a potential natural pH indicator incorporated in natural polymeric films. Food Packag. Shelf Life 2019, 19, 47–55. [Google Scholar] [CrossRef]
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a colorimetric pH indicator based on bacterial cellulose nanofibers and red cabbage (Brassica oleraceae) extract. Carbohydr. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef]
- Costa, M.J.; Maciel, L.C.; Teixeira, J.A.; Vicente, A.A.; Cerqueira, M.A. Use of edible films and coatings in cheese preservation: Opportunities and challenges. Food Res. Int. 2018, 107, 84–92. [Google Scholar] [CrossRef]
- Guitián, M.V.; Ibarguren, C.; Soria, M.C.; Hovanyecz, P.; Banchio, C.; Audisio, M.C. Anti-Listeria monocytogenes effect of bacteriocin-incorporated agar edible coatings applied on cheese. Int. Dairy J. 2019, 97, 92–98. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Cui, H. Moringa oil/chitosan nanoparticles embedded gelatin nanofibers for food packaging against Listeria monocytogenes and Staphylococcus aureus on cheese. Food Packag. Shelf Life 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Küçük, G.S.; Çelik, Ö.F.; Mazi, B.G.; Türe, H. Evaluation of alginate and zein films as a carrier of natamycin to increase the shelf life of kashar cheese. Packag. Technol. Sci. 2020, 33, 39–48. [Google Scholar] [CrossRef]
- Incoronato, A.L.; Conte, A.; Buonocore, G.G.; Del Nobile, M.A. Agar hydrogel with silver nanoparticles to prolong the shelf life of Fior di Latte cheese. J. Dairy Sci. 2011, 94, 1697–1704. [Google Scholar] [CrossRef]
- Khorshidian, N.; Yousefi, M.; Khanniri, E.; Mortazavian, A.M. Potential application of essential oils as antimicrobial preservatives in cheese. Innov. Food Sci. Emerg. Technol. 2018, 45, 62–72. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Saha, N.; Saha, P. Characterization of Bacterial Cellulose Produced using Media Containing Waste Apple Juice. Appl. Biochem. Microbiol. 2018, 54, 649–657. [Google Scholar] [CrossRef]
- Fuleki, T.; Francis, F.J. Quantitative Methods for Anthocyanins. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Rizk, E.M.; Azouz, A.; Lobna, A.M.H. Evaluation of red cabbage anthocyanin pigments and its potential uses as antioxidant and natural food colorants. Arab Univ. J. Agric. Sci. 2009, 17, 361–372. [Google Scholar]
- Kuswandi, B.; Oktaviana, R.; Abdullah, A.; Heng, L.Y. A Novel On-Package Sticker Sensor Based on Methyl Red for Real-Time Monitoring of Broiler Chicken Cut Freshness. Packag. Technol. Sci. 2013, 27, 69–81. [Google Scholar] [CrossRef]
- Souza, A.C.; Goto, G.E.O.; Mainardi, J.A.; Coelho, A.C.V.; Tadini, C.C. Cassava starch composite films incorporated with cinnamon essential oil: Antimicrobial activity, microstructure, mechanical and barrier properties. LWT Food Sci. Technol. 2013, 54, 346–352. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, B.; Chu, J.; Zhang, P. Silica in situ enhanced PVA/chitosan biodegradable films for food packages. Carbohydr. Polym. 2018, 184, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Krki, N.; Lazi, V.; Savati, S.; Šoji, B.; Petrovi, L.; Šuput, D. Application of chitosan coating with oregano essential oil on dry fermented sausage. J. Food Nutr. Res. 2012, 51, 60–68. [Google Scholar]
- Villegas, C.; Torres, A.; Rios, M.; Rojas, A.; Romero, J.; de Dicastillo, C.L.; Valenzuela, X.; Galotto, M.J.; Guarda, A. Supercritical impregnation of cinnamaldehyde into polylactic acid as a route to develop antibacterial food packaging materials. Food Res. Int. 2017, 99, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.D.W.; Peyron, S.; Rigou, P.; Chalier, P. Rapid quantification of clove (Syzygium aromaticum) and spearmint (Mentha spicata) essential oils encapsulated in a complex organic matrix using an ATR-FTIR spectroscopic method. PLoS ONE 2018, 13, e0207401. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Ding, Y.; Ye, X.; Ding, Y. Antibacterial Effect of Cinnamon Oil Combined with Thyme or Clove Oil. Agric. Sci. China 2011, 10, 1482–1487. [Google Scholar] [CrossRef]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Development and evaluation of chitosan based active nanocomposite films containing bacterial cellulose nanocrystals and silver nanoparticles. Food Hydrocoll. 2018, 84, 414–423. [Google Scholar] [CrossRef]
- Rawat, S. Food Spoilage: Microorganisms and their prevention. Asian J. Plant Sci. 2015, 5, 47–56. [Google Scholar]
- Banjara, N.; Suhr, M.J.; Hallen-Adams, H.E. Diversity of Yeast and Mold Species from a Variety of Cheese Types. Curr. Microbiol. 2015, 70, 792–800. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Jiang, Y.; Chai, Z.; Li, P.; Cheng, Y.; Jing, H.; Leng, X. Synergistic Antimicrobial Activities of Natural Essential Oils with Chitosan Films. J. Agric. Food Chem. 2011, 59, 12411–12419. [Google Scholar] [CrossRef]
- Cava, R.; Nowak, E.; Taboada, A.; Marin-Iniesta, F. Antimicrobial activity of clove and cinnamon essential oils against Listeria monocytogenes in pasteurized milk. J. Food Prot. 2007, 70, 2757–2763. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Hiremath, N.; Jacob, H. Efficacy of antimicrobial properties of polylactide/cinnamon oil film with and without high-pressure treatment against Listeria monocytogenes and Salmonella typhimurium inoculated in chicken sample. Food Packag. Shelf Life 2016, 10, 72–78. [Google Scholar] [CrossRef]
- Frank, K.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Alginate Biocomposite Films Incorporated with Cinnamon Essential Oil Nanoemulsions: Physical, Mechanical, and Antibacterial Properties. Available online: https://www.hindawi.com/journals/ijps/2018/1519407/ (accessed on 25 February 2020).
- da Silva, R.; Sierakowski, M.R.; Bassani, H.P.; Zawadzki, S.F.; Pirich, C.L.; Ono, L.; de Freitas, R.A. Hydrophilicity improvement of mercerized bacterial cellulose films by polyethylene glycol graft. Int. J. Biol. Macromol. 2016, 86, 599–605. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.F.; de Oliveira, F.S.M.; Caetano, V.F.; Vinhas, G.M.; Cardoso, S.A.; da Silva, C.F.; de Oliveira, F.S.M.; Caetano, V.F.; Vinhas, G.M.; Cardoso, S.A. Orange essential oil as antimicrobial additives in poly(vinyl chloride) films. Polímeros 2018, 28, 332–338. [Google Scholar] [CrossRef]
- Ghosh, T.; Teramoto, Y.; Katiyar, V. Influence of Nontoxic Magnetic Cellulose Nanofibers on Chitosan Based Edible Nanocoating: A Candidate for Improved Mechanical, Thermal, Optical, and Texture Properties. J. Agric. Food Chem. 2019, 67, 4289–4299. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Saha, N.; Brodnjak, U.V.; Saha, P. Bacterial cellulose based greener packaging material: A bioadhesive polymeric film. Mater. Res. Express 2018, 5, 115405. [Google Scholar] [CrossRef]
- Lee, J.-W.; Son, S.-M.; Hong, S.-I. Characterization of protein-coated polypropylene films as a novel composite structure for active food packaging application. J. Food Eng. 2008, 86, 484–493. [Google Scholar] [CrossRef]
- Siracusa, V.; Romani, S.; Gigli, M.; Mannozzi, C.; Cecchini, J.; Tylewicz, U.; Lotti, N. Characterization of Active Edible Films based on Citral Essential Oil, Alginate and Pectin. Materials 2018, 11, 1980. [Google Scholar] [CrossRef]
- Taqi, A.; Mutihac, L.; Stamatin, I. Physical and Barrier Properties of Apple Pectin/Cassava Starch Composite Films Incorporating Laurus nobilis L. Oil and Oleic Acid. J. Food Process. Preserv. 2014, 38, 1982–1993. [Google Scholar] [CrossRef]
- Drake, M.A.; Delahunty, C.M. Chapter 20—Sensory Character of Cheese and Its Evaluation. In Cheese, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 517–545. ISBN 978-0-12-417012-4. [Google Scholar]
- Insider, E.B. Business Ignore Expiry Dates—Here Are 12 Science-Backed Ways to Tell if Your Food Has Really Gone off. Available online: https://www.sciencealert.com/how-to-tell-if-food-s-really-gone-bad-to-avoid-wasting-it (accessed on 10 January 2020).
- Saravani, M.; Ehsani, A.; Aliakbarlu, J.; Ghasempour, Z. Gouda cheese spoilage prevention: Biodegradable coating induced by Bunium persicum essential oil and lactoperoxidase system. Food Sci. Nutr. 2019, 7, 959–968. [Google Scholar] [CrossRef]
- Oliveira, R.B.A.; Margalho, L.P.; Nascimento, J.S.; Costa, L.E.O.; Portela, J.B.; Cruz, A.G.; Sant’Ana, A.S. Processed cheese contamination by spore-forming bacteria: A review of sources, routes, fate during processing and control. Trends Food Sci. Technol. 2016, 57, 11–19. [Google Scholar] [CrossRef]
- Ahmadiani, N.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Anthocyanins Contents, Profiles, and Color Characteristics of Red Cabbage Extracts from Different Cultivars and Maturity Stages. J. Agric. Food Chem. 2014, 62, 7524–7531. [Google Scholar] [CrossRef] [PubMed]
- Ehsannia, S.; Sanjabi, M.R. Physicochemical, microbiological and spoilage analysis of probiotic processed cheese analogues with reduced emulsifying salts during refrigerated storage. J. Food Sci. Technol. 2016, 53, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, B.C. The interaction between yeasts and bacteria in dairy environments. Int. J. Food Microbiol. 2001, 69, 37–44. [Google Scholar] [CrossRef]
- Darnay, L.; Németh, Á.; Koncz, K.; Monspart-Sényi, J.; Pásztor-Huszár, K.; Friedrich, L.; Laczay, P. Effect of different O2/CO2 permeable foils on aging of semi-hard goat cheese. Int. Dairy J. 2019, 90, 114–118. [Google Scholar] [CrossRef]
- Stone, B.A.; Svensson, B.; Collins, M.E.; Rastall, R.A. Polysaccharide Degradation. In Glycoscience: Chemistry and Chemical Biology; Fraser-Reid, B.O., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 2325–2375. ISBN 978-3-540-30429-6. [Google Scholar]
- Abdalla, A.L.; Regitano, J.B.; Tornisielo, V.L.; Marchese, L.; Peçanha, M.R.S.R.; Vitti, D.M.S.S.; Smith, T. Biodegradation of polyethylene glycol (PEG) in three tropical soils using radio labelled PEG. Anim. Feed Sci. Technol. 2005, 122, 187–193. [Google Scholar] [CrossRef]
- Polyvinylpyrrolidone Safety Data Sheet. Available online: http://www.ampolymer.com/SDS/PolyvinylpyrrolidoneSDS.html (accessed on 26 September 2019).
- Othman, M.; Rashid, H.; Jamal, N.A.; Shaharuddin, S.I.S.; Sulaiman, S.; Hairil, H.S.; Khalid, K.; Zakaria, M.N. Effect of Cinnamon Extraction Oil (CEO) for Algae Biofilm Shelf-Life Prolongation. Polymers 2018, 11, 4. [Google Scholar] [CrossRef]
- Benkerroum, H.; Patel, K. Antimicrobial Activity of Garlic, Cinnamon, and Clove against a Complex Mixture of Soil Bacteria. Available online: http://esrjournal.org/ojs/index.php/esrjournal/article/view/315 (accessed on 15 January 2020).
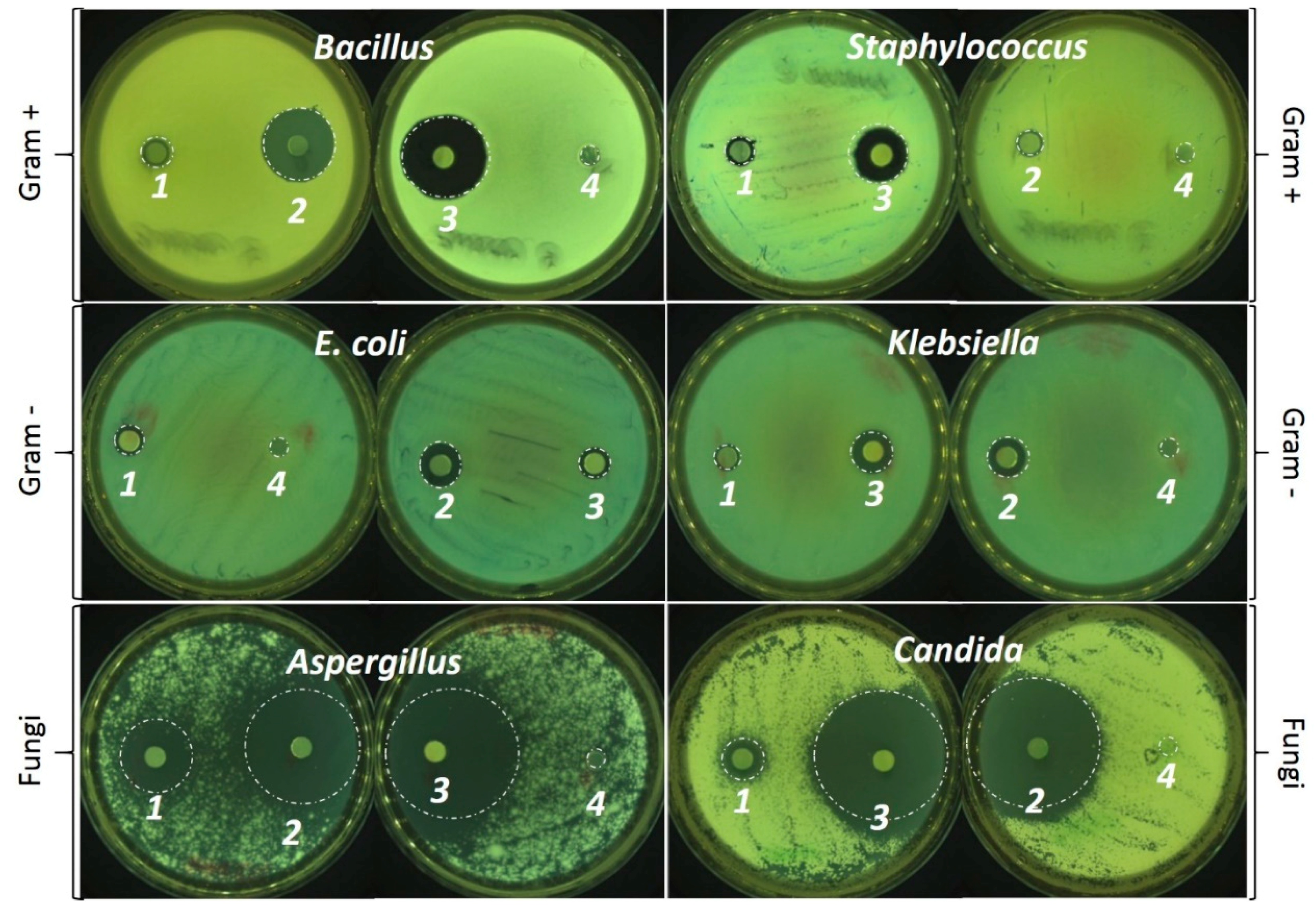
| Films | Inhibition Zones (mm2) | |||||
|---|---|---|---|---|---|---|
| Gram +ve | Gram –ve | Fungi | ||||
| S. aureus | B. cereus | E. coli | Klebsiella. sp | C. albicans | Aspergillus. Sp | |
| CLEO | 4.80 a** ± 0.84 | 28.92 a*** ± 10.74 | 9.60 a,b*** ± 1.37 | 26.89 a*** ± 11.21 | 74.14 a*** ± 8.17 | 257.02 a*** ± 57.26 |
| CiEO | 21.73 a,b** ± 7.56 | 279.08 b*** ± 62.20 | 39.55 c*** ± 8.76 | 45.62 a*** ± 9.85 | 1040.95 b*** ± 123.29 | 796.48 b*** ± 94.09 |
| CLiEO | 54.64 b** ± 15.77 | 237.76 b*** ± 38.49 | 29.02 b,c*** ± 6.30 | 108.55 b*** ± 24.71 | 965.38 b*** ± 74.45 | 1093.47 c*** ± 84.83 |
| PVP-CMC-BC-GG (Control) | 0 | 0 | 0 | 0 | 0 | 0 |
| Films | L* | a* | b* | C* | h0 | ∆L | ∆a | ∆b | ∆C | ∆H | ΔE | Br | % Haze |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLEO# | 5.43 | 0.20 | 0.25 | 0.32 | 5.09 | 91.00 | 0.63 | 32.10 | −26.6 | 18.90 | 29.00 | 2.21 | 6.84 |
| CiEO# | 5.31 | −0.68 | 27.64 | 28.40 | 10.40 | 79.00 | −0.2 | 57.20 | −0.14 | −57.2 | 57.00 | 2.00 | 6.63 |
| CLiEO# | 5.42 | −0.38 | 12.61 | 13.10 | 10.70 | 90.00 | 0.04 | 42.20 | −16.7 | −38.7 | 42.00 | 2.17 | 5.95 |
| Films | OTR | Contact Angle (Ɵ) | |
|---|---|---|---|
| (cm3/m2 × day × 0.1 MPa) | Upper Side | Lower Side | |
| CLEO | 31.5 (8.129E-12 *) | 50.7 ± 3.62 | 58.01 ± 7 |
| CiEO | 36.6 (7.360E-12 *) | 42.4 ± 1.25 | 53.5 ± 5.66 |
| CLiEO | 10.07 (2.24E-12 *) | 55.01 ± 8.85 | 67.47 ± 6.5 |
| PVP-CMC-BC-GG (Control) | 21.88 (4.998E-12 *) | 76.16 ± 1.1 | 46.4 ± 0.8 |
| Films | E (% Increase) | σ (% Increase) | ε (% Decrease) |
|---|---|---|---|
| CLEO | 48.5 ± 2.11 | 27.2 ± 17.7 | 69.08 ± 2.04 |
| CiEO | 86.4 ± 14.5 | 35.5 ± 1.65 | 68.32 ± 4.65 |
| CLiEO | 99.6 ± 22.64 | 51.1 ± 15.23 | 65.8 ± 7.13 |
| Films | L* | a* | b* | C* | h0 | ∆L | ∆a | ∆b | ∆C | ∆H | ΔE | Br | % Haze |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLEO * | |||||||||||||
| 10th d | 66.80 | −0.79 | 4.83 | 4.89 | 99.32 | - | - | - | - | - | - | 33.47 | 50.23 |
| 20th d | 65.47 | −0.88 | 5.91 | 5.98 | 98.45 | - | - | - | - | - | - | 31.20 | 51.41 |
| 30th d | 67.46 | −1.44 | 9.60 | 9.71 | 98.53 | - | - | - | - | - | - | 52.53 | 31.42 |
| CiEO * | |||||||||||||
| 10th d | 66.93 | −1.20 | 7.18 | 7.28 | 99.54 | - | - | - | - | - | - | 32.34 | 51.38 |
| 20th d | 63.87 | −1.60 | 8.73 | 8.87 | 100.36 | - | - | - | - | - | - | 27.91 | 52.53 |
| 30th d | 64.56 | −1.36 | 10.44 | 10.53 | 97.44 | - | - | - | - | - | - | 53.37 | 27.65 |
| CLiEO * | |||||||||||||
| 10th d | 66.42 | −1.12 | 7.65 | 7.73 | 98.33 | - | - | - | - | - | - | 31.48 | 51.23 |
| 20th d | 51.16 | −1.35 | 9.37 | 9.46 | 98.18 | - | - | - | - | - | - | 15.84 | 55.52 |
| 30th d | 63.57 | −0.88 | 9.96 | 10.00 | 95.02 | - | - | - | - | - | - | 54.55 | 26.76 |
| Control * | |||||||||||||
| 10th d | 65.93 | −0.16 | 3.46 | 3.47 | 92.65 | - | - | - | - | - | - | 33.03 | 49.95 |
| 20th d | 64.77 | −0.18 | 3.66 | 3.66 | 92.76 | - | - | - | - | - | - | 31.50 | 51.28 |
| 30th d | 55.01 | −0.51 | 6.73 | 6.75 | 94.30 | - | - | - | - | - | - | 54.30 | 19.82 |
| PE * | |||||||||||||
| 10th d | 55.00 | 0.16 | −0.55 | 0.57 | 286.00 | - | - | - | - | - | - | 28.30 | 34.50 |
| 20th d | 26.69 | 3.09 | 13.03 | 13.39 | 76.75 | - | - | - | - | - | - | 2.88 | 79.24 |
| 30th d | 60.86 | 0.52 | 3.82 | 3.86 | 82.32 | - | - | - | - | - | - | 26.86 | 49.99 |
| Acid * | 58.24 | 51.60 | −7.20 | 52.10 | 352.05 | - | - | - | - | - | - | 29.61 | 85.00 |
| Base * | 77.80 | −4.10 | 55.40 | 55.55 | 94.30 | - | - | - | - | - | - | 16.25 | 90.41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandyopadhyay, S.; Saha, N.; Zandraa, O.; Pummerová, M.; Sáha, P. Essential Oil Based PVP-CMC-BC-GG Functional Hydrogel Sachet for ‘Cheese’: Its Shelf Life Confirmed with Anthocyanin (Isolated from Red Cabbage) Bio Stickers. Foods 2020, 9, 307. https://doi.org/10.3390/foods9030307
Bandyopadhyay S, Saha N, Zandraa O, Pummerová M, Sáha P. Essential Oil Based PVP-CMC-BC-GG Functional Hydrogel Sachet for ‘Cheese’: Its Shelf Life Confirmed with Anthocyanin (Isolated from Red Cabbage) Bio Stickers. Foods. 2020; 9(3):307. https://doi.org/10.3390/foods9030307
Chicago/Turabian StyleBandyopadhyay, Smarak, Nabanita Saha, Oyunchimeg Zandraa, Martina Pummerová, and Petr Sáha. 2020. "Essential Oil Based PVP-CMC-BC-GG Functional Hydrogel Sachet for ‘Cheese’: Its Shelf Life Confirmed with Anthocyanin (Isolated from Red Cabbage) Bio Stickers" Foods 9, no. 3: 307. https://doi.org/10.3390/foods9030307
APA StyleBandyopadhyay, S., Saha, N., Zandraa, O., Pummerová, M., & Sáha, P. (2020). Essential Oil Based PVP-CMC-BC-GG Functional Hydrogel Sachet for ‘Cheese’: Its Shelf Life Confirmed with Anthocyanin (Isolated from Red Cabbage) Bio Stickers. Foods, 9(3), 307. https://doi.org/10.3390/foods9030307