Qishta—A Lebanese Heat Concentrated Dairy Product Characteristics and Production Procedures
Abstract
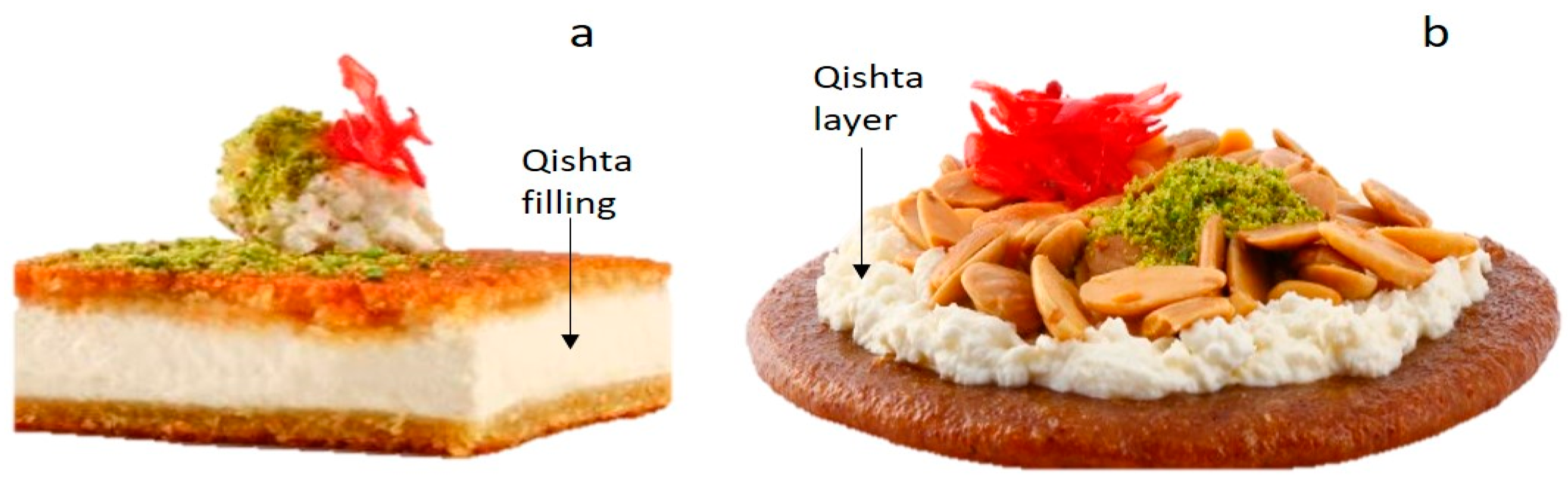
1. Introduction
2. Materials and Methods
2.1. Materials
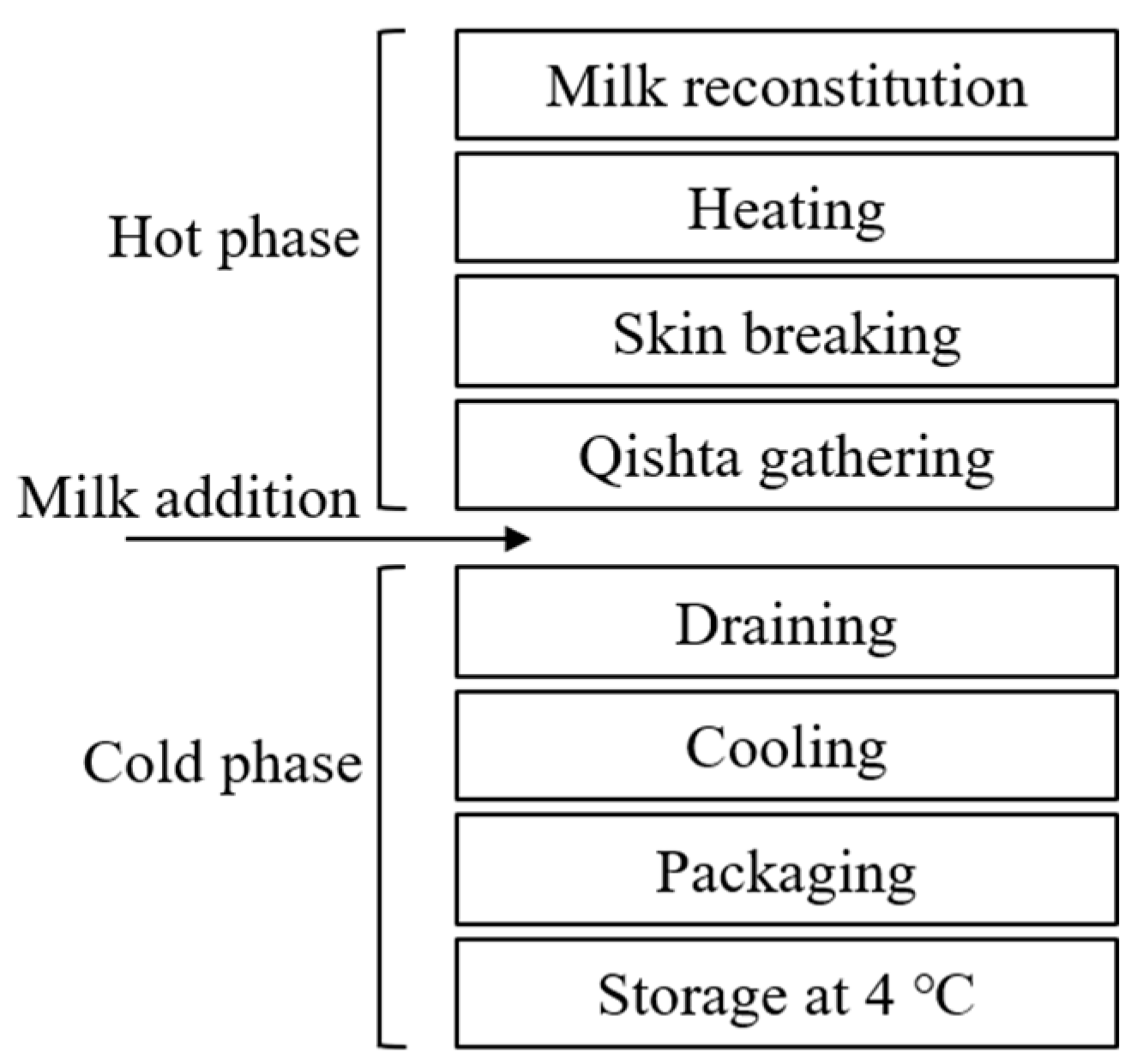
2.2. Qishta Preparation Procedure
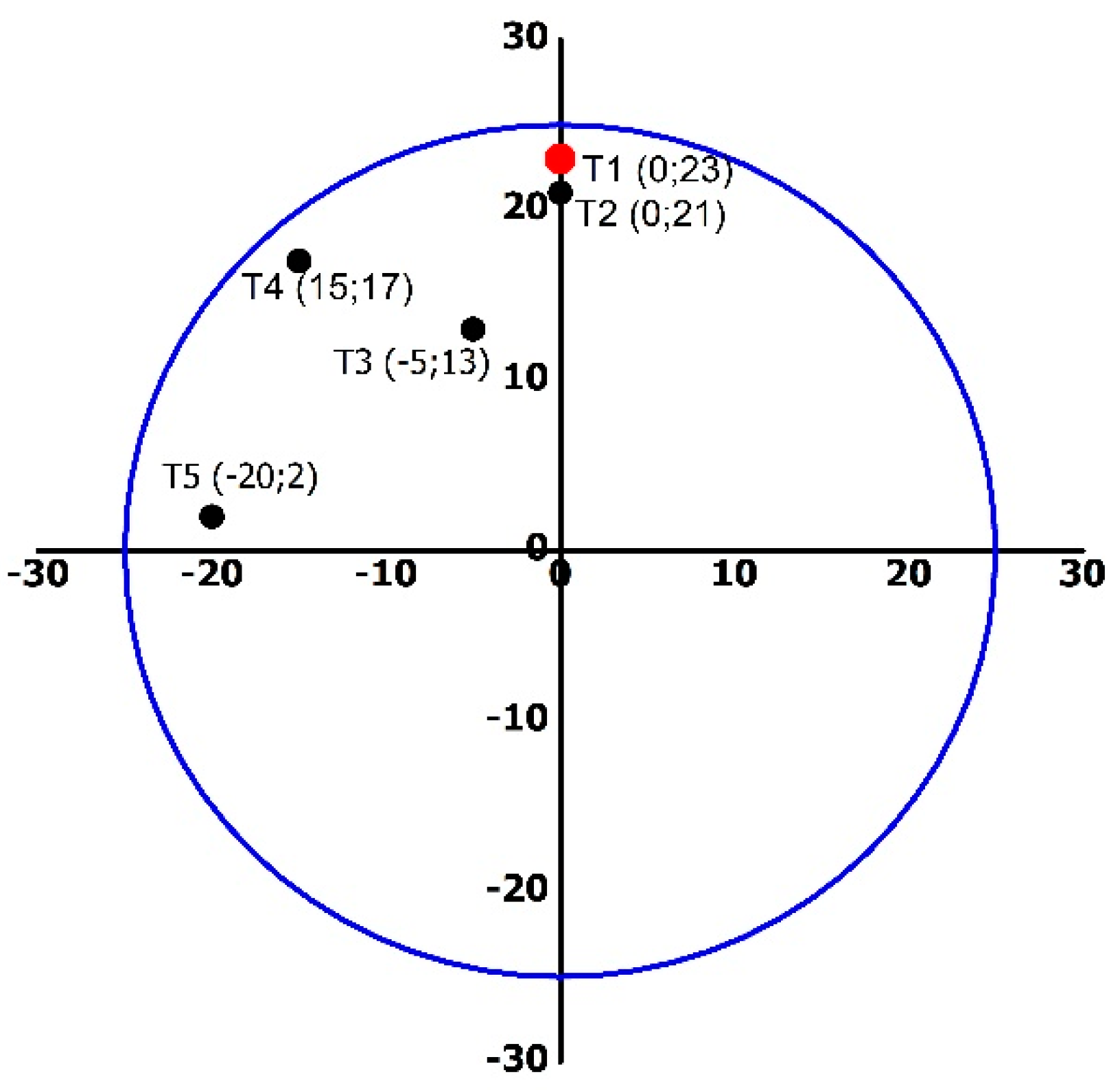
2.3. Temperature Distribution
2.4. Physicochemical Analysis for Qishta
2.5. Static Light Scattering
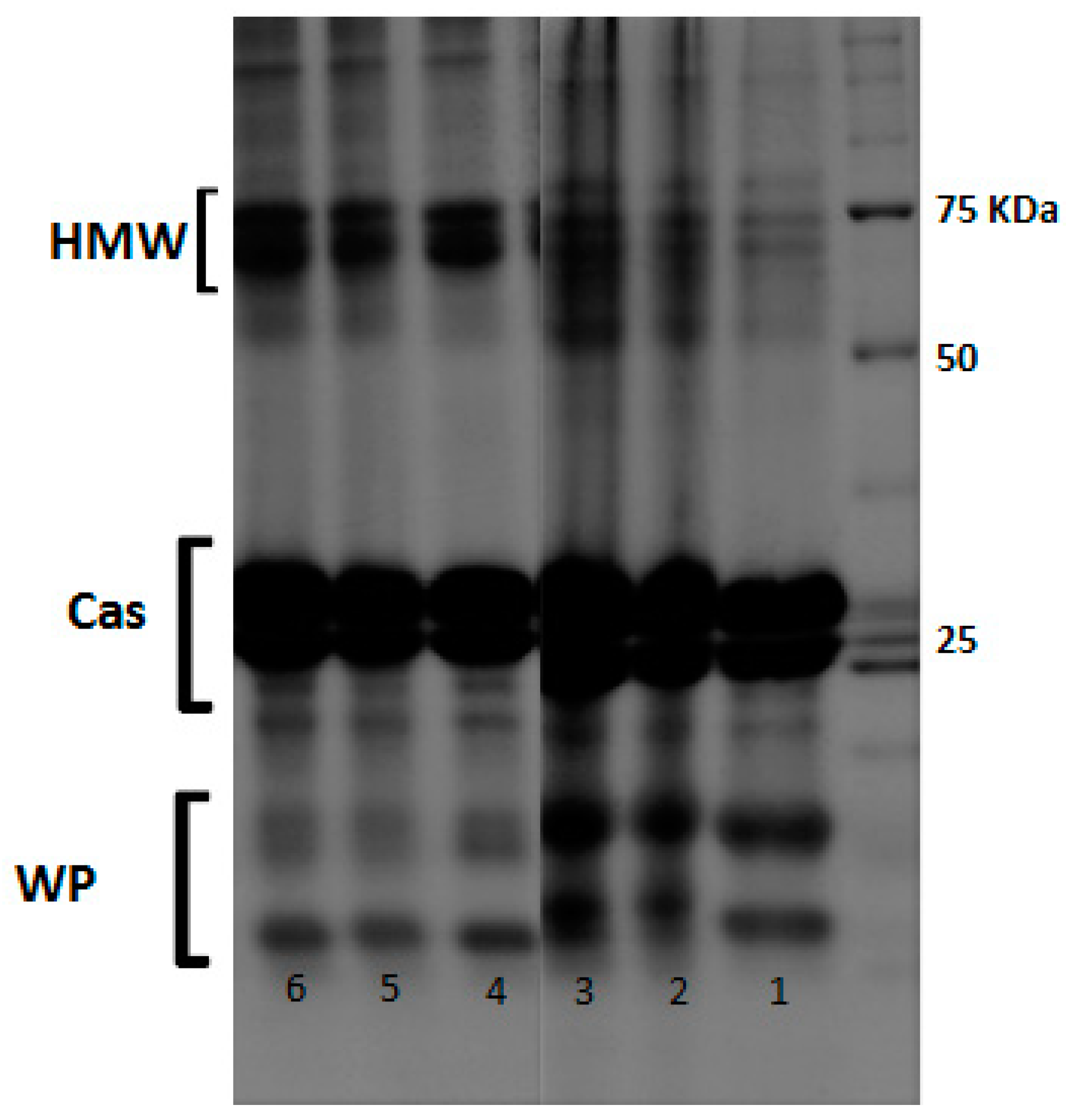
2.6. SDS-PAGE Analysis
2.7. Milk Fat Effect
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Qishta
3.2. Temperature Distribution Profile
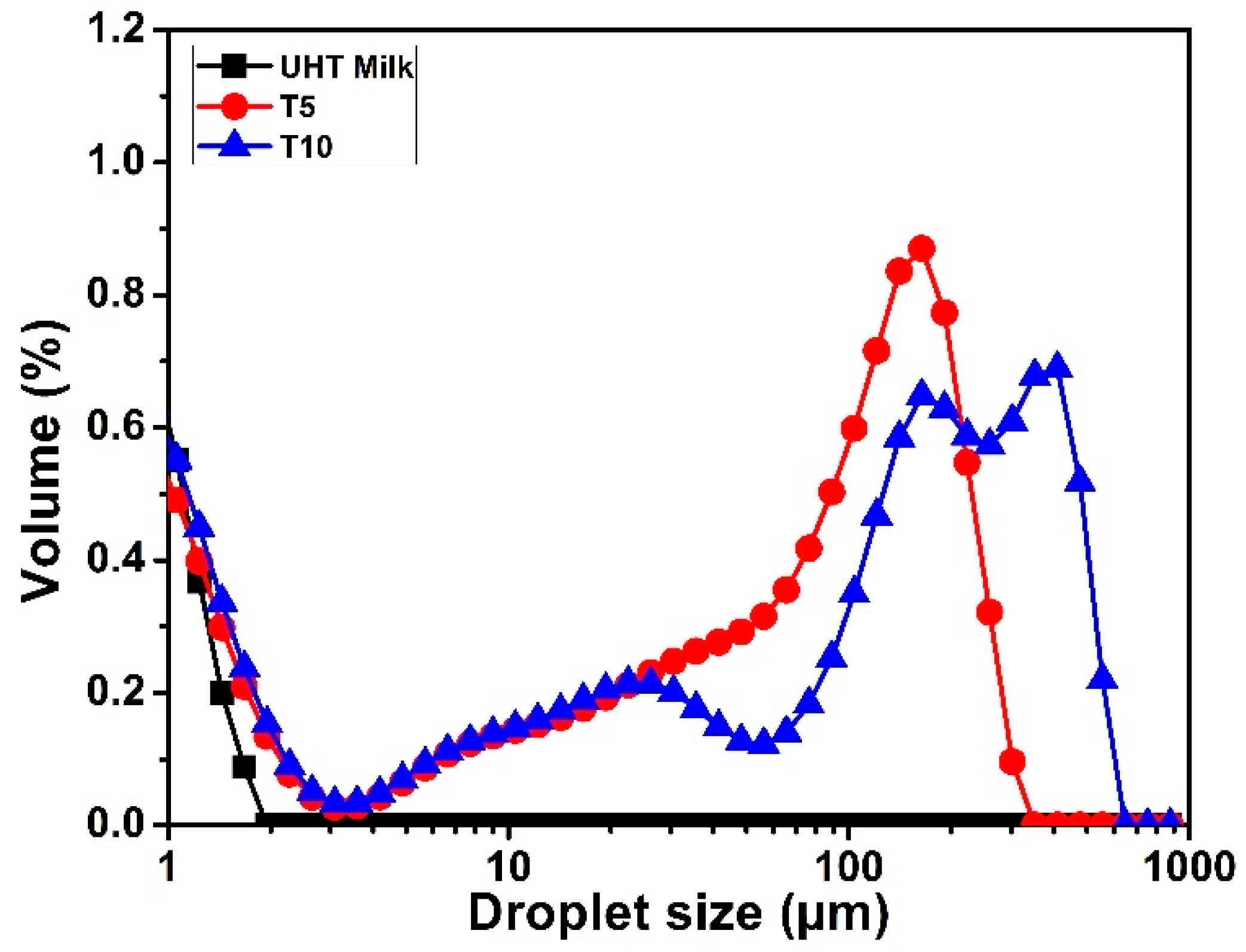
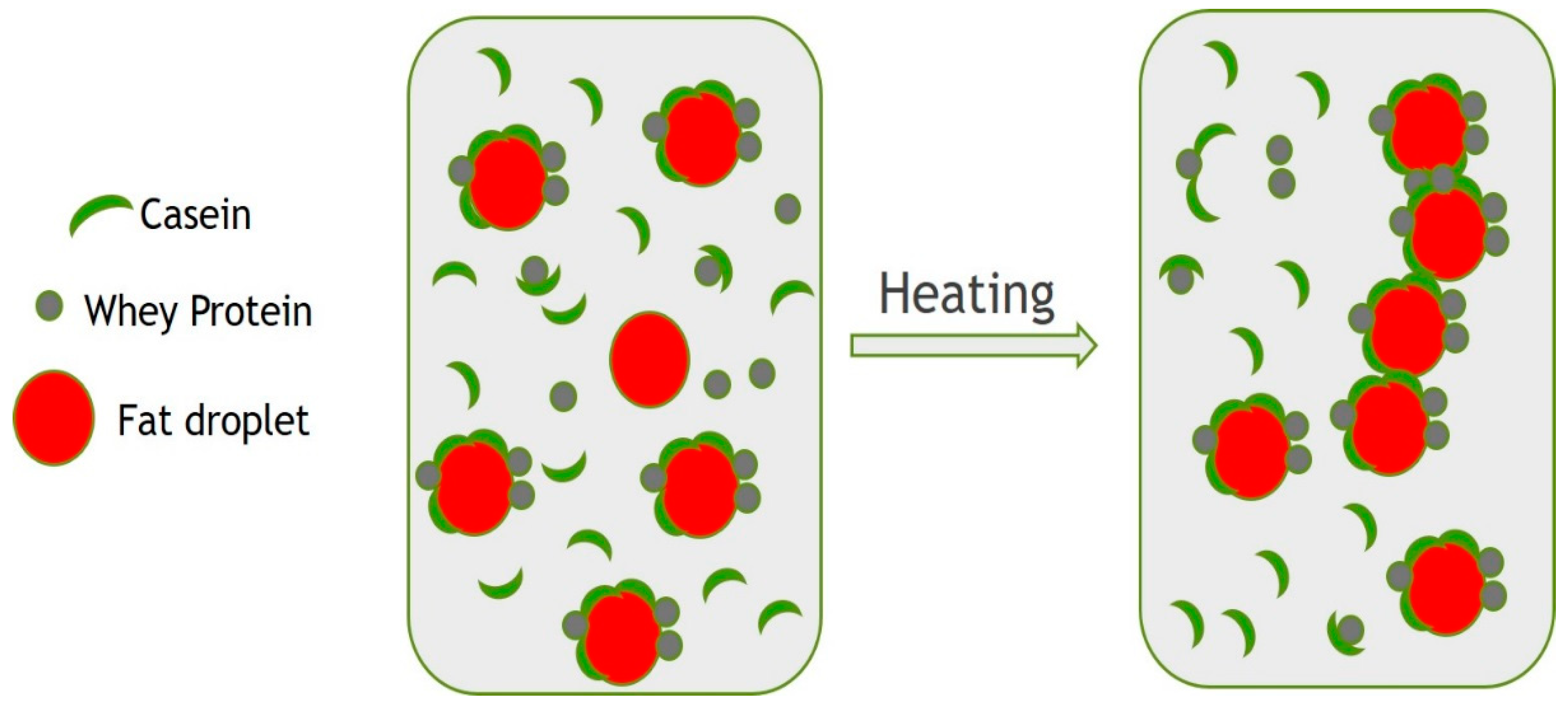
3.3. Emulsion Stability to Heat Treatment
Static Light Scattering (SLS)
3.4. Identification of Major Proteins in Qishta
3.5. Effect of Temperature on Milk Fats
3.6. Effect of Fat on Qishta Formation
Skim Milk Experiment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arja, R.; Haddad, E.; Mouawad, H.; Serhan, H. La filière lait au Liban. Options Méditerranéennes 2001, 32, 148–158. [Google Scholar]
- Kassaify, Z.G.; Najjar, M.; Toufeili, I.; Malek, A. Microbiological and chemical profile of Lebanese qishta [heat-coagulated milk]. EMHJ East. Mediterr. Health J. 2010, 16, 926–931. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seçkin, A.K.; Gursoy, O.; Kinik, O.; Akbulut, N. Conjugated linoleic acid (CLA) concentration, fatty acid composition and cholesterol content of some Turkish dairy products. LWT Food Sci. Technol. 2005, 38, 909–915. [Google Scholar] [CrossRef]
- Radovanovic, M.; Nedeljkovic, A.; Bogdanovic, M.; Miocinovic, J.; Pudja, P. Composition and protein distribution of top and lower layers of kajmak. In Proceedings of the 24th International Scientific-Expert-Conference of Agriculture and Food Industry, Sarajevo, Bosnia and Herzegovina, 25–28 September 2013; pp. 171–175. [Google Scholar]
- Alqaisi Shawabkeh, O.; Ndambi, O.; Hemme, T. Development of milk production and the dairy industry in Jordan. Nutr. Ecol. Econ. Eval. Dairy Farming Syst. Feed. Strateg. Semi-Arid Environ. 2009, 21, 31. [Google Scholar]
- Amárita, F.; Fernández, C.R.; Alkorta, F. Hybrid biosensors to estimate lactose in milk. Anal. Chim. Acta 1997, 349, 153–158. [Google Scholar] [CrossRef]
- Anema, S.G. Effect of milk concentration on the irreversible thermal denaturation and disulfide aggregation of β-Lactoglobulin. J. Agric. Food Chem. 2000, 48, 4168–4175. [Google Scholar] [CrossRef]
- Maubois, J.L.; Kosikowski, F.V. making ricotta cheese by ultrafiltration. J. Dairy Sci. 1978, 61, 881–884. [Google Scholar] [CrossRef]
- Çakır-Fuller, E. Enhanced heat stability of high protein emulsion systems provided by microparticulated whey proteins. Food Hydrocoll. 2015, 47, 41–50. [Google Scholar] [CrossRef]
- Euston, S.R.; Finnigan, S.R.; Hirst, R.L. Aggregation kinetics of heated whey protein-stabilized emulsions. Food Hydrocoll. 2000, 14, 155–161. [Google Scholar] [CrossRef]
- Singh, H.; Waungana, A. Influence of heat treatment of milk on cheesemaking properties. Int. Dairy J. 2001, 11, 543–551. [Google Scholar] [CrossRef]
- Singh, H.; Havea, P. Thermal denaturation, aggregation and gelation of whey proteins. In Advanced Dairy Chemistry—1 Proteins: Part A/Part B; Fox, P.F., McSweeney, P.L.H., Eds.; Springer: Boston, MA, USA, 2003; pp. 1261–1287. [Google Scholar]
- Singh, H.; Latham, J.M. Heat stability of milk: Aggregation and dissociation of protein at ultra-high temperatures. Int. Dairy J. 1993, 3, 225–237. [Google Scholar] [CrossRef]
- Corredig, M.; Dalgleish, D.G. Effect of temperature and pH on the interactions of whey proteins with casein micelles in skim milk. Food Res. Int. 1996, 29, 49–55. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Fox, P.F. “Heat Treatment of Milk | Heat Stability of Milk”. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 744–749. [Google Scholar]
- Tolkach, A.; Kulozik, U. Reaction kinetic pathway of reversible and irreversible thermal denaturation beta-lactoglobulin. Lait 2007, 87, 301–315. [Google Scholar] [CrossRef]
- Raikos, V. Effect of heat treatment on milk protein functionality at emulsion interfaces. A review. Food Hydrocoll. 2010, 24, 259–265. [Google Scholar] [CrossRef]
- Dickinson, E. Food Emulsions: Principles, Practices, and Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; Volume 20, p. 609. [Google Scholar]
- Dalgleish, D.G. Adsorption of protein and the stability of emulsions. Trends Food Sci. Technol. 1997, 8, 1–6. [Google Scholar] [CrossRef]
- Hunt, J.A.; Dalgleish, D.G. Adsorption behaviour of whey protein isolate and caseinate in soya oil-in-water emulsions. Food Hydrocoll. 1994, 8, 175–187. [Google Scholar] [CrossRef]
- Bayarri, S.; Smith, T.; Hollowood, T.; Hort, J. The role of rheological behaviour in flavour perception in model oil/water emulsions. Eur. Food Res. Technol. 2007, 226, 161–168. [Google Scholar] [CrossRef]
- Jensen, R.G.; Ferris, A.M.; Lammi-Keefe, C.J. The composition of milk fat1. J. Dairy Sci. 1991, 74, 3228–3243. [Google Scholar] [CrossRef]
- Ye, A.; Singh, H.; Taylor, M.W.; Anema, S. Interactions of whey proteins with milk fat globule membrane proteins during heat treatment of whole milk. Le Lait 2004, 84, 269–283. [Google Scholar] [CrossRef]
- Kim, H.Y.; Jimenez-Flores, R. Heat-induced interactions between the proteins of milk fat globule membrane and skim milk. J. Dairy Sci. 1995, 78, 24–35. [Google Scholar] [CrossRef]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Heat-Induced Changes in Milk. In Dairy Chemistry and Biochemistry; Springer International Publishing: Cham, Switzerland, 2015; pp. 345–375. [Google Scholar] [CrossRef]
- Houlihan, A.V.; Goddard, P.A.; Nottingham, S.M.; Kitchen, B.J.; Masters, C.J. Interactions between the bovine milk fat globule membrane and skim milk components on heating whole milk. J. Dairy Res. 1992, 59, 187–195. [Google Scholar] [CrossRef]




| Sample | Moisture% | Fat% | FDM% 1 | Protein% | PDM% 2 | Lactose% | Ash% |
|---|---|---|---|---|---|---|---|
| Raw Milk | 87.4 | 3.6 | 31 | 3.2 | 27.5 | 4.8 | 1 |
| Qishta | 68 ± 2 | 11.7 ± 0.6 | 36.6 ± 2.1 | 12.1 ± 0.7 | 37.2 ± 1.5 | 5.4 ± 0.2 | 1.6 ± 0.2 |
| Red Milk | 82.0 ± 0.1 | 4.2 ± 0.1 | 21 ± 1 | 4.0 ± 0.2 | 20.0 ± 1.7 | 7.0 ± 0.2 | 0.8 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najib, M.; Hallab, M.W.; Hallab, K.; Hallab, Z.; Delaplace, G.; Hamze, M.; Chihib, N.-E. Qishta—A Lebanese Heat Concentrated Dairy Product Characteristics and Production Procedures. Foods 2020, 9, 125. https://doi.org/10.3390/foods9020125
Najib M, Hallab MW, Hallab K, Hallab Z, Delaplace G, Hamze M, Chihib N-E. Qishta—A Lebanese Heat Concentrated Dairy Product Characteristics and Production Procedures. Foods. 2020; 9(2):125. https://doi.org/10.3390/foods9020125
Chicago/Turabian StyleNajib, Mustapha, Mohamad Walid Hallab, Karim Hallab, Zaher Hallab, Guillaume Delaplace, Monzer Hamze, and Nour-Eddine Chihib. 2020. "Qishta—A Lebanese Heat Concentrated Dairy Product Characteristics and Production Procedures" Foods 9, no. 2: 125. https://doi.org/10.3390/foods9020125
APA StyleNajib, M., Hallab, M. W., Hallab, K., Hallab, Z., Delaplace, G., Hamze, M., & Chihib, N.-E. (2020). Qishta—A Lebanese Heat Concentrated Dairy Product Characteristics and Production Procedures. Foods, 9(2), 125. https://doi.org/10.3390/foods9020125




