The Effect of Wet Milling and Cryogenic Milling on the Structure and Physicochemical Properties of Wheat Bran
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Wheat Bran Processing
2.2.1. Wet Milling
2.2.2. Cryogenic Milling
2.3. Physical Characterisation of Wheat Bran
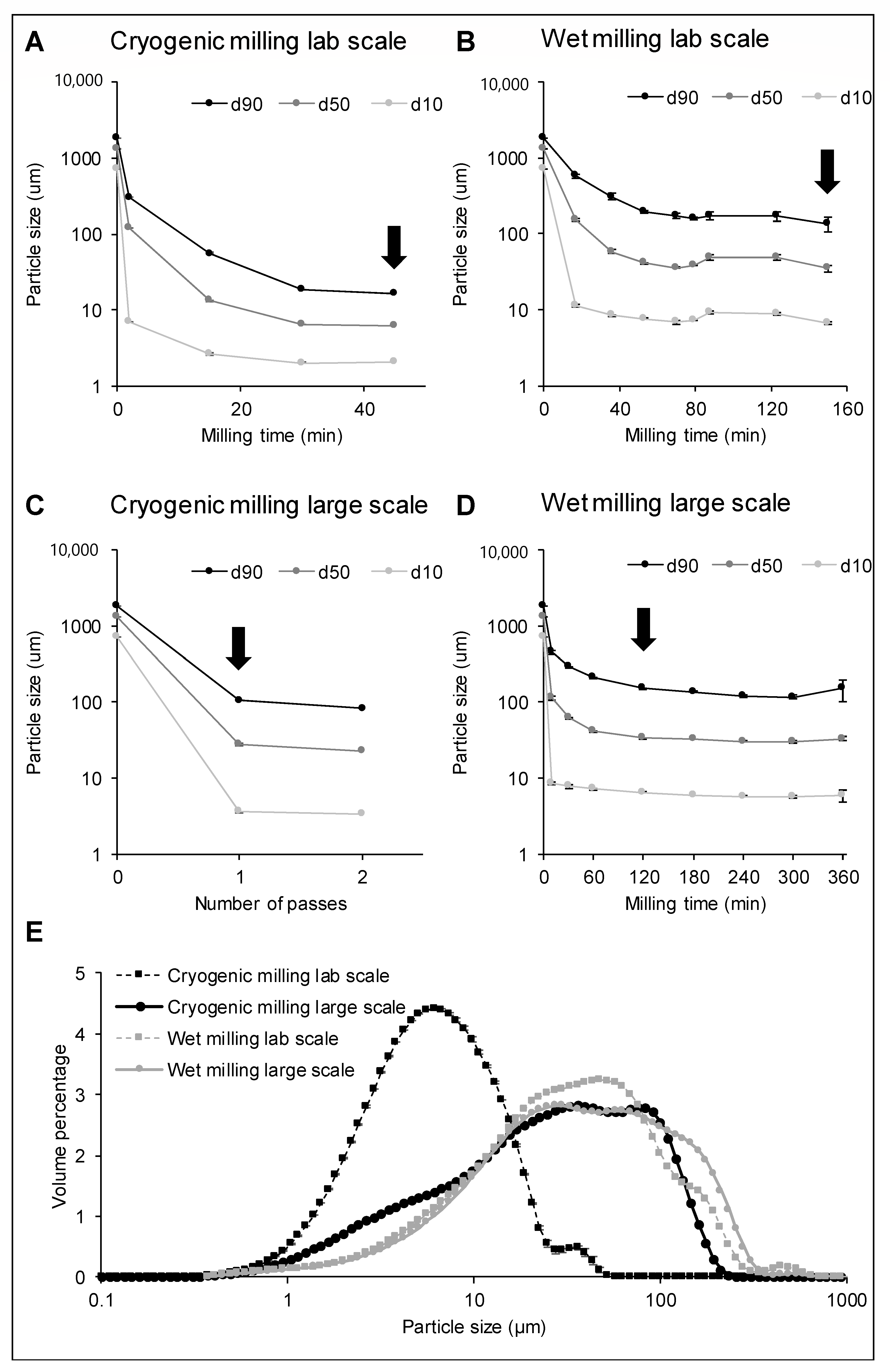
2.3.1. Particle Size Analysis
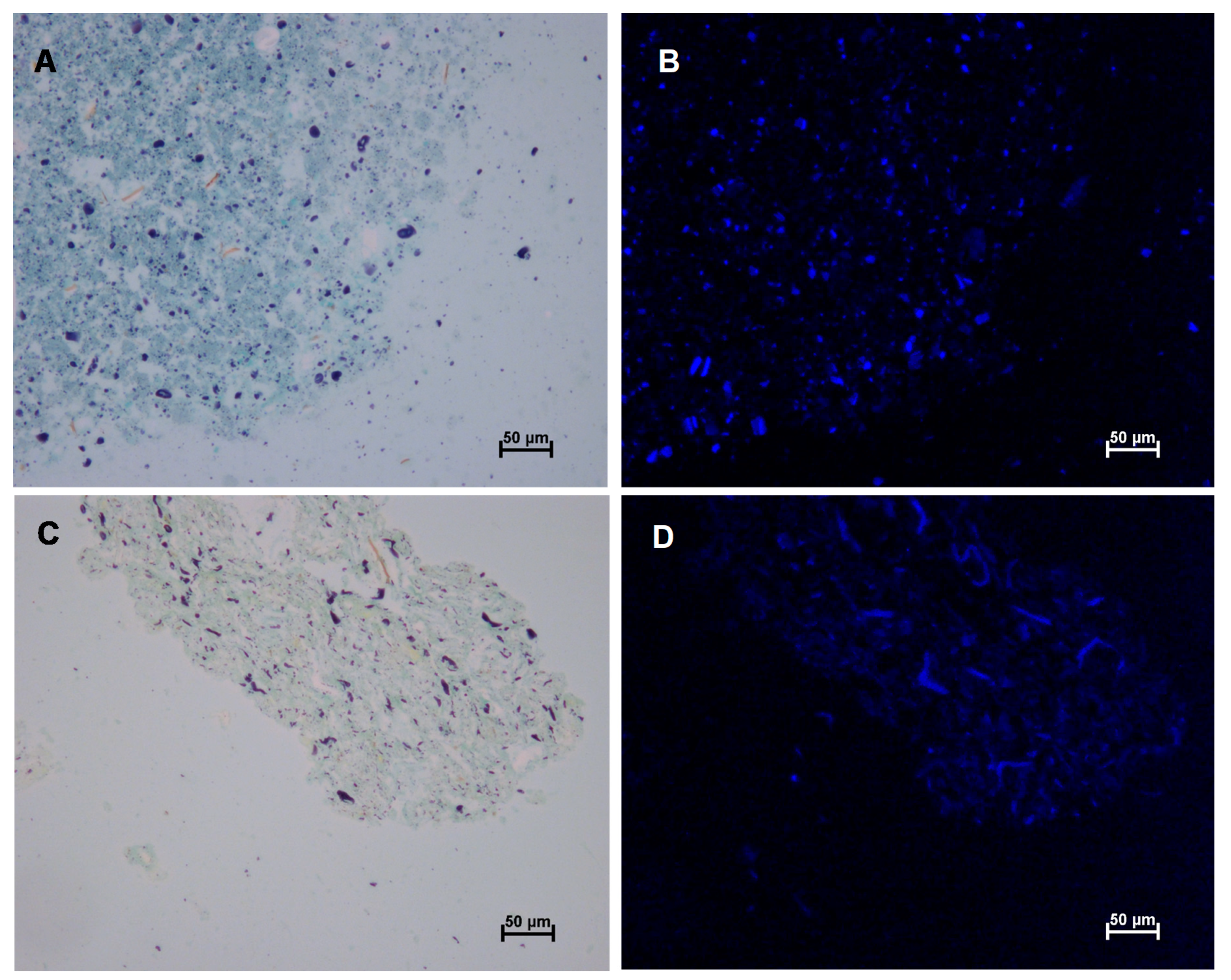
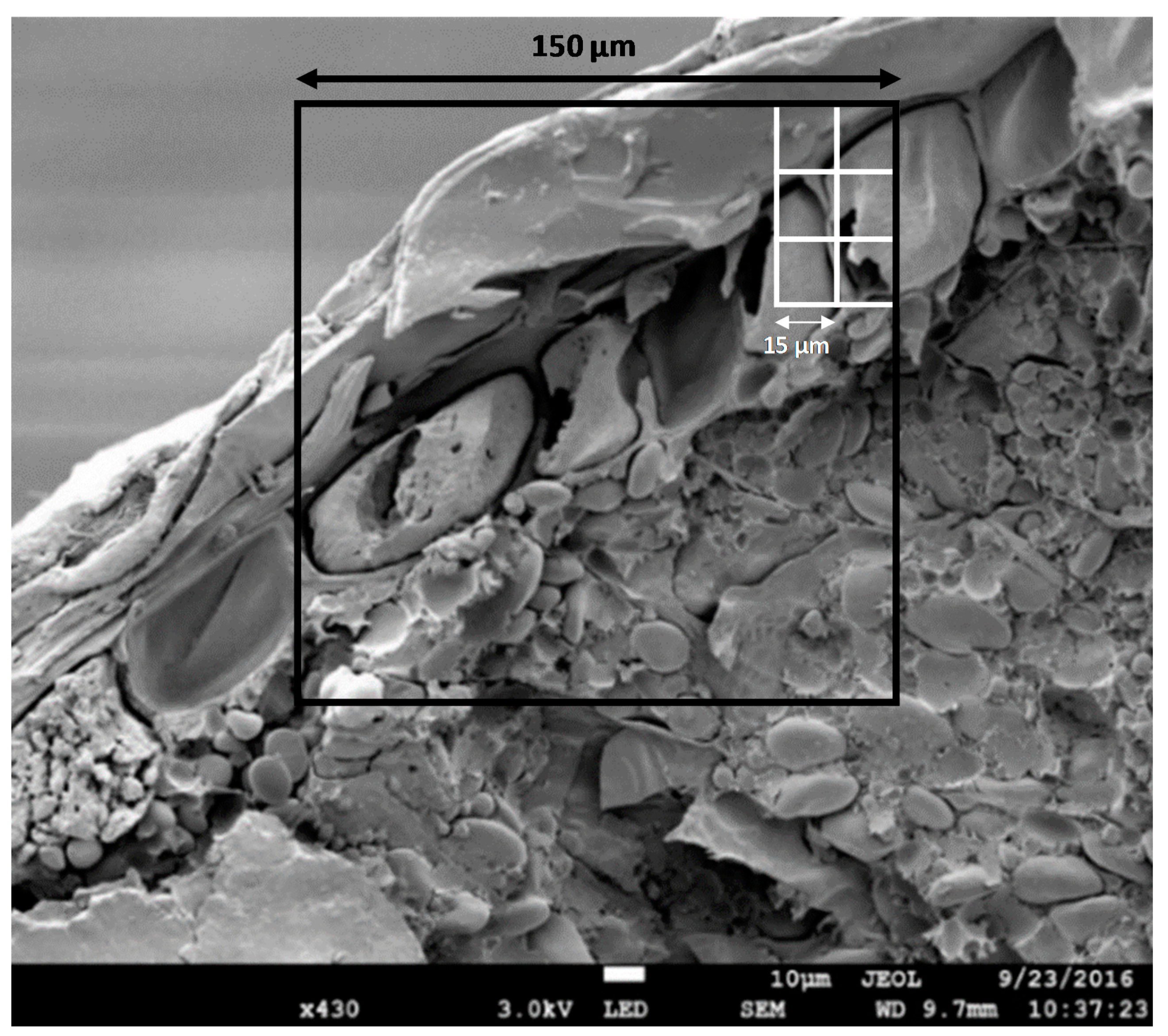
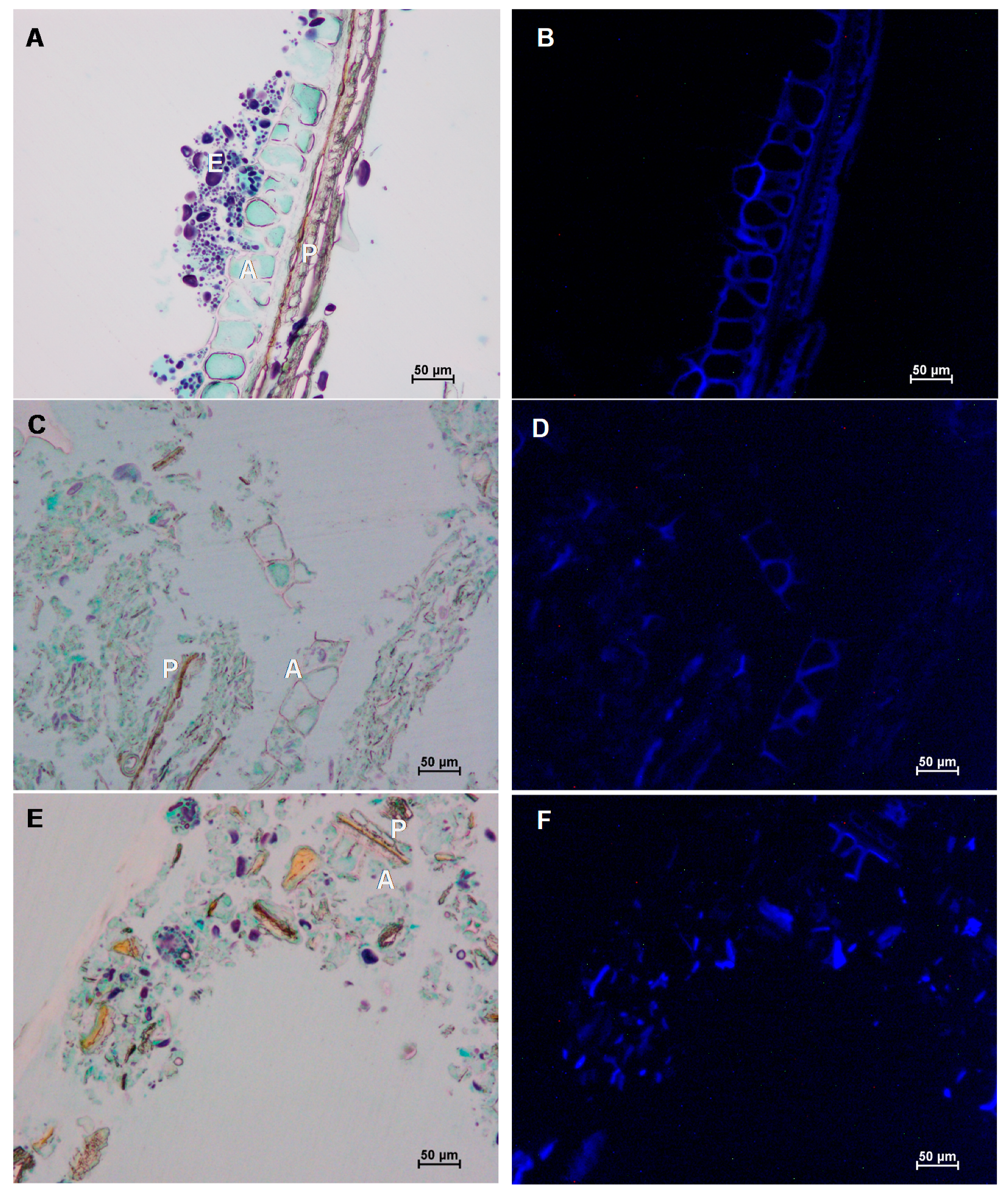
2.3.2. Microscopy
2.3.3. Water Retention Capacity and Extractability
2.3.4. Nitrogen Physisorption
2.4. Chemical Characterisation of the Aqueous Extracts of Wheat Bran
2.5. Statistical Analyses
3. Results and Discussion
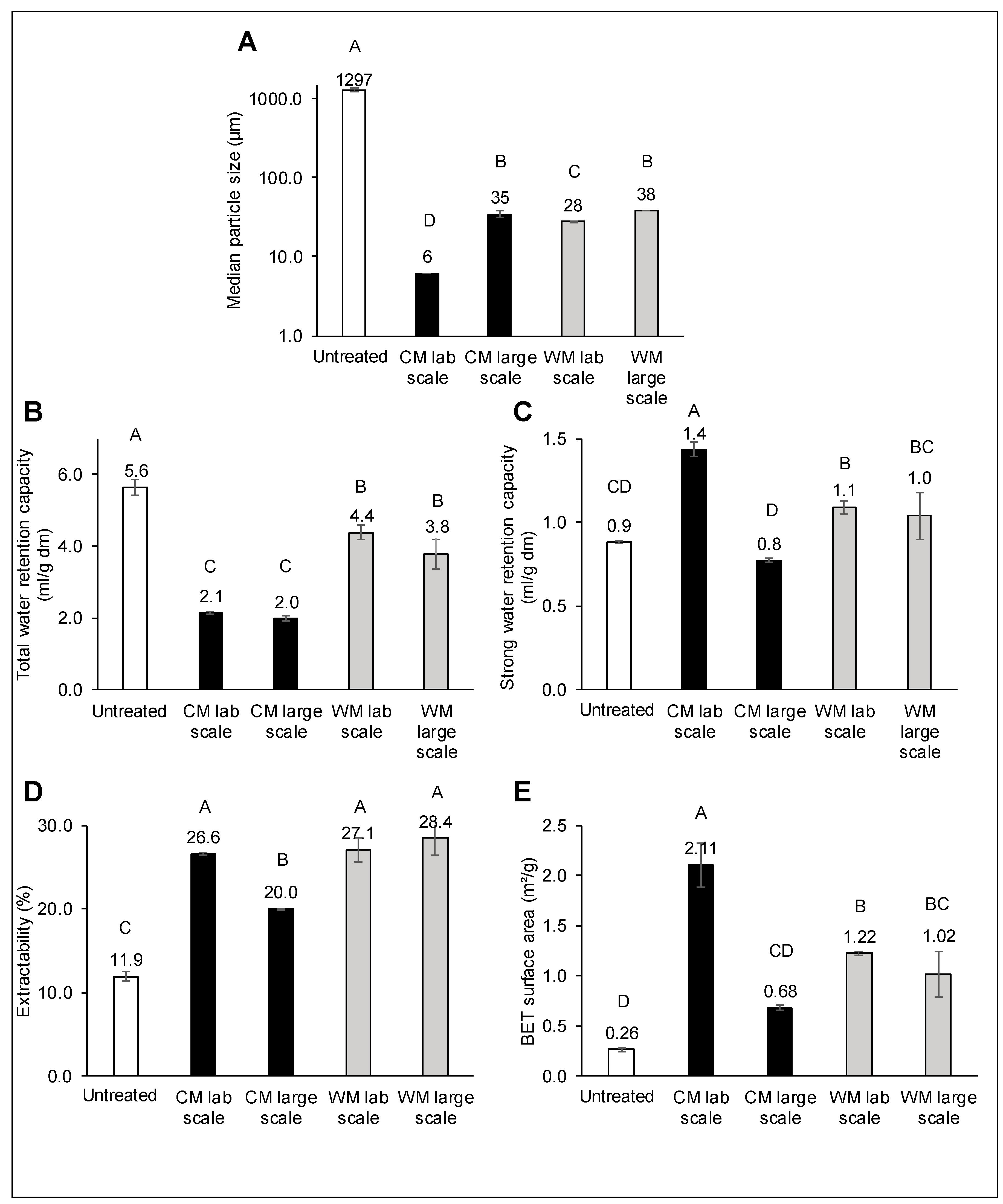
3.1. Effect of Wet and Cryogenic Milling on the Particle Size Distribution of Wheat Bran
3.2. Effect of Wet and Cryogenic Milling on the Structure of Wheat Bran
3.3. Effect of Wet and Cryogenic Milling on the Physical Properties of Wheat Bran
3.4. Effect of Wet and Cryogenic Milling on the Chemical Composition of the Aqueous Extract of Wheat Bran
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Schatzkin, A.; Park, Y.; Leitzmann, M.F.; Hollenbeck, A.R.; Cross, A.J. Prospective study of dietary fiber, whole grain foods, and small intestinal cancer. Gastroenterology 2008, 135, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- De Munter, J.S.L.; Hu, F.B.; Spiegelman, D.; Franz, M.; Van Dam, R.M. Whole grain, bran, and germ intake and risk of type 2 diabetes: A prospective cohort study and systematic review. PLoS Med. 2007, 4, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R.; Gallaher, D.D. Whole grain intake and cardiovascular disease: A review. Curr. Atheroscler. Rep. 2004, 6, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.; Gustafson, J. Health benefits practical aspects of high-fiber diets. Am. J. Clin. Nutr. 1994, 59, 1242S–1247S. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz, Y. Chemical composition of kernel structures. In Wheat: Chemistry and Technology; AACC: St. Paul, MN, USA, 1988; pp. 97–158. [Google Scholar]
- Brouns, F.; Hemery, Y.; Price, R.; Anson, N.M. Wheat aleurone: Separation, composition, health aspects, and potential food use. Crit. Rev. Food Sci. Nutr. 2012, 52, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, N.; Herrick, K.A.; Terry, A.L.; Hughes, J.P. Contribution of Whole Grains to Total Grains Intake among Adults Aged 20 and Over: United States, 2013–2016. NCHS Data Brief 2019, 341, 1–8. [Google Scholar]
- Hemdane, S.; Jacobs, P.J.; Dornez, E.; Verspreet, J.; Delcour, J.A.; Courtin, C.M. Wheat (Triticum aestivum L.) bran in bread making: A critical review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 28–42. [Google Scholar] [CrossRef]
- Sozer, N.; Cicerelli, L.; Heiniö, R.L.; Poutanen, K. Effect of wheat bran addition on invitro starch digestibility, physico-mechanical and sensory properties of biscuits. J. Cereal Sci. 2014, 60, 105–113. [Google Scholar] [CrossRef]
- Campbell, G.M.; Ross, M.; Motoi, L. Bran in Bread: Effects of particle size and level of wheat and oat bran on mixing, proving and baking. In Bubbles in Food 2: Novelty, Health and Luxury; Campbell, G.M., Scanlon, M.G., Pyle, D.L., Eds.; Eagan Press: St. Paul, MN, USA, 2008; pp. 337–354. [Google Scholar]
- Noort, M.W.J.; van Haaster, D.; Hemery, Y.; Schols, H.A.; Hamer, R.J. The effect of particle size of wheat bran fractions on bread quality—Evidence for fibre-protein interactions. J. Cereal Sci. 2010, 52, 59–64. [Google Scholar] [CrossRef]
- Moder, G.J.; Finney, K.F.; Bruinsma, B.L.; Ponte, J.G.; Bolte, L.C. Bread-making potential of straight-grade and whole-wheat flours of Triumph and Eagle-Plainsman V Hard Red Winter Wheats. Cereal Chem. 1984, 61, 269–273. [Google Scholar]
- Zhang, D.; Moore, W. Wheat bran particle size effects on bread baking performance and quality. J. Sci. Food Agric. 1999, 79, 805–809. [Google Scholar] [CrossRef]
- Jacobs, P.J.; Bogaerts, S.; Hemdane, S.; Delcour, J.A.; Courtin, C.M. Impact of wheat bran hydration properties as affected by toasting and degree of milling on optimal dough development in bread making. J. Agric. Food Chem. 2016, 64, 3636–3644. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.J.; Hemdane, S.; Dornez, E.; Delcour, J.A.; Courtin, C.M. Study of hydration properties of wheat bran as a function of particle size. Food Chem. 2015, 179, 296–304. [Google Scholar] [CrossRef]
- De Bondt, Y.; Rosa-Sibakov, N.; Liberloo, I.; Roye, C.; Van de Walle, D.; Dewettinck, K.; Goos, P.; Nordlund, E.; Courtin, C.M. Study into the effect of microfluidisation processing parameters on the physicochemical properties of wheat (Triticum aestivum L.) bran. Food Chem. 2020, 305. [Google Scholar] [CrossRef]
- Rosa, N.N.; Barron, C.; Gaiani, C.; Dufour, C.; Micard, V. Ultra-fine grinding increases the antioxidant capacity of wheat bran. J. Cereal Sci. 2013, 57, 84–90. [Google Scholar] [CrossRef]
- Brewer, L.R.; Kubola, J.; Siriamornpun, S.; Herald, T.J.; Shi, Y.C. Wheat bran particle size influence on phytochemical extractability and antioxidant properties. Food Chem. 2014, 152, 483–490. [Google Scholar] [CrossRef]
- Mendis, M.; Simsek, S. Arabinoxylans and human health. Food Hydrocoll. 2014, 42, 239–243. [Google Scholar] [CrossRef]
- Stevenson, L.; Phillips, F.; O’Sullivan, K.; Walton, J. Wheat bran: Its composition and benefits to health, a European perspective. Int. J. Food Sci. Nutr. 2012, 63, 1001–1013. [Google Scholar] [CrossRef]
- Majzoobi, M.; Pashangeh, S.; Farahnaky, A.; Eskandari, M.H.; Jamalian, J. Effect of particle size reduction, hydrothermal and fermentation treatments on phytic acid content and some physicochemical properties of wheat bran. J. Food Sci. Technol. 2014, 51, 2755–2761. [Google Scholar] [CrossRef]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef]
- Dost, K.; Tokul, O. Determination of phytic acid in wheat and wheat products by reverse phase high performance liquid chromatography. Anal. Chim. Acta 2006, 558, 22–27. [Google Scholar] [CrossRef]
- Bechtel, D.B.; Abécassis, J.; Shewry, P.R.; Evers, A.D. Development, structure, and mechanical properties of the wheat grain. In Wheat Chemistry and Technology; AACC International: St. Paul, MN, USA, 2009. [Google Scholar]
- Hemery, Y.M.; Mabille, F.; Martelli, M.R.; Rouau, X. Influence of water content and negative temperatures on the mechanical properties of wheat bran and its constitutive layers. J. Food Eng. 2010, 98, 360–369. [Google Scholar] [CrossRef]
- Rosa-Sibakov, N.; Sibakov, J.; Lahtinen, P.; Poutanen, K. Wet grinding and microfluidization of wheat bran preparations: Improvement of dispersion stability by structural disintegration. J. Cereal Sci. 2015, 64, 1–10. [Google Scholar] [CrossRef]
- Yang, J.; Wu, Q.; Chen, H.; Zhuang, Y. Influence of micronization on improving phytoestrogenic effects of wheat bran. J. Am. Coll. Nutr. 2015, 34, 224–227. [Google Scholar] [CrossRef]
- Hemery, Y.; Chaurand, M.; Holopainen, U.; Lampi, A.M.; Lehtinen, P.; Piironen, V.; Sadoudi, A.; Rouau, X. Potential of dry fractionation of wheat bran for the development of food ingredients, part I: Influence of ultra-fine grinding. J. Cereal Sci. 2011, 53, 1–8. [Google Scholar] [CrossRef]
- Glenn, G.M.; Johnston, R.K. Moisture-dependent changes in the mechanical properties of isolated wheat bran. J. Cereal Sci. 1992, 15, 223–236. [Google Scholar] [CrossRef]
- Goswami, T.K.; Singh, M. Role of feed rate and temperature in attrition grinding of cumin. J. Food Eng. 2003, 59, 285–290. [Google Scholar] [CrossRef]
- Li, S.; Ge, S.; Huang, Z.; Wang, Q.; Zhao, H.; Pan, H. Cryogenic grinding technology for traditional Chinese herbal medicine. Cryogenics 1991, 31, 136–137. [Google Scholar] [CrossRef]
- Manohar, B.; Sridhar, B.S. Size and shape characterization of conventionally and cryogenically ground turmeric (Curcuma domestica) particles. Powder Technol. 2001, 120, 292–297. [Google Scholar] [CrossRef]
- Roye, C.; Bulckaen, K.; De Bondt, Y.; Liberloo, I.; Van De Walle, D.; Dewettinck, K.; Courtin, C.M. Side-by-side comparison of composition and structural properties of wheat, rye, oat, and maize bran and their impact on in vitro fermentability. Cereal Chem. 2019, 97, 20–33. [Google Scholar] [CrossRef]
- AACC. Approved methods of the American Association of Cereal Chemists; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Langenaeken, N.A.; De Schepper, C.F.; De Schutter, D.P.; Courtin, C.M. Different gelatinization characteristics of small and large barley starch granules impact their enzymatic hydrolysis and sugar production during mashing. Food Chem. 2019, 295, 138–146. [Google Scholar] [CrossRef]
- Courtin, C.M.; Van Den Broeck, H.; Delcour, J.A. Determination of reducing end sugar residues in oligo- and polysaccharides by gas-liquid chromatography. J. Chromatogr. A 2000, 866, 97–104. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Eastwood, M.A.; Robertson, J.A.; Brydon, W.G.; MacDonald, D. Measurement of water-holding properties of fibre and their faecal bulking ability in man. Br. J. Nutr. 1983, 50, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Dornez, E.; Gebruers, K.; Cuyvers, S.; Delcour, J.A.; Courtin, C.M. Impact of wheat flour-associated endoxylanases on arabinoxylan in dough after mixing and resting. J. Agric. Food Chem. 2007, 55, 7149–7155. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Bian, Y.Y.; Zhu, K.X.; Guo, X.N.; Peng, W.; Zhou, H.M. Activation of endogenous phytase and degradation of phytate in wheat bran. J. Agric. Food Chem. 2015, 63, 1082–1087. [Google Scholar] [CrossRef]
- Van Craeyveld, V.; Holopainen, U.; Selinheimo, E.; Poutanen, K.; Delcour, J.A.; Courtin, C.M. Extensive dry ball milling of wheat and rye bran leads to in situ production of arabinoxylan oligosaccharides through nanoscale fragmentation. J. Agric. Food Chem. 2009, 57, 8467–8473. [Google Scholar] [CrossRef]
- Courtin, C.M.; Delcour, J.A. Arabinoxylans and endoxylanases in wheat flour bread-making. J. Cereal Sci. 2002, 35, 225–243. [Google Scholar] [CrossRef]
| Cryogenic Milling | Wet Milling | ||||
|---|---|---|---|---|---|
| No Treatment | Laboratory Scale | Large Scale | Laboratory Scale | Large Scale | |
| Total glucose content after hydrolysis of extract (% dm) | 4.20 (0.13) c | 9.43 (0.46) b | 4.44 (0.01) c | 18.53 (1.41) a | 19.26 (0.82) a |
| Glucose (% dm) | 0.58 (0.06) c | 0.37 (0.01) c | 0.87 (0.02) c | 4.24 (0.18) a | 3.52 (0.37) b |
| Maltose (% dm) | 0.06 (0.05) c | 3.07 (0.21) b | 0.17 (0.01)c | 4.48 (0.21) a | 4.66 (0.41) a |
| Sucrose (% dm) | 1.87 (0.09) b | 2.29 (0.10) a | 1.64 (0.01) c | ND | ND |
| WE-β-glucan (% dm) | 0.14 (0.01) c | 0.96 (0.04) a | 0.28 (0.02) c | 0.64 (0.03) b | 0.61 (0.20) b |
| Fructose (% dm) | 0.46 (0.05) c | 0.21 (0.02) d | 0.66 (0.02) c | 2.70 (0.16) a | 2.20 (0.01) b |
| WE-AX (% dm) | 0.53 (0.02) d | 2.84 (0.16) a | 1.01 (0.01)c | 1.78 (0.13) b | 1.84 (0.01) b |
| WE-protein content (% dm) | 2.91 (0.31) d | 5.35 (0.09) b | 6.50 (0.11) a | 4.25 (0.03) c | 4.64 (0.35) c |
| WE-total phophorus (%dm) | 0.33 (0.02) c | 0.67 (0.04) b | 0.72 (0.01) b | 1.15 (0.06) a | 1.19 (0.24) a |
| 0.83 (0.08) ab | 0.70 (0.05) b | 0.83 (0.00) ab | 0.89 (0.02) a | 0.89 (0.03) a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bondt, Y.; Liberloo, I.; Roye, C.; Windhab, E.J.; Lamothe, L.; King, R.; Courtin, C.M. The Effect of Wet Milling and Cryogenic Milling on the Structure and Physicochemical Properties of Wheat Bran. Foods 2020, 9, 1755. https://doi.org/10.3390/foods9121755
De Bondt Y, Liberloo I, Roye C, Windhab EJ, Lamothe L, King R, Courtin CM. The Effect of Wet Milling and Cryogenic Milling on the Structure and Physicochemical Properties of Wheat Bran. Foods. 2020; 9(12):1755. https://doi.org/10.3390/foods9121755
Chicago/Turabian StyleDe Bondt, Yamina, Inge Liberloo, Chiara Roye, Erich J. Windhab, Lisa Lamothe, Roberto King, and Christophe M. Courtin. 2020. "The Effect of Wet Milling and Cryogenic Milling on the Structure and Physicochemical Properties of Wheat Bran" Foods 9, no. 12: 1755. https://doi.org/10.3390/foods9121755
APA StyleDe Bondt, Y., Liberloo, I., Roye, C., Windhab, E. J., Lamothe, L., King, R., & Courtin, C. M. (2020). The Effect of Wet Milling and Cryogenic Milling on the Structure and Physicochemical Properties of Wheat Bran. Foods, 9(12), 1755. https://doi.org/10.3390/foods9121755






