Protein Signatures to Trace Seafood Contamination and Processing
Abstract
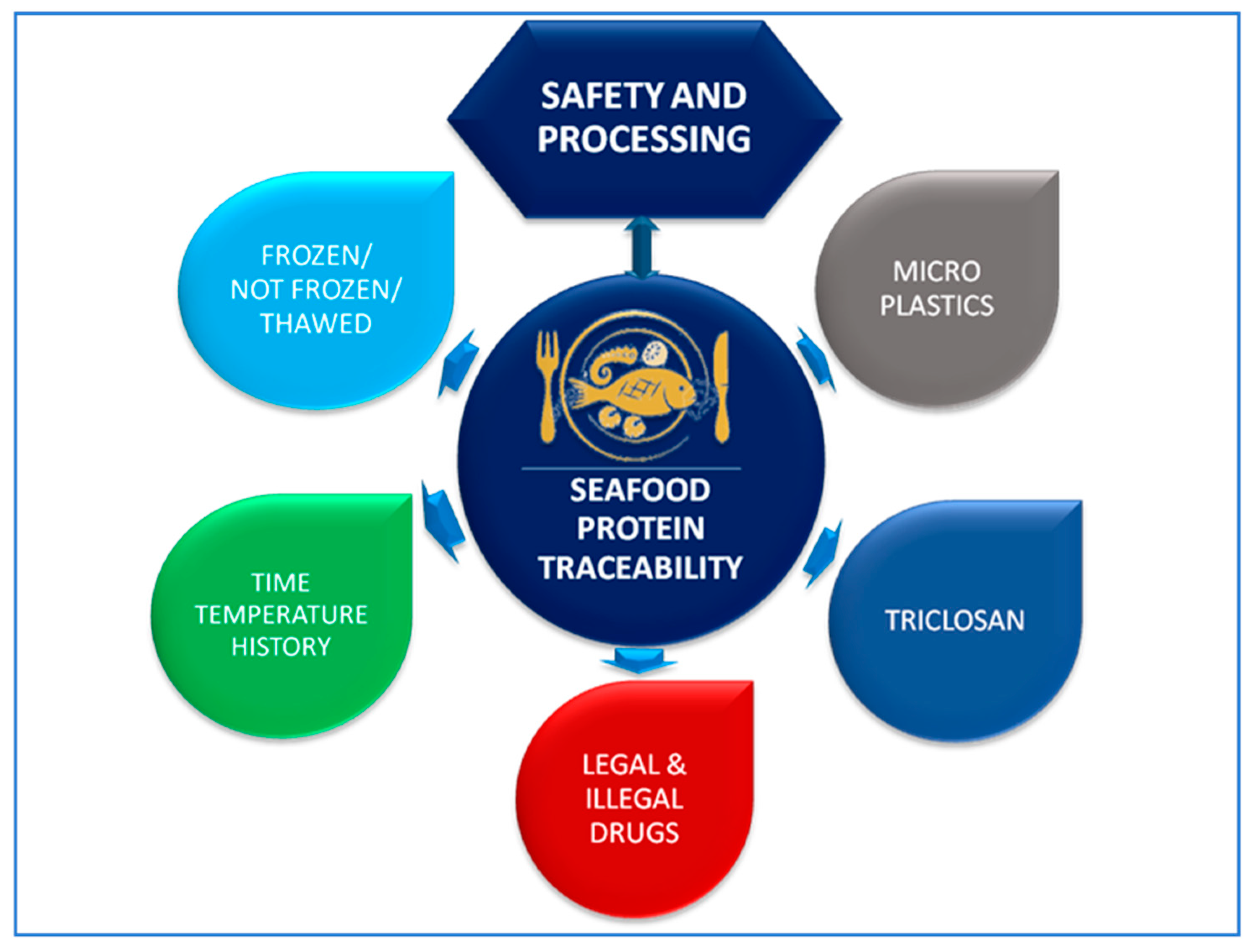
1. Introduction to Traceability and Safety
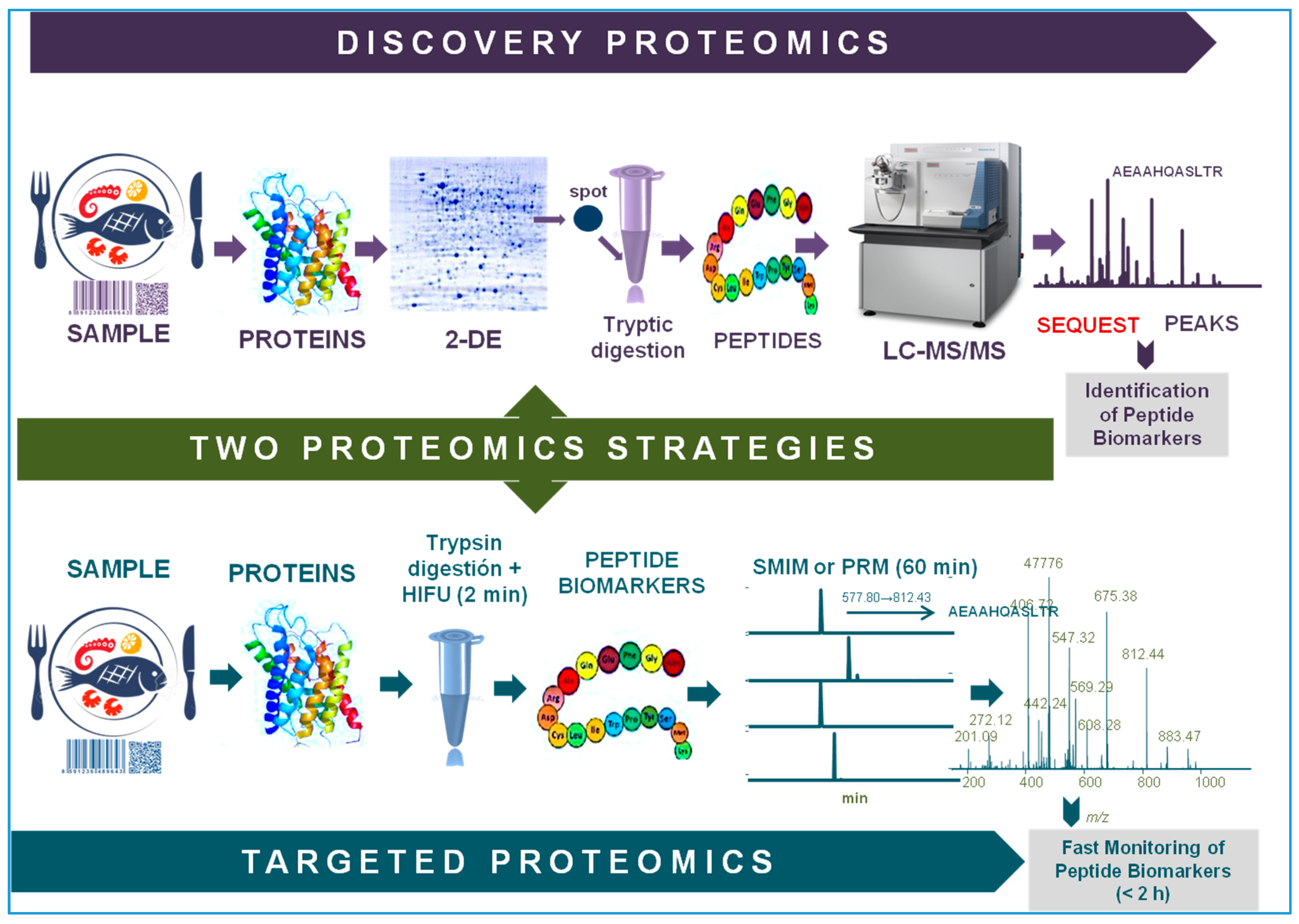
2. Proteomics: Discovery and Targeted Strategies
2.1. Classic Applications of Proteomics to Traceability
2.2. Application of Proteomics to Trace Exposure to Emergent Contaminants
2.2.1. Tracing Exposure to Microplastics
2.2.2. Proteomic Markers to Trace Exposure to Triclosan
2.2.3. Tracing Exposure to Medicines and Recreational Drugs by Proteomics
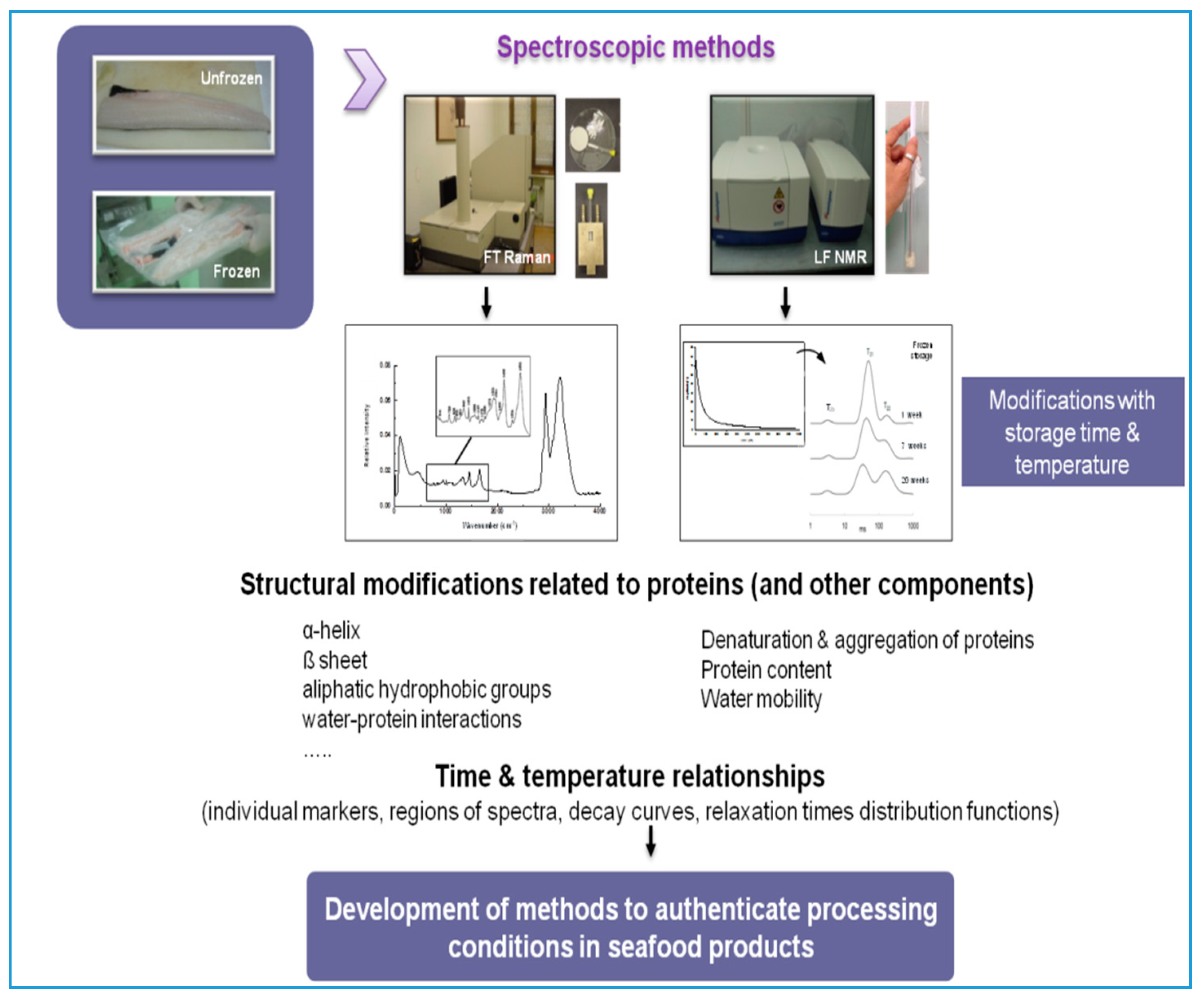
3. Vibrational and LF-NMR Spectroscopy
3.1. Vibrational Spectroscopy
3.2. Low-Field Nuclear Magnetic Resonance (LF-NMR) Relaxometry
3.3. Application of Spectroscopic Techniques to Trace Protein Changes in Frozen Fish Muscle
3.3.1. Time–Temperature History of Frozen Fish
3.3.2. Discrimination of Fresh and Frozen–Thawed Fish
4. Conclusions and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- EU Commission. Regulation (EC) No 178/2002 of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off. J. Eur. Communities 2002, L31, 1–24. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32002R0178&from=EN (accessed on 25 November 2020).
- Covaci, A.; Voorspoels, S.; Schepens, P.; Jorens, P.; Blust, R.; Neels, H. The Belgian PCB/dioxin crisis-8 years later. An overview. Environ. Toxicol. Pharmacol. 2008, 25, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Buzby, J.C.; Chandran, R. The Belgian Dioxin Crisis and Its Effects on Agricultural Production and Exports; International Trade and Food Safety, AER-828; United States Department of Agriculture: Washington, DC, USA, 2003; pp. 125–136. Available online: http://www.grains.org/buyingselling/barley (accessed on 25 November 2020).
- Gracia, A.; De-Magistris, T. Consumer preferences for food labeling: What ranks first? Food Control 2016, 61, 39–46. [Google Scholar] [CrossRef]
- Martinez, I.; Aursand, M.; Erikson, U.; Singstad, T.E.; Veliyulin, E.; Van Der Zwaag, C. Destructive and non-destructive analytical techniques for authentication and composition analyses of foodstuffs. Trends Food Sci. Technol. 2003, 14, 489–498. [Google Scholar] [CrossRef]
- Martinez, I.; Friis, T.J.; Careche, M. Post mortem muscle protein degradation during ice-storage of Arctic (Pandalus borealis) and tropical (Penaeus japonicus and Penaeus monodon) shrimps: A comparative electrophoretic and immunological study. J. Sci. Food Agric. 2001, 81, 1199–1208. [Google Scholar] [CrossRef]
- Martinez, I.; Friis, T.J. Application of proteome analysis to seafood authentication. Proteomics 2004, 4, 347–354. [Google Scholar] [CrossRef]
- EU Commission. Regulation (EC). No 2074/2005 of 5 December 2005 laying down implementing measures for certain products under Regulation (EC). No 853/2004 of the European Parliament and of the Council and for the organisation of official controls under Regulatio. Off. J. Eur. Communities 2005, L338, 27–59. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:338:0027:0059:EN:PDF (accessed on 25 November 2020).
- O’Farrell, P.H. High Resolution two-dimensional electrophoresis of Proteins. J. Biol. Chem. 1975, 250, 4007–4021. [Google Scholar] [CrossRef]
- Pandey, A.; Mann, M. Proteomics to study genes and genomes. Nature 2000, 405, 837–846. [Google Scholar] [CrossRef]
- Geng, F.; Huang, X.; Majumder, K.; Zhu, Z.; Cai, Z.; Ma, M. Mass spectrometry and two-dimensional electrophoresis to characterize the glycosylation of hen egg white ovomacroglobulin. J. Agric. Food Chem. 2015, 63, 8209–8215. [Google Scholar] [CrossRef]
- Mayer, K.; Albrecht, S.; Schaller, A. Targeted analysis of protein phosphorylation by 2D electrophoresis. Methods Mol. Biol. 2015, 1306, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.; Da Rocha, A.P.; Roepstorff, P.; Rogowska-Wrzesinska, A. Fish proteins as targets of ferrous-catalyzed oxidation: Identification of protein carbonyls by fluorescent labeling on two-dimensional gels and MALDI-TOF/TOF mass spectrometry. J. Agric. Food Chem. 2011, 59, 7962–7977. [Google Scholar] [CrossRef] [PubMed]
- Carrera, M.; Cañas, B.; Piñeiro, C.; Vázquez, J.; Gallardo, J.M. De novo mass spectrometry sequencing and characterization of species-specific peptides from nucleoside diphosphate kinase B for the classification of commercial fish species belonging to the family merlucciidae. J. Proteome Res. 2007, 6, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fonslow, B.R.; Shan, B.; Baek, M.C.; Yates, J.R. Protein analysis by shotgun/bottom-up proteomics. Chem. Rev. 2013, 113, 2343–2394. [Google Scholar] [CrossRef] [PubMed]
- Wolters, D.A.; Washburn, M.P.; Yates, J.R. An automated multidimensional protein identification technology for shotgun proteomics. Anal. Chem. 2001, 73, 5683–5690. [Google Scholar] [CrossRef]
- Carrera, M.; Ezquerra-Brauer, J.M.; Aubourg, S.P. Characterization of the jumbo squid (Dosidicus gigas) skin by-product by shotgun proteomics and protein-based bioinformatics. Mar. Drugs 2020, 18, 31. [Google Scholar] [CrossRef]
- Eng, J.K.; McCormack, A.L.; Yates, J.R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 1994, 5, 976–989. [Google Scholar] [CrossRef]
- Perkins, D.N.; Pappin, D.J.C.; Creasy, D.M.; Cottrell, J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 1999, 20, 3551–3567. [Google Scholar] [CrossRef]
- Geer, L.Y.; Markey, S.P.; Kowalak, J.A.; Wagner, L.; Xu, M.; Maynard, D.M.; Yang, X.; Shi, W.; Bryant, S.H. Open mass spectrometry search algorithm. J. Proteome Res. 2004, 3, 958–964. [Google Scholar] [CrossRef]
- Craig, R.; Beavis, R.C. TANDEM: Matching proteins with tandem mass spectra. Bioinformatics 2004, 20, 1466–1467. [Google Scholar] [CrossRef]
- Keller, A.; Nesvizhskii, A.I.; Kolker, E.; Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef] [PubMed]
- Käll, L.; Canterbury, J.D.; Weston, J.; Noble, W.S.; MacCoss, M.J. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 2007, 4, 923–925. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, A.; Wilm, M.; Mann, M. Peptide sequencing by mass spectrometry for homology searches and cloning of genes. J. Protein Chem. 1997, 16, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Bern, M.; Kil, Y.J.; Becker, C. Byonic: Advanced peptide and protein identification software. Curr. Protoc. Bioinform. 2012, 40, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, K.; Hendrie, C.; Liang, C.; Li, M.; Doherty-Kirby, A.; Lajoie, G. PEAKS: Powerful software for peptide de novo sequencing by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2337–2342. [Google Scholar] [CrossRef] [PubMed]
- Robotham, S.A.; Horton, A.P.; Cannon, J.R.; Cotham, V.C.; Marcotte, E.M.; Brodbelt, J.S. UVnovo: A de novo sequencing algorithm using single series of fragment ions via chromophore tagging and 351 nm ultraviolet photodissociation mass spectrometry. Anal. Chem. 2016, 88, 3990–3997. [Google Scholar] [CrossRef]
- Mueller, L.N.; Rinner, O.; Schmidt, A.; Letarte, S.; Bodenmiller, B.; Brusniak, M.Y.; Vitek, O.; Aebersold, R.; Müller, M. SuperHirn—A novel tool for high resolution LC-MS-based peptide/protein profiling. Proteomics 2007, 7, 3470–3480. [Google Scholar] [CrossRef] [PubMed]
- Mateos, J.; Landeira-Abia, A.; Fafián-Labora, J.A.; Fernández-Pernas, P.; Lesende-Rodríguez, I.; Fernández-Puente, P.; Fernández-Moreno, M.; Delmiro, A.; Martín, M.A.; Blanco, F.J.; et al. iTRAQ-based analysis of progerin expression reveals mitochondrial dysfunction, reactive oxygen species accumulation and altered proteostasis. Stem Cell Res. Ther. 2015, 6, 119. [Google Scholar] [CrossRef]
- Robotti, E.; Marengo, E. 2D-DIGE and fluorescence image analysis. Methods Mol. Biol. 2018, 1664, 25–39. [Google Scholar] [CrossRef]
- Stryiński, R.; Mateos, J.; Pascual, S.; González, Á.F.; Gallardo, J.M.; Łopieńska-Biernat, E.; Medina, I.; Carrera, M. Proteome profiling of L3 and L4 Anisakis simplex development stages by TMT-based quantitative proteomics. J. Proteom. 2019, 201, 1–11. [Google Scholar] [CrossRef]
- López-Ferrer, D.; Ramos-Fernández, A.; Martínez-Bartolomé, S.; García-Ruiz, P.; Vázquez, J. Quantitative proteomics using 16O/18O labeling and linear ion trap mass spectrometry. Proteomics 2006, 6 (Suppl. 1), 4–11. [Google Scholar] [CrossRef]
- Ong, S.E.; Blagoev, B.; Kratchmarova, I.; Kristensen, D.B.; Steen, H.; Pandey, A.; Mann, M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteom. 2002, 1, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Fornelli, L.; Toby, T.K.; Schachner, L.F.; Doubleday, P.F.; Srzenti, K.; Dehart, C.J.; Kelleher, N.L. Top-down proteomics: Where we are, where we are going? J. Proteom. 2018, 175, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Carrera, M.; Cañas, B.; Vázquez, J.; Gallardo, J.M. Extensive de novo sequencing of new parvalbumin isoforms using a novel combination of bottom-up proteomics, accurate molecular mass measurement by FTICR-MS, and selected MS/MS ion monitoring. J. Proteome Res. 2010, 9, 4393–4406. [Google Scholar] [CrossRef]
- Carrera, M.; Eguiraun, H.; Cañas, B.; Martinez, I.; Piñeiro, C. Proteomics: Contribution of proteomics techniques to understanding the interrelationship between food and health. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldra, F., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands; Oxford Academic Press: Oxford, UK, 2015; Volume 4, pp. 554–560. [Google Scholar] [CrossRef]
- Martinez, I.; Ofstad, R.; Olsen, R.L. Myosin isoforms in red and white muscles of some marine teleost fishes. J. Muscle Res. Cell Motil. 1990, 11, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, Y.; Kobayashi, T.; Watabe, S.; Hashimoto, K. Mapping of fish myosin light chains by two-dimensional gel electrophoresis. Comp. Biochem. Physiol. Part B Comp. Biochem. 1990, 95, 341–345. [Google Scholar] [CrossRef]
- Martinez, I.; Christiansen, J.S. Myofibrillar proteins in developing white muscle of the Arctic charr, Salvelinus alpinus (L.). Comp. Biochem. Physiol. Part B Biochem. 1994, 107, 11–20. [Google Scholar] [CrossRef]
- Martinez, I.; Ofstad, R.; Olsen, R.L. Intraspecific myosin light chain polymorphism in the white muscle of herring (Clupea harengus harengus, L.). FEBS Lett. 1990, 265, 23–26. [Google Scholar] [CrossRef]
- Martinez, I.; Bang, B.; Hatlen, B.; Blix, P. Myofibrillar proteins in skeletal muscles of parr, smolt and adult atlantic salmon (Salmo salar L.). Comparison with another salmonid, the arctic charr Salvelinus alpinus (L.). Comp. Biochem. Physiol. Part B Comp. Biochem. 1993, 106, 1021–1028. [Google Scholar] [CrossRef]
- Wulff, T.; Nielsen, M.E.; Deelder, A.M.; Jessen, F.; Palmblad, M. Authentication of fish products by large-scale comparison of tandem mass spectra. J. Proteome Res. 2013, 12, 5253–5259. [Google Scholar] [CrossRef]
- Martinez, I.; Solberg, C.; Lauritzsen, C.; Ofstad, R. Two-dimensional electrophoretic analyses of cod (Gadus morhua, L.) whole muscle proteins, water soluble fraction and surimi. Effect of the addition of CaCl2 and MgCl2 during the washing procedure. Appl. Theor. Electrophor. 1992, 2, 201–206. [Google Scholar] [PubMed]
- Kjærsgård, I.V.H.; Jessen, F. Proteome analysis elucidating post-mortem changes in cod (Gadus morhua) muscle proteins. J. Agric. Food Chem. 2003, 51, 3985–3991. [Google Scholar] [CrossRef] [PubMed]
- Kjærsgård, I.V.H.; Nørrelykke, M.R.; Jessen, F. Changes in cod muscle proteins during frozen storage revealed by proteome analysis and multivariate data analysis. Proteomics 2006, 6, 1606–1618. [Google Scholar] [CrossRef] [PubMed]
- Terova, G.; Addis, M.F.; Preziosa, E.; Pisanu, S.; Pagnozzi, D.; Biosa, G.; Gornati, R.; Bernardini, G.; Roggio, T.; Saroglia, M. Effects of postmortem storage temperature on sea bass (Dicentrarchus labrax) muscle protein degradation: Analysis by 2-D DIGE and MS. Proteomics 2011, 11, 2901–2910. [Google Scholar] [CrossRef] [PubMed]
- Kjærsgård, I.V.H.; Nørrelykke, M.R.; Baron, C.P.; Jessen, F. Identification of carbonylated protein in frozen rainbow trout (Oncorhynchus mykiss) fillets and development of protein oxidation during frozen storage. J. Agric. Food Chem. 2006, 54, 9437–9446. [Google Scholar] [CrossRef]
- Jessen, F.; Lametsch, R.; Bendixen, E.; Kjærsgård, I.V.H.; Jørgensen, B.M. Extracting information from two-dimensional electrophoresis gels by partial least squares regression. Proteomics 2002, 2, 32–35. [Google Scholar] [CrossRef]
- Kjærsgård, I.V.H.; Jessen, F. Two-dimensional gel electrophoresis detection of protein oxidation in fresh and tainted rainbow trout muscle. J. Agric. Food Chem. 2004, 52, 7101–7107. [Google Scholar] [CrossRef]
- Campos, A.M.O.; de Almeida, A.M. Top-down proteomics and farm animal and aquatic sciences. Proteomes 2016, 4, 38. [Google Scholar] [CrossRef]
- Ortea, I.; O’Connor, G.; Maquet, A. Review on proteomics for food authentication. J. Proteom. 2016, 147, 212–225. [Google Scholar] [CrossRef]
- Piñeiro, C.; Carrera, M.; Cañas, B.; Lekube, X.; Martinez, I. Proteomics and food analysis: Principles, techniques, and applications. In Handbook of Food Analysis, 3rd ed.; Nollet, L.M., Toldrá, F., Eds.; CRC Press: Boca Raton, FL, USA, 2015; Chapter 18; pp. 369–391. [Google Scholar] [CrossRef]
- Rodríguez, E.M.; Ortea, I. Food authentication of seafood species. In Proteomics in Food Science; Colgrave, M.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 331–342. [Google Scholar] [CrossRef]
- Carrera, M.; Cañas, B.; Gallardo, J.M. Proteomic identification of commercial fish species. In Proteomics in Food Science; Colgrave, M.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 317–330. [Google Scholar] [CrossRef]
- Martinez, I.; James, F.; Loréal, H. Application of Modern Analytical Techniques to Ensure Seafood Safety and Authenticity; FAO Fisheries Technical Paper 455; FAO: Rome, Italy, 2005. [Google Scholar] [CrossRef]
- Méndez, L.; Pazos, M. Chapter 18—Proteomics to assess fish quality and bioactivity. In Proteomics in Food Science; Colgrave, M.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 297–316. [Google Scholar] [CrossRef]
- Rodrigues, P.M.; Campos, A.; Kuruvilla, J.; Schrama, D.; Cristobal, S. Proteomics in aquaculture: Quality and safety. In Proteomics in Food Science; Colgrave, M.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 279–296. [Google Scholar] [CrossRef]
- Vaibhav, V.; Thompson, E.; Raftos, D.; Haynes, P.A. Proteomic analysis of disease in Sydney rock oysters. In Proteomics in Food Science; Colgrave, M.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 343–358. [Google Scholar] [CrossRef]
- Rodrigues, P.M.; Silva, T.S.; Dias, J.; Jessen, F. Proteomics in aquaculture: Applications and trends. J. Proteom. 2012, 75, 4325–4345. [Google Scholar] [CrossRef]
- Carrera, M.; Piñeiro, C.; Martinez, I. Proteomic strategies applied to farming conditions in aquaculture. Foods 2020, 9, 1050. [Google Scholar] [CrossRef] [PubMed]
- Fernández Robledo, J.A.; Yadavalli, R.; Allam, B.; Pales Espinosa, E.; Gerdol, M.; Greco, S.; Stevick, R.J.; Gómez-Chiarri, M.; Zhang, Y.; Heil, C.A.; et al. From the raw bar to the bench: Bivalves as models for human health. Dev. Comp. Immunol. 2019, 92, 260–282. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.; Puiseux-Dao, S.; Edery, M. Toxins and stress in fish: Proteomic analyses and response network. Toxicon 2011, 57, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.J.; Chen, J.; Huang, Z.A.; Zhuang, L.; Peng, L.Z.; Shi, Y.H. Influence of acute cadmium exposure on the liver proteome of a teleost fish, ayu (Plecoglossus altivelis). Mol. Biol. Rep. 2012, 39, 2851–2859. [Google Scholar] [CrossRef]
- Biales, A.D.; Bencic, D.C.; Villeneuve, D.L.; Ankley, G.T.; Lattier, D.L. Proteomic analysis of zebrafish brain tissue following exposure to the pesticide prochloraz. Aquat. Toxicol. 2011, 105, 618–628. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, S.; Ji, C.; Li, F.; Cong, M.; Shan, X.; Wu, H. iTRAQ-based proteomic analysis on the mitochondrial responses in gill tissues of juvenile olive flounder Paralichthys olivaceus exposed to cadmium. Environ. Pollut. 2020, 257, 113591. [Google Scholar] [CrossRef]
- Pacitti, D.; Lawan, M.M.; Sweetman, J.; Martin, S.A.M.; Feldmann, J.; Secombes, C.J. Selenium Supplementation in fish: A combined chemical and biomolecular study to understand Sel-Plex assimilation and impact on selenoproteome expression in rainbow trout (Oncorhynchus mykiss). PLoS ONE 2015, 10, e0127041. [Google Scholar] [CrossRef]
- Ferain, A.; Bonnineau, C.; Neefs, I.; De Saeyer, N.; Lemaire, B.; Cornet, V.; Larondelle, Y.; De Schamphelaere, K.A.C.; Debier, C.; Rees, J.-F. Exploring the interactions between polyunsaturated fatty acids and cadmium in rainbow trout liver cells: A genetic and proteomic study. Aquat. Toxicol. 2018, 205, 100–113. [Google Scholar] [CrossRef]
- Dang, T.T.; Jessen, F.; Martens, H.J.; Gringer, N.; Olsen, K.; Bøknæs, N.; Orlien, V. Proteomic and microscopic approaches in understanding mechanisms of shell-loosening of shrimp (Pandalus borealis) induced by high pressure and protease. Food Chem. 2019, 289, 729–738. [Google Scholar] [CrossRef]
- Green, D.S.; Colgan, T.J.; Thompson, R.C.; Carolan, J.C. Exposure to microplastics reduces attachment strength and alters the haemolymph proteome of blue mussels (Mytilus edulis). Environ. Pollut. 2019, 246, 423–434. [Google Scholar] [CrossRef]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Goïc, N.L.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jeong, C.B.; Lee, J.S.; Wang, D.; Wang, M. Transgenerational proteome plasticity in resilience of a marine copepod in response to environmentally relevant concentrations of cicroplastics. Environ. Sci. Technol. 2019, 53, 8426–8436. [Google Scholar] [CrossRef] [PubMed]
- Magni, S.; Della Torre, C.; Garrone, G.; D’Amato, A.; Parenti, C.C.; Binelli, A. First evidence of protein modulation by polystyrene microplastics in a freshwater biological model. Environ. Pollut. 2019, 250, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Careche, M.; Sánchez-Alonso, I. Quality and quality changes assessment of processed fish. In Trends in Fish Processing Technologies; Borda, D., Nicolau, A., Raspor, P., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 255–276. [Google Scholar] [CrossRef]
- Sánchez-Valencia, J.; Sánchez-Alonso, I.; Martinez, I.; Careche, M. Low-field nuclear magnetic resonance of proton (1H LF NMR) relaxometry for monitoring the time and temperature history of frozen hake (Merluccius merluccius L.) muscle. Food Bioprocess Technol. 2015, 8, 2137–2145. [Google Scholar] [CrossRef]
- Karoui, R.; Lefur, B.; Grondin, C.; Thomas, E.; Demeulemester, C.; De Baerdemaeker, J.; Guillard, A.S. Mid-infrared spectroscopy as a new tool for the evaluation of fish freshness. Int. J. Food Sci. Technol. 2007, 42, 57–64. [Google Scholar] [CrossRef]
- Hassoun, A.; Karoui, R. Quality evaluation of fish and other seafood by traditional and nondestructive instrumental methods: Advantages and limitations. Crit. Rev. Food Sci. Nutr. 2017, 57, 1976–1998. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alonso, I.; Moreno, P.; Careche, M. Low field nuclear magnetic resonance (LF NMR) spectroscopic analysis of hake (Merluccius merluccius, L.) upon freezing. A possibility for authentication of fresh vs thawed muscle. In Proceedings of the 4th Trans-Atlantic Fisheries Technology Conference (TAFT), Clearwater Beach, FL, USA, 30 October–2 November 2012. [Google Scholar]
- Herrero, A.M.; Carmona, P.; García, M.L.; Solas, M.T.; Careche, M. Ultrastructural changes and structure and mobility of myowater in frozen-stored hake (Merluccius merluccius L.) muscle: Relationship with functionality and texture. J. Agric. Food Chem. 2005, 53, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Careche, M.; Herrero, A.M.; Rodriguez-Casado, A.; Del Mazo, M.L.; Carmona, P. Structural changes of hake (Merluccius merluccius L.) fillets: Effects of freezing and frozen storage. J. Agric. Food Chem. 1999, 47, 952–959. [Google Scholar] [CrossRef]
- Careche, M.; Sánchez-Alonso, I.; González-Muñoz, I.; Navas, A.; Tejada, M. LF NMR relaxometry can be used to verify that fish have been subjected to freezing in order to comply with EU regulation about prevention of parasite infection. In Proceedings of the 46th WEFTA Meeting, Split, Croatia, 12–14 October 2016. [Google Scholar]
- Felberg, H.S.; Hagen, L.; Slupphaug, G.; Batista, I.; Nunes, M.L.; Olsen, R.L.; Martinez, I. Partial characterisation of gelatinolytic activities in herring (Clupea harengus) and sardine (Sardina pilchardus) possibly involved in post-mortem autolysis of ventral muscle. Food Chem. 2010, 119, 675–683. [Google Scholar] [CrossRef]
- Felberg, H.S.; Slizyté, R.; Mozuraityte, R.; Dahle, S.W.; Olsen, R.L.; Martinez, I. Proteolytic activities of ventral muscle and intestinal content of North Sea herring (Clupea harengus) with full and emptied stomachs. Food Chem. 2009, 116, 40–46. [Google Scholar] [CrossRef]
- Skog, T.-E.; Hylland, K.; Torstensen, B.E.; Berntssen, M.H.G. Salmon farming affects the fatty acid composition and taste of wild saithe Pollachius virens L. Aquac. Res. 2003, 34, 999–1007. [Google Scholar] [CrossRef]
- Simmons, D.B.D.; Miller, J.; Clarence, S.; McCallum, E.S.; Balshine, S.; Chandramouli, B.; Cosgrove, J.; Sherry, J.P. Altered expression of metabolites and proteins in wild and caged fish exposed to wastewater effluents in situ. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Li, W.; Yao, Z.; Sun, L.; Hu, W.; Cao, J.; Lin, W.; Lin, X. Proteomics analysis reveals a potential antibiotic cocktail therapy strategy for Aeromonas hydrophila infection in biofilm. J. Proteome Res. 2016, 15, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M.; Bini, L.; Magni, S.; Rizzo, A.; Ghilardi, A.; Landi, C.; Armini, A.; Del Giacco, L.; Binelli, A. Exposure to cocaine and its main metabolites altered the protein profile of zebrafish embryos. Environ. Pollut. 2018, 232, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- SAPEA. A Scientific Perspective on Microplastics in Nature and Society. Science Advice for Policy by European Academies—SAPEA; Evidence Review Report; SAPEA: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Lusher, A.; Hollman, P.; Mandoza-Hill, J. Microplastics in Fisheries and Aquaculture; FAO Fisheries and Aquaculture Technical Paper 615; FAO: Rome, Italy, 2017; Available online: http://www.fao.org/3/a-i7677e.pdf (accessed on 25 November 2020).
- Musatadi, M.; González-Gaya, B.; Irazola, M.; Prieto, A.; Etxebarria, N.; Olivares, M.; Zuloaga, O. Focused ultrasound-based extraction for target analysis and suspect screening of organic xenobiotics in fish muscle. Sci. Total Environ. 2020, 740, 139894. [Google Scholar] [CrossRef]
- Olaniyan, L.W.B.; Mkwetshana, N.; Okoh, A.I. Triclosan in water, implications for human and environmental health. Springerplus 2016, 5, 1639. [Google Scholar] [CrossRef]
- Agüera, A.; Fernández-Alba, A.R.; Piedra, L.; Mézcua, M.; Gómez, M.J. Evaluation of triclosan and biphenylol in marine sediments and urban wastewaters by pressurized liquid extraction and solid phase extraction followed by gas chromatography mass spectrometry and liquid chromatography mass spectrometry. Anal. Chim. Acta 2003, 480, 193–205. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Li, J.; Mao, L.; Nguyen, S.H.; Duarte, T.; Coin, L.; Bond, P.; Yuan, Z.; Guo, J. Triclosan at environmentally relevant concentrations promotes horizontal transfer of multidrug resistance genes within and across bacterial genera. Environ. Int. 2018, 121, 1217–1226. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Zhang, S.; Bond, P.; Yuan, Z.; Guo, J. Triclosan at environmental concentrations can enhance the spread of extracellular antibiotic resistance genes through transformation. Sci. Total Environ. 2020, 713, 136621. [Google Scholar] [CrossRef]
- Riva, C.; Cristoni, S.; Binelli, A. Effects of triclosan in the freshwater mussel Dreissena polymorpha: A proteomic investigation. Aquat. Toxicol. 2012, 118–119, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Falisse, E.; Voisin, A.S.; Silvestre, F. Impacts of triclosan exposure on zebrafish early-life stage: Toxicity and acclimation mechanisms. Aquat. Toxicol. 2017, 189, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Küster, A.; Adler, N. Pharmaceuticals in the environment: Scientific evidence of risks and its regulation. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130587. [Google Scholar] [CrossRef]
- Zuccato, E.; Chiabrando, C.; Castiglioni, S.; Bagnati, R.; Fanelli, R. Estimating community drug abuse by wastewater analysis. Environ. Health Perspect. 2008, 116, 1027–1032. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drug Addition. Perspectives on Drugs. Update—Wastewater Analysis and Drugs: A European Multi-City Study. 2020. Available online: https://www.emcdda.europa.eu/system/files/publications/2757/POD_Wastewateranalysis_update2020.pdf (accessed on 25 November 2020).
- Brodin, T.; Fick, J.; Jonsson, M.; Klaminder, J. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science 2013, 339, 814–815. [Google Scholar] [CrossRef] [PubMed]
- European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Communities 2010, 66, 1–27. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02000L0060-20141120&from=EN (accessed on 25 November 2020).
- European Union. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive). Off. J. Eur. Communities 2008, L164, 19–40. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008L0056&from=EN (accessed on 25 November 2020).
- Martin, J.M.; Saaristo, M.; Tan, H.; Bertram, M.G.; Nagarajan-Radha, V.; Dowling, D.K.; Wong, B.B.M. Field-realistic antidepressant exposure disrupts group foraging dynamics in mosquitofish. Biol. Lett. 2019, 15, 20190615. [Google Scholar] [CrossRef]
- Hampel, M.; Alonso, E.; Aparicio, I.; Santos, J.L.; Leaver, M. Hepatic proteome analysis of Atlantic salmon (Salmo salar) after exposure to environmental concentrations of human pharmaceuticals. Mol. Cell. Proteom. 2015, 14, 371–381. [Google Scholar] [CrossRef]
- Yan, S.; Wang, M.; Liang, X.; Martyniuk, C.J.; Zha, J.; Wang, Z. Environmentally relevant concentrations of carbamazepine induce liver histopathological changes and a gender-specific response in hepatic proteome of Chinese rare minnows (Gobiocypris rarus). Environ. Pollut. 2018, 243, 480–491. [Google Scholar] [CrossRef]
- Ribas, J.L.C.; Sherry, J.P.; Zampronio, A.R.; Silva de Assis, H.C.; Simmons, D.B.D. Inhibition of immune responses and related proteins in Rhamdia quelen exposed to diclofenac. Environ. Toxicol. Chem. 2017, 36, 2092–2107. [Google Scholar] [CrossRef] [PubMed]
- Binelli, A.; Marisa, I.; Fedorova, M.; Hoffmann, R.; Riva, C. First evidence of protein profile alteration due to the main cocaine metabolite (benzoylecgonine) in a freshwater biological model. Aquat. Toxicol. 2013, 140–141, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Li-Chan, E. Introduction to Vibrational Spectroscopy in Food Science. In Applications of Vibrational Spectroscopy in Food Science; Part One: Introduction and Basic Concepts; Li-Chan, E., Chalmers, J.M., Griffiths, P.R., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2010; Volume 1, pp. 3–29. [Google Scholar]
- Griffiths, P.R. Introduction to the Theory and Instrumentation of Vibrational Spectroscopy. In Applications of Vibrational Spectroscopy in Food Science; Part One: Introduction and Basic Concepts; Li-Chan, E., Chalmers, J.M., Griffiths, P.R., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2010; Volume 1, pp. 31–46. [Google Scholar]
- Lohumi, S.; Lee, S.; Lee, H.; Cho, B.-K. A review of vibrational spectroscopic techniques for the detection of food authenticity and adulteration. Trends Food Sci. Technol. 2015, 46, 85–98. [Google Scholar] [CrossRef]
- Li-Chan, E. The applications of Raman spectroscopy in food science. Trends Food Sci. Technol. 1996, 7, 361–370. [Google Scholar] [CrossRef]
- Li-Chan, E.; Chalmers, J.M.; Griffiths, P.R. (Eds.) Instrumentation and Fundamental Applications. In Applications of Vibrational Spectroscopy in Food Science; John Wiley & Sons Ltd.: Chichester, UK, 2010; Volume 1, pp. 1–346. [Google Scholar]
- Li-Chan, E.; Chalmers, J.M.; Griffiths, P.R. (Eds.) Analysis of Food Drink and Related Materials. In Applications of Vibrational Spectroscopy in Food Science; John Wiley & Sons Ltd.: Chichester, UK, 2010; Volume 2, pp. 347–715. [Google Scholar]
- Uddin, M.; Okazaki, E. Applications of Vibrational Spectroscopy to the Analysis of Fish and Other Aquatic Food Products. In Applications of Vibrational Spectroscopy in Food Science; Part Four: Examples of Applications for Food Analysis and Quality Assurance; Li-Chan, E., Chalmers, J.M., Griffiths, P.R., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2010; Volume II, pp. 439–459. [Google Scholar]
- Carmona, P.; Sánchez-Alonso, I.; Careche, M. Chemical changes during freezing and frozen storage of fish investigated by vibrational spectroscopy. In Applications of Vibrational Spectroscopy in Food Science; Part Three: Fundamental Research to Elucidate Properties and Processing-Induced Changes; Li-Chan, E., Chalmers, J.M., Griffiths, P.R., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2010; Volume 1, pp. 229–240. [Google Scholar]
- Gudjónsdóttir, M.; Arason, S.; Rustad, T. The effects of pre-salting methods on water distribution and protein denaturation of dry salted and rehydrated cod—A low-field NMR study. J. Food Eng. 2011, 104, 23–29. [Google Scholar] [CrossRef]
- Erikson, U.; Standal, I.B.; Aursand, I.G.; Veliyulin, E.; Aursand, M. Use of NMR in fish processing optimization: A review of recent progress. Magn. Reson. Chem. 2012, 50, 471–480. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M. Recent developments in the food quality detected by non-invasive nuclear magnetic resonance technology. Crit. Rev. Food Sci. Nutr. 2019, 59, 2202–2213. [Google Scholar] [CrossRef]
- Hills, B.P.; Takacs, S.F.; Belton, P.S. The effects of proteins on the proton N.M.R. transverse relaxation time of water. Mol. Phys. 1989, 67, 919–937. [Google Scholar] [CrossRef]
- Belton, P. Spectroscopic approaches to the understanding of water in foods. Food Rev. Int. 2011, 27, 170–191. [Google Scholar] [CrossRef]
- Duflot, M.; Sánchez-Alonso, I.; Duflos, G.; Careche, M. LF 1H NMR T2 relaxation rate as affected by water addition, NaCl and pH in fresh, frozen and cooked minced hake. Food Chem. 2019, 277, 229–237. [Google Scholar] [CrossRef]
- Grujić, R.; Petrović, L.; Pikula, B.; Amidžić, L. Definition of the optimum freezing rate.1. Investigation of structure and ultrastructure of beef M. longissimus dorsi frozen at different freezing rates. Meat Sci. 1993, 33, 301–318. [Google Scholar] [CrossRef]
- Becker, B.R.; Fricke, B.A. Freezing. Principles. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2003; pp. 2706–2711. [Google Scholar]
- Howgate, P. Fish. In Food Microscopy; Vaughan, J.G., Ed.; Academic Press Inc.: London, UK, 1979; pp. 343–392. [Google Scholar]
- Shenouda, S.Y.K. Theories of protein denaturation during frozen storage of fish flesh. Adv. Food Res. 1980, 26, 275–311. [Google Scholar]
- Haard, N.F. Biochemical reactions in fish muscle during frozen storage. In Seafood Science and Technology; Fishing News Books; Bligh, E.G., Ed.; Blackwell Scientific Publications: Toronto, ON, Canada, 1992; pp. 176–209. [Google Scholar]
- Sánchez-Alonso, I.; Carmona, P.; Careche, M. Vibrational spectroscopic analysis of hake (Merluccius merluccius L.) lipids during frozen storage. Food Chem. 2012, 132, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.M.; Carmona, P.; Careche, M. Raman Spectroscopic study of structural changes in hake (Merluccius merluccius L.) muscle proteins during frozen storage. J. Agric. Food Chem. 2004, 52, 2147–2153. [Google Scholar] [CrossRef] [PubMed]
- Lambelet, P.; Renevey, F.; Kaabi, C.; Raemy, A. Low-field nuclear magnetic resonance relaxation study of stored or processed cod. J. Agric. Food Chem. 1995, 43, 1462–1466. [Google Scholar] [CrossRef]
- Nott, K.P.; Evans, S.D.; Hall, L.D. Quantitative magnetic resonance imaging of fresh and frozen thawed trout. Magn. Reson. Imaging 1999, 17, 445–455. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Moreno, P.; Careche, M. Low field nuclear magnetic resonance (LF-NMR) relaxometry in hake (Merluccius merluccius, L.) muscle after different freezing and storage conditions. Food Chem. 2014, 153, 250–257. [Google Scholar] [CrossRef]
- Gudjónsdóttir, M.; Karlsdóttir, M.G.; Arason, S.; Rustad, T. Injection of fish protein solutions of fresh saithe (Pollachius virens) fillets studied by low field nuclear magnetic resonance and physicochemical measurements. J. Food Sci. Technol.-Mysore. 2013, 50, 228–238. [Google Scholar] [CrossRef]
- Yano, S.; Tanaka, M.; Suzuki, N.; Kanzaki, Y. Texture change of beef and salmon meats caused by refrigeration and use of pulse NMR as an index of taste. Food Sci. Technol. Res. 2002, 8, 137–143. [Google Scholar] [CrossRef]
- Aursand, I.G.; Veliyulin, E.; Bocker, U.; Ofstad, R.; Rustad, T.; Erikson, U. Water and salt distribution in Atlantic salmon (Salmo salar) studied by low-field 1H NMR, 1H and 23Na MRI and light microscopy: Effects of raw material quality and brine salting. J. Agric. Food Chem. 2009, 57, 46–54. [Google Scholar] [CrossRef]
- Nikoo, M.; Regenstein, J.M.; Ghomi, M.R.; Benjakul, S.; Yang, N.; Xu, X. Study of the combined effects of a gelatin-derived cryoprotective peptide and a non-peptide antioxidant in a fish mince model system. LWT Food Sci. Technol. 2015, 60, 358–364. [Google Scholar] [CrossRef]
- Steen, C.; Lambelet, P. Texture changes in frozen cod mince measured by low-field nuclear magnetic resonance spectroscopy. J. Sci. Food Agric. 1997, 75, 268–272. [Google Scholar] [CrossRef]
- Jensen, K.N.; Guldager, H.S.; Jørgensen, B.M. Three-way modelling of NMR relaxation profiles from thawed cod muscle. J. Aquat. Food Prod. Technol. 2002, 11, 201–214. [Google Scholar] [CrossRef]
- Burgaard, M.G.; Jørgensen, B.M. Effect of temperature on quality-related changes in cod (Gadus morhua) during short- and long-term frozen storage. J. Aquat. Food Prod. Technol. 2010, 19, 249–263. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Martinez, I.; Sánchez-Valencia, J.; Careche, M. Estimation of freezing storage time and quality changes in hake (Merluccius merluccius, L.) by low field NMR. Food Chem. 2012, 135, 1626–1634. [Google Scholar] [CrossRef]
- Fu, B.; Labuza, T.P. Shelf-life prediction: Theory and application. Food Control. 1993, 4, 125–133. [Google Scholar] [CrossRef]
- Taoukis, P.S.; Labuza, T.P.; Saguy, I.S. Kinetics of food deterioration and shelf-life prediction. In The Handbook of Food Engineering Practice; Valentas, K.J., Rotstein, E., Singh, R.D., Eds.; CRC Press: New York, NY, USA, 1997; pp. 2–75. [Google Scholar]
- Careche, M.; Sánchez-alonso, I.; Martinez, I. Estimation of quality in frozen fish by low field NMR. In Modern Magnetic Resonance, 2nd ed.; Webb, P.G.A., Ed.; Springer International Publishing: Basel, Switzerland, 2018. [Google Scholar] [CrossRef]
- Sánchez-Valencia, J.; Sánchez-Alonso, I.; Martinez, I.; Careche, M. Estimation of frozen storage time or temperature by kinetic modeling of the Kramer shear resistance and water holding capacity (WHC) of hake (Merluccius merluccius, L.) muscle. J. Food Eng. 2014, 120, 37–43. [Google Scholar] [CrossRef]
- Careche, M.; Carmona, P.; Sánchez-Alonso, I. Monitoring the time and temperature history of frozen hake (Merluccius merluccius, L.) muscle by FTIR spectroscopy of the lipid fraction. Food Bioprocess Technol. 2015, 8, 112–119. [Google Scholar] [CrossRef][Green Version]
- Velioglu, H.M.; Temiz, H.T.; Boyaci, I.H. Differentiation of fresh and frozen-thawed fish samples using Raman spectroscopy coupled with chemometric analysis. Food Chem. 2015, 172, 283–290. [Google Scholar] [CrossRef]
- EU Commission. Regulation (EU) No 1276/2011 of 8 December 2011. Off. J. Eur. Union 2011, 8, 39–41. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:327:0039:0041:EN:PDF (accessed on 25 November 2020).
| Technique | Advantages | Disadvantages |
|---|---|---|
| Proteomics techniques | ||
| Discovery proteomics | Protein spots are visible. Hundreds to thousands of polypeptides can be separated and analyzed in a single gel. Relative spot quantification is easy. Protein quantification is possible using differential labeling (e.g., fluorophores). Suitable to identify post-translational modifications. It is the most relevant method for the separation and de novo MS sequencing of incompletely sequenced organisms. | Expensive equipment. Manual work and high skills required. Time consuming (days). Gel-based approach allows the visualization of a relatively low number of proteins (<1000). Problems with the separation of poorly soluble, hydrophobic proteins and very low or very high relative molecular mass Mr proteins. Salt ions may interfere with protein separation. Suppression of signal by highly abundant proteins. Limited reproducibility. Smaller dynamic range than other separation methods. |
| Proteome wide MS/MS | Identification and quantification of thousands of proteins (>10,000) in a single experiment. This approach presents high sensitivity and reproducibility. It is automated. High-throughput experiments. | Loss of information on protein isoforms. It is difficult to identify proteins from species with unsequenced genomes. Manual work is required for unsequenced genomes. MS identifies only peptides: protein identification is indirect by comparison with the peptides originated from known proteins contained in databases. No match with experimentally measured Mr and pH. |
| Targeted MS/MS | High sensitivity and reproducibility. Automation is possible. | Low protein identification rate (<10%). |
| Spectroscopic techniques | ||
| Spectroscopic techniques (in general) | Little or no sample preparation. They may be applied in a non-destructive or non-invasive way (i.e., in whole muscle). Low analysis cost. Short analysis time (seconds to minutes). Low amount of sample required. No need of high technical skills. Suitable for routine analysis. There is equipment for field analysis (i.e., suitable for laboratory, in situ and industry routine analyses). Alternative to wet chemistry. | No distinction among proteins in complex samples. Low sensitivity. Sometimes, initial high cost in equipment. Spectral data pre-treatment may be needed prior to data analysis. Portable instruments available only for selected applications. |
| Infrared and Raman spectroscopy | Highly specific. IR and Raman spectroscopy are complementary to each other. Minimum interference from water (Raman). Attenuated total reflectance (ATR) measurement mode overcomes the problem of strong water absorption in IR. Several indicators may be analyzed simultaneously. They provide analytical, structural, qualitative and quantitative information. Well-known secondary structure-spectral correlations. Libraries for compounds are available. | It requires skilled personnel. Fluorescence can hide the spectrum (Raman). Strong water absorption may be a problem (IR). Spectral data analysis is complex. The spatial variability of the sample may not be correctly taken into account. |
| Low-field NMR | Good relation with water-holding capacity and texture attributes. | Difficult interpretation in some scenarios. |
| Technique | Application | Species | References |
|---|---|---|---|
| Discovery proteomics | |||
| Authentication | Numerous | [7,36,42,50,51,52,53,54,55] | |
| Species and stock identification | Numerous | [37,38,39,40,41] | |
| Quality, bioactivity and safety | Numerous | [56,57] | |
| Production method (wild vs. farmed) | Numerous | [54,57,58,59,60] | |
| Exposure to biotoxins | Bivalves, fish species | [61,62] | |
| Exposure to environmental pollutants | Ayu, rainbow trout | [63,64,65,66,67] | |
| Postmortem changes | Shrimp, cod, seabass | [6,43,44,45,46] | |
| Freezing and frozen storage | Cod, rainbow trout | [47,48,49] | |
| Surimi manufacture and rigor status | Cod | [43] | |
| High pressure and enzymatic deshelling | Shrimp | [68] | |
| Exposure to microplastics | Oysters, blue mussels, copepods, zebra mussel | [69,70,71,72] | |
| Spectroscopic techniques | |||
| IR 1, Raman 2 | Time–temperature history of frozen fish | European hake | [73] |
| LF-NMR | Time–temperature history of frozen fish | European hake | [73,74] |
| IR 1 | Discrimination fresh/frozen–thawed | European hake, several | [75,76,77] |
| Raman 2 | Discrimination fresh/frozen–thawed | European hake | [78,79] |
| LF-NMR | Discrimination fresh/frozen–thawed | European hake | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, I.; Sánchez-Alonso, I.; Piñeiro, C.; Careche, M.; Carrera, M. Protein Signatures to Trace Seafood Contamination and Processing. Foods 2020, 9, 1751. https://doi.org/10.3390/foods9121751
Martinez I, Sánchez-Alonso I, Piñeiro C, Careche M, Carrera M. Protein Signatures to Trace Seafood Contamination and Processing. Foods. 2020; 9(12):1751. https://doi.org/10.3390/foods9121751
Chicago/Turabian StyleMartinez, Iciar, Isabel Sánchez-Alonso, Carmen Piñeiro, Mercedes Careche, and Mónica Carrera. 2020. "Protein Signatures to Trace Seafood Contamination and Processing" Foods 9, no. 12: 1751. https://doi.org/10.3390/foods9121751
APA StyleMartinez, I., Sánchez-Alonso, I., Piñeiro, C., Careche, M., & Carrera, M. (2020). Protein Signatures to Trace Seafood Contamination and Processing. Foods, 9(12), 1751. https://doi.org/10.3390/foods9121751







