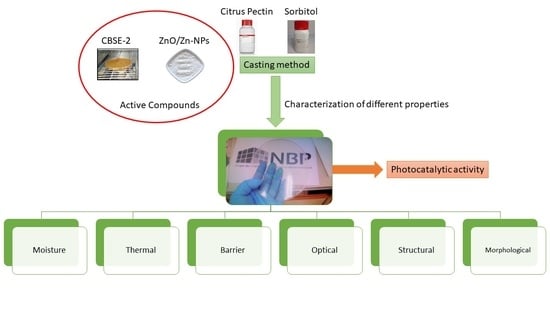
Pectin-Based Films with Cocoa Bean Shell Waste Extract and ZnO/Zn-NPs with Enhanced Oxygen Barrier, Ultraviolet Screen and Photocatalytic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. CBS Extract Preparation
2.3. Synthesis of ZnO/Zn-NPs
2.4. Pectin-Based Film Preparation
2.5. Pectin-Based Film Characterisation
2.5.1. Moisture Content (MC)
2.5.2. Oxygen Transmission Rate (OTR)
2.5.3. Optical Properties
2.5.4. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy (ATR–FTIR)
2.5.5. X-ray Diffraction (XRD)
2.5.6. Field Emission Scanning Electron Microscopy (FESEM)
2.5.7. Photocatalytic Activity
2.6. Statistical Analysis
3. Results
3.1. Moisture Content
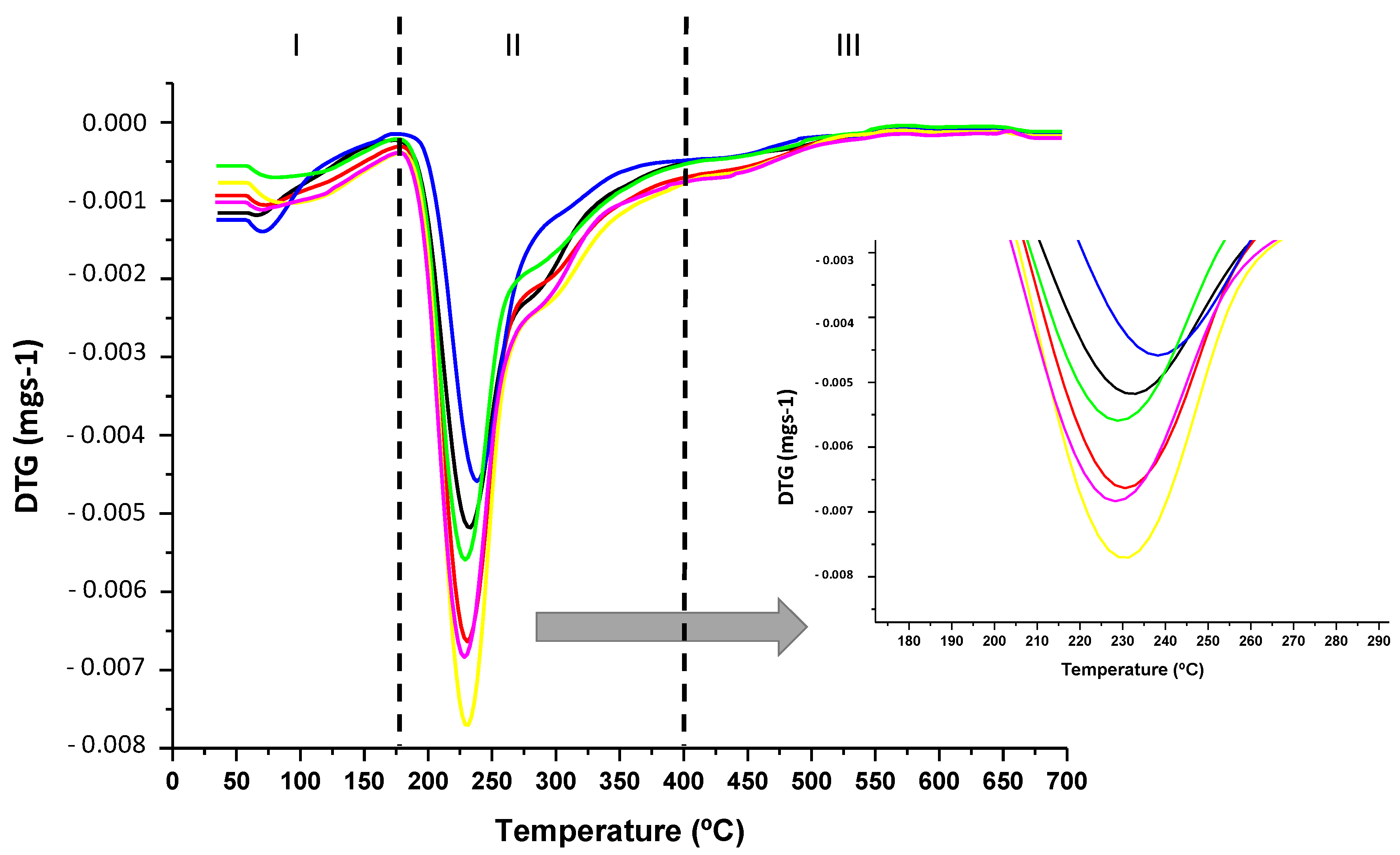
3.2. Thermal Properties
3.3. Oxygen Transmission Rate (OTR)
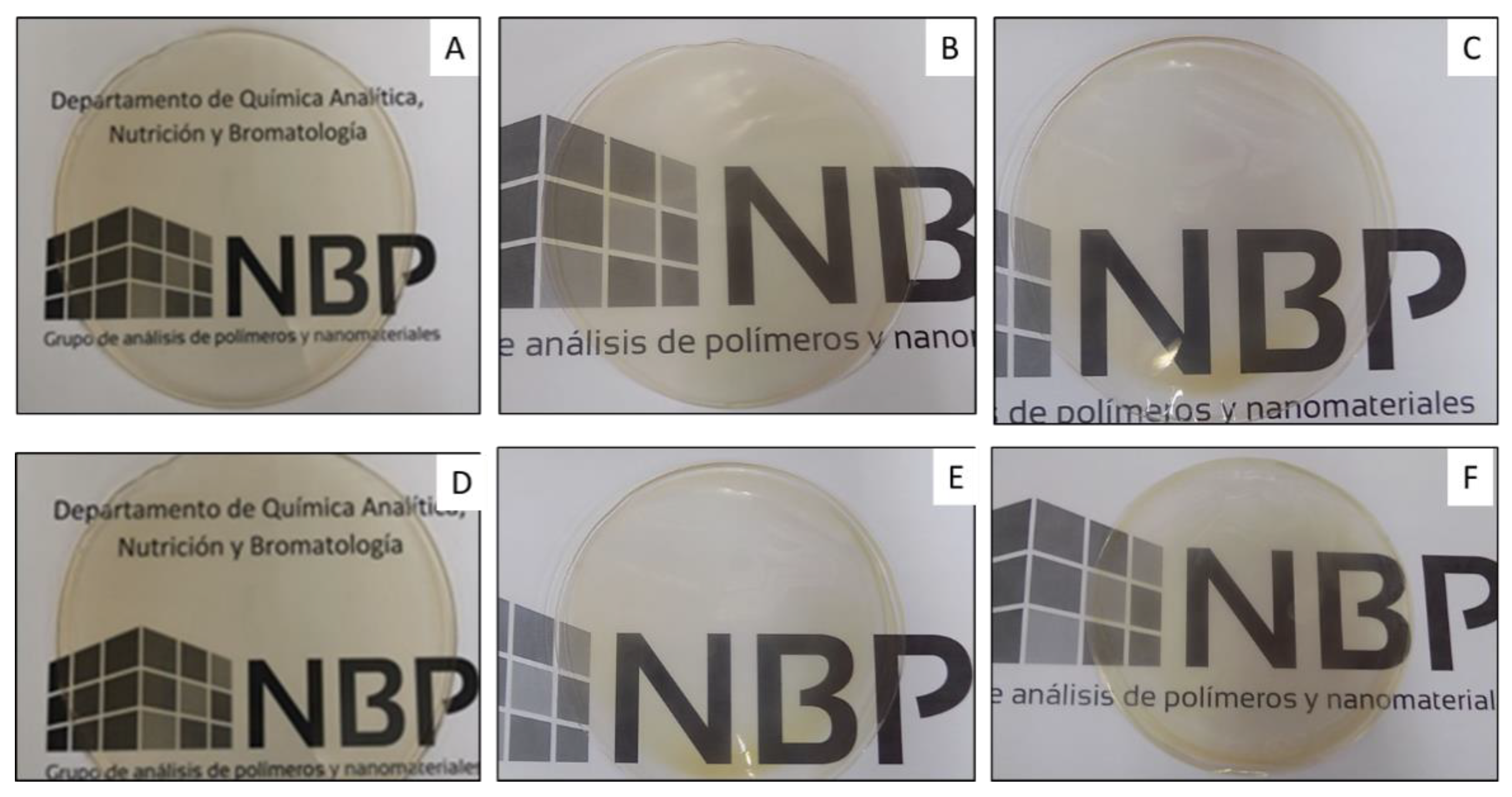
3.4. Optical Properties
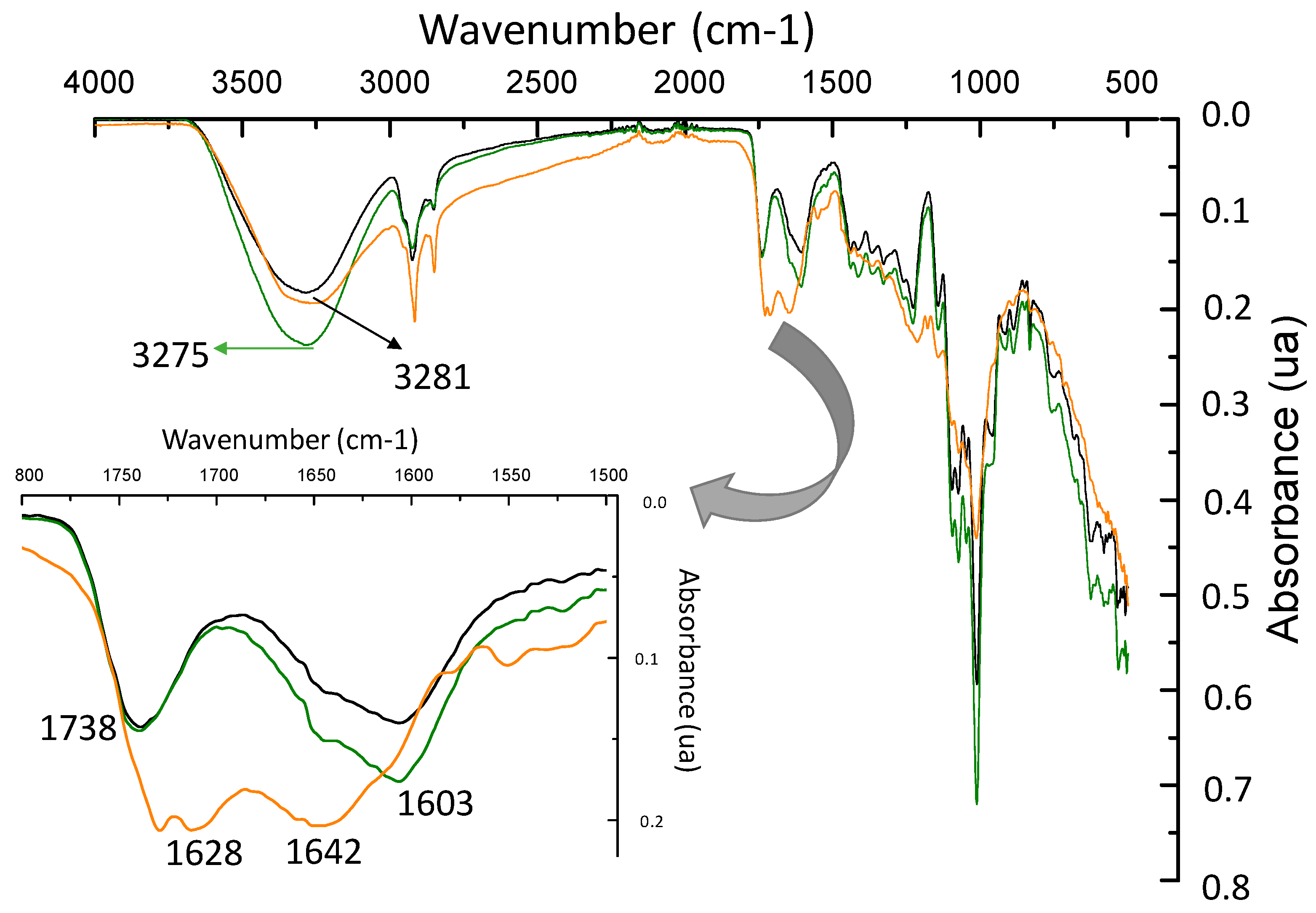
3.5. ATR-FTIR Analysis
3.6. X-ray Diffraction Analysis
3.7. Morphological Analysis
3.8. Photocatalytic Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sivakanthan, S.; Rajendran, S.; Gamage, A.; Madhujith, T.; Mani, S. Antioxidant and antimicrobial applications of biopolymers: A review. Food Res. Int. 2020, 136, 109327. [Google Scholar] [CrossRef]
- Gao, H.-X.; He, Z.; Sun, Q.; He, Q.; Zeng, W.-C. A functional polysaccharide film forming by pectin, chitosan, and tea polyphenols. Carbohydr. Polym. 2019, 215, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mellinas, C.; Valdés, A.; Ramos, M.; Burgos, N.; Del Carmen Garrigós, M.; Jiménez, A. Active edible films: Current state and future trends. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Mendes, J.F.; Norcino, L.B.; Martins, H.H.A.; Manrich, A.; Otoni, C.G.; Carvalho, E.E.N.; Piccoli, R.H.; Oliveira, J.E.; Pinheiro, A.C.M.; Mattoso, L.H.C. Correlating emulsion characteristics with the properties of active starch films loaded with lemongrass essential oil. Food Hydrocoll. 2020, 100, 105428. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Vázquez, M.; Velazquez, G. Cellulose-glycerol-polyvinyl alcohol composite films for food packaging: Evaluation of water adsorption, mechanical properties, light-barrier properties and transparency. Carbohydr. Polym. 2018, 195, 432–443. [Google Scholar] [CrossRef]
- Kalkan, S.; Otağ, M.R.; Engin, M.S. Physicochemical and bioactive properties of edible methylcellulose films containing Rheum ribes L. extract. Food Chem. 2020, 307, 125524. [Google Scholar] [CrossRef]
- Lin, D.; Huang, Y.; Liu, Y.; Luo, T.; Xing, B.; Yang, Y.; Yang, Z.; Wu, Z.; Chen, H.; Zhang, Q.; et al. Physico-mechanical and structural characteristics of starch/polyvinyl alcohol/nano-titania photocatalytic antimicrobial composite films. LWT 2018, 96, 704–712. [Google Scholar] [CrossRef]
- Singh, T.P.; Chatli, M.K.; Sahoo, J. Development of chitosan based edible films: Process optimization using response surface methodology. J. Food Sci. Technol. 2015, 52, 2530–2543. [Google Scholar] [CrossRef]
- Sani, I.K.; Pirsa, S.; Tağı, Ş. Preparation of chitosan/zinc oxide/Melissa officinalis essential oil nano-composite film and evaluation of physical, mechanical and antimicrobial properties by response surface method. Polym. Test. 2019, 79, 106004. [Google Scholar] [CrossRef]
- Bernhardt, D.C.; Pérez, C.D.; Fissore, E.N.; De’Nobili, M.D.; Rojas, A.M. Pectin-based composite film: Effect of corn husk fiber concentration on their properties. Carbohydr. Polym. 2017, 164, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Almasi, H.; Azizi, S.; Amjadi, S. Development and characterization of pectin films activated by nanoemulsion and Pickering emulsion stabilized marjoram (Origanum majorana L.) essential oil. Food Hydrocoll. 2020, 99, 105338. [Google Scholar] [CrossRef]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent Trends in the Use of Pectin from Agro-Waste Residues as a Natural-Based Biopolymer for Food Packaging Applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef] [PubMed]
- Marenda, F.R.B.; Mattioda, F.; Demiate, I.M.; de Francisco, A.; de Oliveira Petkowicz, C.L.; Canteri, M.H.G.; de Mello Castanho Amboni, R.D. Advances in Studies Using Vegetable Wastes to Obtain Pectic Substances: A Review. J. Polym. Environ. 2019, 27, 549–560. [Google Scholar] [CrossRef]
- Cataldo, V.A.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Coffee grounds as filler for pectin: Green composites with competitive performances dependent on the UV irradiation. Carbohydr. Polym. 2017, 170, 198–205. [Google Scholar] [CrossRef]
- Espitia, P.J.; Du, W.X.; de Jesús Avena-Bustillos, R.; Soares, N.D.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Kalathaki, I.; Alba, K.; Muhamedsalih, H.; Kontogiorgos, V. Fabrication and characterisation of metal-doped pectin films. Food Hydrocoll. 2019, 92, 259–266. [Google Scholar] [CrossRef]
- Noreen, A.; Nazli, Z.-H.; Akram, J.; Rasul, I.; Mansha, A.; Yaqoob, N.; Iqbal, R.; Tabasum, S.; Zuber, M.; Zia, K.M. Pectins functionalized biomaterials; a new viable approach for biomedical applications: A review. Int. J. Biol. Macromol. 2017, 101, 254–272. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.-C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Giosafatto, C.V.L.; Di Pierro, P.; Gunning, P.; Mackie, A.; Porta, R.; Mariniello, L. Characterization of Citrus pectin edible films containing transglutaminase-modified phaseolin. Carbohydr. Polym. 2014, 106, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.D.; Pérez, L.L.; Salcedo, J.M.; Córdoba, L.P.; do Amaral Sobral, P.J. Production and characterization of films based on blends of chitosan from blue crab (Callinectes sapidus) waste and pectin from Orange (Citrus sinensis Osbeck) peel. Int. J. Biol. Macromol. 2017, 98, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Akhter, R.; Masoodi, F.A.; Wani, T.A.; Rather, S.A. Functional characterization of biopolymer based composite film: Incorporation of natural essential oils and antimicrobial agents. Int. J. Biol. Macromol. 2019, 137, 1245–1255. [Google Scholar] [CrossRef]
- Šešlija, S.; Nešić, A.; Ružić, J.; Kalagasidis Krušić, M.; Veličković, S.; Avolio, R.; Santagata, G.; Malinconico, M. Edible blend films of pectin and poly(ethylene glycol): Preparation and physico-chemical evaluation. Food Hydrocoll. 2018, 77, 494–501. [Google Scholar] [CrossRef]
- Porta, R.; Di Pierro, P.; Sabbah, M.; Regalado-Gonzales, C.; Mariniello, L.; Kadivar, M.; Arabestani, A. Blend films of pectin and bitter vetch (Vicia ervilia) proteins: Properties and effect of transglutaminase. Innov. Food Sci. Emerg. Technol. 2016, 36, 245–251. [Google Scholar] [CrossRef]
- Eghbal, N.; Degraeve, P.; Oulahal, N.; Yarmand, M.S.; Mousavi, M.E.; Gharsallaoui, A. Low methoxyl pectin/sodium caseinate interactions and composite film formation at neutral pH. Food Hydrocoll. 2017, 69, 132–140. [Google Scholar] [CrossRef]
- Kodoth, A.K.; Ghate, V.M.; Lewis, S.A.; Prakash, B.; Badalamoole, V. Pectin-based silver nanocomposite film for transdermal delivery of Donepezil. Int. J. Biol. Macromol. 2019, 134, 269–279. [Google Scholar] [CrossRef]
- Lorevice, M.V.; Otoni, C.G.; de Moura, M.R.; Mattoso, L.H.C. Chitosan nanoparticles on the improvement of thermal, barrier, and mechanical properties of high- and low-methyl pectin films. Food Hydrocoll. 2016, 52, 732–740. [Google Scholar] [CrossRef]
- Chaichi, M.; Hashemi, M.; Badii, F.; Mohammadi, A. Preparation and characterization of a novel bionanocomposite edible film based on pectin and crystalline nanocellulose. Carbohydr. Polym. 2017, 157, 167–175. [Google Scholar] [CrossRef]
- Nešić, A.; Gordić, M.; Davidović, S.; Radovanović, Ž.; Nedeljković, J.; Smirnova, I.; Gurikov, P. Pectin-based nanocomposite aerogels for potential insulated food packaging application. Carbohydr. Polym. 2018, 195, 128–135. [Google Scholar] [CrossRef]
- Borzi, F.; Torrieri, E.; Wrona, M.; Nerín, C. Polyamide modified with green tea extract for fresh minced meat active packaging applications. Food Chem. 2019, 300, 125242. [Google Scholar] [CrossRef]
- Heras-Mozos, R.; Muriel-Galet, V.; López-Carballo, G.; Catalá, R.; Hernández-Muñoz, P.; Gavara, R. Development and optimization of antifungal packaging for sliced pan loaf based on garlic as active agent and bread aroma as aroma corrector. Int. J. Food Microbiol. 2019, 290, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, H.; Youssef, A.M.; Darwish, N.A.; Abou-Kandil, A.I. Eco-friendly polymer composites for green packaging: Future vision and challenges. Compos. Part B Eng. 2019, 172, 16–25. [Google Scholar] [CrossRef]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Garrigós, M.C.; Jiménez, A. Natural additives and agricultural wastes in biopolymer formulations for food packaging. Front. Chem. 2014, 2, 6. [Google Scholar] [CrossRef]
- Panak Balentić, J.; Ačkar, Đ.; Jokić, S.; Jozinović, A.; Babić, J.; Miličević, B.; Šubarić, D.; Pavlović, N. Cocoa Shell: A By-Product with Great Potential for Wide Application. Molecules 2018, 23, 1404. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Navarro, S.L.B.; Rodrigues, C.E.C. Cocoa shell and its compounds: Applications in the food industry. Trends Food Sci. Technol. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, A.; Isopencu, G.; Busuioc, C.; Popa, O.M.; Dietrich, P.; Socaciu-Siebert, L. Bacterial cellulose films with ZnO nanoparticles and propolis extracts: Synergistic antimicrobial effect. Sci. Rep. 2019, 9, 17687. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Safety assessment of the substance zinc oxide, nanoparticles, for use in food contact materials. EFSA J. 2016, 14, 4408. [Google Scholar]
- Indumathi, M.P.; Rajarajeswari, G.R. Mahua oil-based polyurethane/chitosan/nano ZnO composite films for biodegradable food packaging applications. Int. J. Biol. Macromol. 2019, 124, 163–174. [Google Scholar]
- Chaudhary, P.; Fatima, F.; Kumar, A. Relevance of Nanomaterials in Food Packaging and its Advanced Future Prospects. J. Inorg. Organomet. Polym. Mater. 2020, 1, 3. [Google Scholar] [CrossRef]
- Soubhagya, A.S.; Moorthi, A.; Prabaharan, M. Preparation and characterization of chitosan/pectin/ZnO porous films for wound healing. Int. J. Biol. Macromol. 2020, 157, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.M.P.; Dang, T.M.Q.; Tran, T.X.; Rachtanapun, P. Effects of Zinc Oxide Nanoparticles on the Properties of Pectin/Alginate Edible Films. Int. J. Polym. Sci. 2018, 2018, 5645797. [Google Scholar] [CrossRef]
- Kodoth, A.K.; Ghate, V.M.; Lewis, S.A.; Badalamoole, V. Application of pectin-zinc oxide hybrid nanocomposite in the delivery of a hydrophilic drug and a study of its isotherm, kinetics and release mechanism. Int. J. Biol. Macromol. 2018, 115, 418–430. [Google Scholar] [CrossRef]
- Kodoth, A.K.; Badalamoole, V. Pectin Based Graft Copolymer–ZnO Hybrid Nanocomposite for the Adsorptive Removal of Crystal Violet. J. Polym. Environ. 2019, 27, 2040–2053. [Google Scholar] [CrossRef]
- Mellinas, A.C.; Jiménez, A.; Garrigós, M.C. Optimization of microwave-assisted extraction of cocoa bean shell waste and evaluation of its antioxidant, physicochemical and functional properties. LWT 2020, 127, 109361. [Google Scholar] [CrossRef]
- Younis, H.G.R.; Zhao, G. Physicochemical properties of the edible films from the blends of high methoxyl apple pectin and chitosan. Int. J. Biol. Macromol. 2019, 131, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Dash, K.K.; Ali, N.A.; Das, D.; Mohanta, D. Thorough evaluation of sweet potato starch and lemon-waste pectin based-edible films with nano-titania inclusions for food packaging applications. Int. J. Biol. Macromol. 2019, 139, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Shahmohammadi Jebel, F.; Almasi, H. Morphological, physical, antimicrobial and release properties of ZnO nanoparticles-loaded bacterial cellulose films. Carbohydr. Polym. 2016, 149, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Wu, H.; Jiao, C.; Jiang, Y.; Liu, R.; Xiao, D.; Lu, J.; Zhang, Z.; Shen, G.; Li, S. Investigation of the structural and physical properties, antioxidant and antimicrobial activity of pectin-konjac glucomannan composite edible films incorporated with tea polyphenol. Food Hydrocoll. 2019, 94, 128–135. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Jiang, W.; Qi, J.-R.; Liao, J.; Wan, Z.; Liang, W.; Huang, J.; Cao, Y.; Xiao, J.; Yang, X.-Q. Structural characterization of pectin-bismuth complexes and their aggregation in acidic conditions. Int. J. Biol. Macromol. 2020, 154, 788–794. [Google Scholar] [CrossRef]
- Wang, C.; Chang, T.; Dong, S.; Zhang, D.; Ma, C.; Chen, S.; Li, H. Biopolymer films based on chitosan/potato protein/linseed oil/ZnO NPs to maintain the storage quality of raw meat. Food Chem. 2020, 332, 127375. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Properties and characterization of bionanocomposite films prepared with various biopolymers and ZnO nanoparticles. Carbohydr. Polym. 2014, 106, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Vishnu Chandar, J.; Shanmugan, S.; Mutharasu, D.; Azlan, A.A. Impact of ZnO Nanoparticles on Thermal Properties of Poly(3-hydroxybutyrate-co-10 mol % 3-hydroxyhexanoate) Copolymer. Polym. Sci. Ser. A 2019, 61, 504–513. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Peltzer, M.; Dominici, F.; Jiménez, A.; del Carmen Garrigós, M.; Kenny, J.M. Influence of thymol and silver nanoparticles on the degradation of poly(lactic acid) based nanocomposites: Thermal and morphological properties. Polym. Degrad. Stab. 2014, 108, 158–165. [Google Scholar] [CrossRef]
- Shankar, S.; Tanomrod, N.; Rawdkuen, S.; Rhim, J.-W. Preparation of pectin/silver nanoparticles composite films with UV-light barrier and properties. Int. J. Biol. Macromol. 2016, 92, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Wang, L.-F.; Rhim, J.-W. Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of PLA-based nanocomposite films. Mater. Sci. Eng. C 2018, 93, 289–298. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Soren, S.; Kumar, S.; Mishra, S.; Jena, P.K.; Verma, S.K.; Parhi, P. Evaluation of antibacterial and antioxidant potential of the zinc oxide nanoparticles synthesized by aqueous and polyol method. Microb. Pathog. 2018, 119, 145–151. [Google Scholar] [CrossRef]
- Shabaani, M.; Rahaiee, S.; Zare, M.; Jafari, S.M. Green synthesis of ZnO nanoparticles using loquat seed extract; Biological functions and photocatalytic degradation properties. LWT 2020, 134, 110133. [Google Scholar] [CrossRef]
- Janani, N.; Zare, E.N.; Salimi, F.; Makvandi, P. Antibacterial tragacanth gum-based nanocomposite films carrying ascorbic acid antioxidant for bioactive food packaging. Carbohydr. Polym. 2020, 247, 116678. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Peponi, L. Polyurethane based on PLA and PCL incorporated with catechin: Structural, thermal and mechanical characterization. Eur. Polym. J. 2017, 89, 174–184. [Google Scholar] [CrossRef]
- Correa, J.P.; Molina, V.; Sanchez, M.; Kainz, C.; Eisenberg, P.; Massani, M.B. Improving ham shelf life with a polyhydroxybutyrate/polycaprolactone biodegradable film activated with nisin. Food Packag. Shelf Life 2017, 11, 31–39. [Google Scholar] [CrossRef]
- Nanni, A.; Messori, M. A comparative study of different winemaking by-products derived additives on oxidation stability, mechanical and thermal proprieties of polypropylene. Polym. Degrad. Stab. 2018, 149, 9–18. [Google Scholar] [CrossRef]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Use of herbs, spices and their bioactive compounds in active food packaging. RSC Adv. 2015, 5, 40324–40335. [Google Scholar] [CrossRef]
- Jha, P. Effect of plasticizer and antimicrobial agents on functional properties of bionanocomposite films based on corn starch-chitosan for food packaging applications. Int. J. Biol. Macromol. 2020, 160, 571–582. [Google Scholar] [CrossRef]
- Maryam Adilah, Z.A.; Jamilah, B.; Nur Hanani, Z.A. Functional and antioxidant properties of protein-based films incorporated with mango kernel extract for active packaging. Food Hydrocoll. 2018, 74, 207–218. [Google Scholar] [CrossRef]
- Dairi, N.; Ferfera-Harrar, H.; Ramos, M.; Garrigós, M.C. Cellulose acetate/AgNPs-organoclay and/or thymol nano-biocomposite films with combined antimicrobial/antioxidant properties for active food packaging use. Int. J. Biol. Macromol. 2019, 121, 508–523. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Development of novel nano-biocomposite antioxidant films based on poly (lactic acid) and thymol for active packaging. Food Chem. 2014, 162, 149–155. [Google Scholar] [CrossRef]
- Noshirvani, N.; Ghanbarzadeh, B.; Rezaei Mokarram, R.; Hashemi, M. Novel active packaging based on carboxymethyl cellulose-chitosan-ZnO NPs nanocomposite for increasing the shelf life of bread. Food Packag. Shelf Life 2017, 11, 106–114. [Google Scholar] [CrossRef]
- Vartiainen, J.; Tammelin, T.; Pere, J.; Tapper, U.; Harlin, A. Biohybrid barrier films from fluidized pectin and nanoclay. Carbohydr. Polym. 2010, 82, 989–996. [Google Scholar] [CrossRef]
- Farahnaky, A.; Sharifi, S.; Imani, B.; Dorodmand, M.M.; Majzoobi, M. Physicochemical and mechanical properties of pectin-carbon nanotubes films produced by chemical bonding. Food Packag. Shelf Life 2018, 16, 8–14. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Puglia, D.; Terenzi, A.; Berglund, L.A.; Kenny, J.M. Microstructure and nonisothermal cold crystallization of PLA composites based on silver nanoparticles and nanocrystalline cellulose. Polym. Degrad. Stab. 2012, 97, 2027–2036. [Google Scholar] [CrossRef]
- Espitia, P.J.; Soares, N.D.; Teófilo, R.F.; dos Reis Coimbra, J.S.; Vitor, D.M.; Batista, R.A.; Ferreira, S.O.; de Andrade, N.J.; Medeiros, E.A. Physical–mechanical and antimicrobial properties of nanocomposite films with pediocin and ZnO nanoparticles. Carbohydr. Polym. 2013, 94, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Pirsa, S. Biodegradable film based on pectin/Nano-clay/methylene blue: Structural and physical properties and sensing ability for measurement of vitamin C. Int. J. Biol. Macromol. 2020, 163, 666–675. [Google Scholar] [CrossRef]
- Adilah, A.N.; Jamilah, B.; Noranizan, M.A.; Hanani, Z.A.N. Utilization of mango peel extracts on the biodegradable films for active packaging. Food Packag. Shelf Life 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Wani, A.A.; Shah, M.A. Effect of plant extracts on the techno-functional properties of biodegradable packaging films. Trends Food Sci. Technol. 2018, 80, 141–154. [Google Scholar] [CrossRef]
- Luzi, F.; Di Michele, A.; Torre, L.; Puglia, D. Active role of ZnO nanorods in thermomechanical and barrier performance of poly(vinyl alcohol-co-ethylene) formulations for flexible packaging. Polymers 2019, 11, 922. [Google Scholar] [CrossRef]
- Mendes, J.F.; Martins, J.T.; Manrich, A.; Sena Neto, A.R.; Pinheiro, A.C.M.; Mattoso, L.H.C.; Martins, M.A. Development and physical-chemical properties of pectin film reinforced with spent coffee grounds by continuous casting. Carbohydr. Polym. 2019, 210, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Nešić, A.; Ružić, J.; Gordić, M.; Ostojić, S.; Micić, D.; Onjia, A. Pectin-polyvinylpyrrolidone films: A sustainable approach to the development of biobased packaging materials. Compos. Part B Eng. 2017, 110, 56–61. [Google Scholar] [CrossRef]
- Viana, R.M.; Sá, N.M.; Barros, M.O.; de Fátima Borges, M.; Azeredo, H.M. Nanofibrillated bacterial cellulose and pectin edible films added with fruit purees. Carbohydr. Polym. 2018, 196, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.H.F.; Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Moates, G.K.; Wellner, N.; Waldron, K.W.; Azeredo, H.M.C. Pectin extraction from pomegranate peels with citric acid. Int. J. Biol. Macromol. 2016, 88, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Mzoughi, Z.; Abdelhamid, A.; Rihouey, C.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Optimized extraction of pectin-like polysaccharide from Suaeda fruticosa leaves: Characterization, antioxidant, anti-inflammatory and analgesic activities. Carbohydr. Polym. 2018, 185, 127–137. [Google Scholar] [CrossRef]
- Kpodo, F.M.; Agbenorhevi, J.K.; Alba, K.; Bingham, R.J.; Oduro, I.N.; Morris, G.A.; Kontogiorgos, V. Pectin isolation and characterization from six okra genotypes. Food Hydrocoll. 2017, 72, 323–330. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Labbafi, M.; Saeid Hosseini, S.; Hojjati, M. Pistachio green hull pectin: Optimization of microwave-assisted extraction and evaluation of its physicochemical, structural and functional properties. Food Chem. 2019, 271, 663–672. [Google Scholar] [CrossRef]
- Sheikh, M.; Asghari, M.; Afsari, M. Effect of tiny amount of zinc oxide on morphological and thermal properties of nanocomposite PEBA thin films. Alex. Eng. J. 2018, 57, 3661–3669. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Krishnakumar, C.; Arulmozhi, P.; Mahadevan, S.; Parameswari, N. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Glycosmis pentaphylla (Retz.) DC. Microb. Pathog. 2018, 116, 44–48. [Google Scholar] [CrossRef]
- Arciniegas-Grijalba, P.A.; Patiño-Portela, M.C.; Mosquera-Sánchez, L.P.; Guerra Sierra, B.E.; Muñoz-Florez, J.E.; Erazo-Castillo, L.A.; Rodríguez-Páez, J.E. ZnO-based nanofungicides: Synthesis, characterization and their effect on the coffee fungi Mycena citricolor and Colletotrichum sp. Mater. Sci. Eng. C 2019, 98, 808–825. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, K.; Thakur, N.; Chauhan, S.; Chauhan, M.S. The effect of shape and size of ZnO nanoparticles on their antimicrobial and photocatalytic activities: A green approach. Bull. Mater. Sci. 2020, 43, 20. [Google Scholar] [CrossRef]
- Lin, S.-T.; Thirumavalavan, M.; Jiang, T.-Y.; Lee, J.-F. Synthesis of ZnO/Zn nano photocatalyst using modified polysaccharides for photodegradation of dyes. Carbohydr. Polym. 2014, 105, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Sridevi, H.; Varadavenkatesan, T.; Vinayagam, R.; Selvaraj, R. Photocatalytic zinc oxide nanoparticles synthesis using Peltophorum pterocarpum leaf extract and their characterization. Optik 2019, 185, 248–255. [Google Scholar] [CrossRef]
- Raja, A.; Ashokkumar, S.; Pavithra Marthandam, R.; Jayachandiran, J.; Khatiwada, C.P.; Kaviyarasu, K.; Ganapathi Raman, R.; Swaminathan, M. Eco-friendly preparation of zinc oxide nanoparticles using Tabernaemontana divaricata and its photocatalytic and antimicrobial activity. J. Photochem. Photobiol. B Biol. 2018, 181, 53–58. [Google Scholar] [CrossRef]
- Rafaie, H.A.; Nor, R.M.; Azmina, M.S.; Ramli, N.I.T.; Mohamed, R. Decoration of ZnO microstructures with Ag nanoparticles enhanced the catalytic photodegradation of methylene blue dye. J. Environ. Chem. Eng. 2017, 5, 3963–3972. [Google Scholar] [CrossRef]
- Ali, J.; Irshad, R.; Li, B.; Tahir, K.; Ahmad, A.; Shakeel, M.; Khan, N.U.; Khan, Z.U.H. Synthesis and characterization of phytochemical fabricated zinc oxide nanoparticles with enhanced antibacterial and catalytic applications. J. Photochem. Photobiol. B Biol. 2018, 183, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Kaneyuki, T.; Mori, A.; Packer, L. Antioxidant Activities of Pomegranate Fruit Extract and Its Anthocyanidins: Delphinidin, Cyanidin, and Pelargonidin. J. Agric. Food Chem. 2002, 50, 166–171. [Google Scholar] [CrossRef] [PubMed]

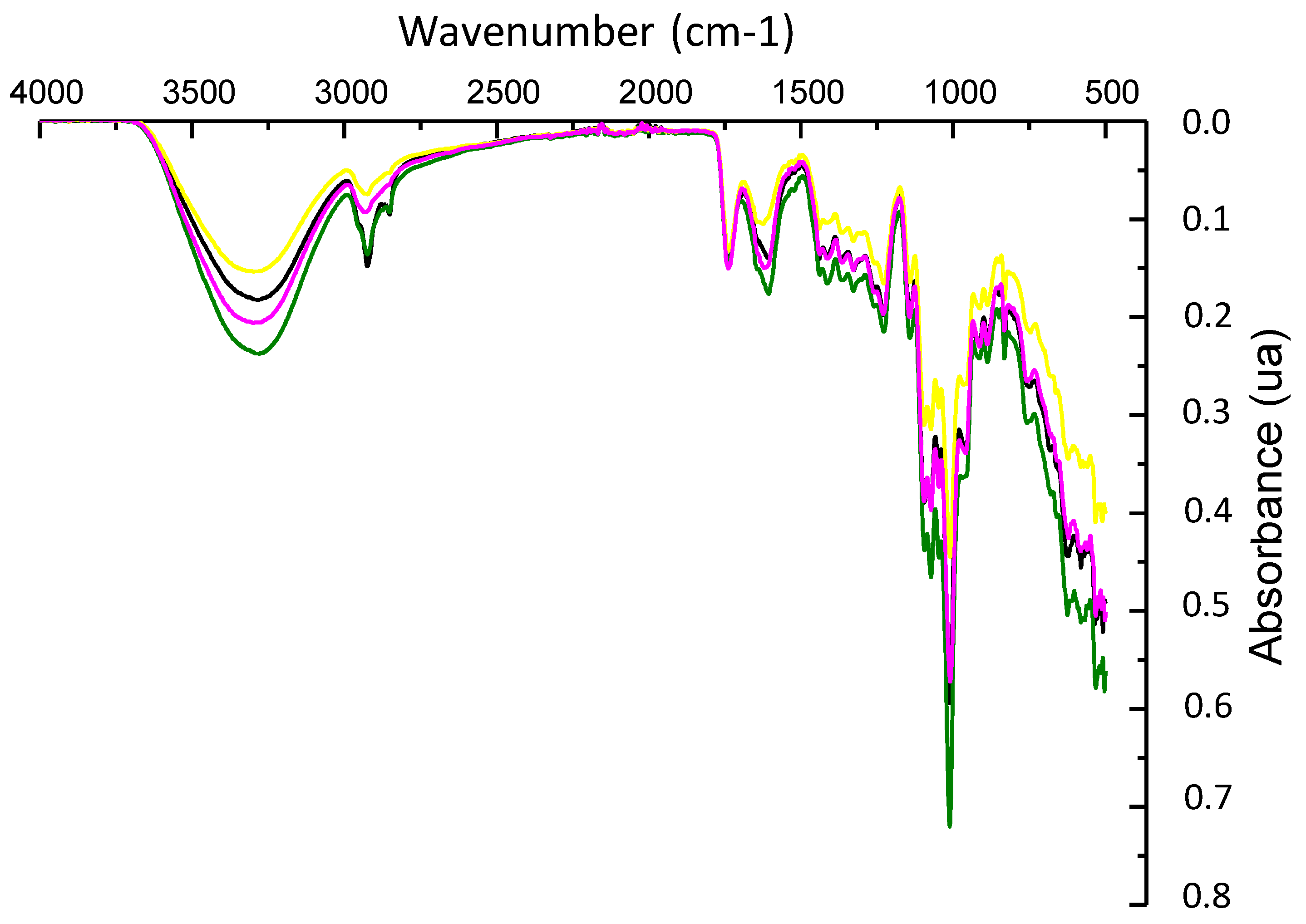
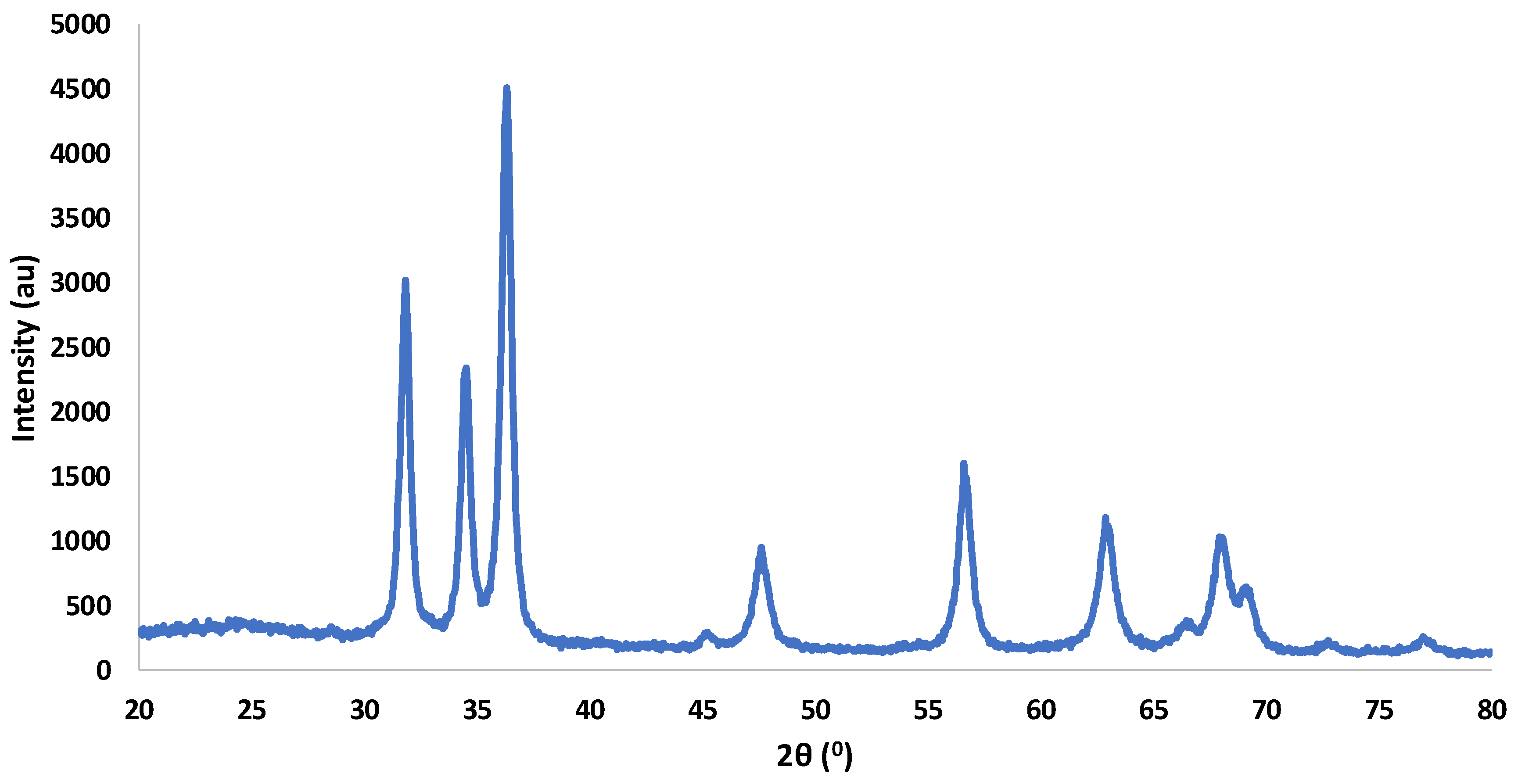


| Formulation | CBSE-2 (wt%) | ZnO/Zn-NPs (wt%) | Moisture (%) | Thickness * (mm) | OTR.e (cm3 mm m−2 day) | Degradation Efficiency (%) |
|---|---|---|---|---|---|---|
| PC (control) | - | - | 18 ± 3 a | 0.087 ± 0.022 a | 0.51 ± 0.07 a | 4 ± 2 a |
| PC-1ZnO | - | 1 | 13 ± 1 a | 0.073 ± 0.031 a | 0.32 ± 0.02 b | 72 ± 7 b |
| PC-3ZnO | - | 3 | 14 ± 3 a | 0.070 ± 0.024 a | 0.51 ± 0.09 a | 87 ± 4 c |
| PC-5E | 5 | - | 13 ± 1 a | 0.078 ± 0.016 a | 0.63 ± 0.01 c | 39 ± 6 d |
| PC-5E-1ZnO | 5 | 1 | 17 ± 1 a | 0.071 ± 0.011 a | 0.36 ± 0.02 b | 84 ± 4 bc |
| PC-5E-3ZnO | 5 | 3 | 16 ± 4 a | 0.085 ± 0.015 a | 0.35 ± 0.13 ab | 88 ± 2 c |
| Formulation | Tmax (°C) | Tg (°C) | OOT (°C) |
|---|---|---|---|
| PC | 231 ± 1 a | 52 ± 1 a | 202 ± 1 a |
| PC-1ZnO | 229 ± 1 a | 51 ± 2 a | 202 ± 1 a |
| PC-3ZnO | 239 ± 2 b | 51 ± 1 a | 214 ± 2 b |
| PC-5E | 229 ± 1 a | 53 ± 2 a | 206 ± 1 c |
| PC-5E-1ZnO | 230 ± 1 a | 53 ± 1 a | 205 ± 1 c |
| PC-5E-3ZnO | 229 ± 1 a | 52 ± 1 a | 207 ± 2 c |
| Formulation | L* | a* | b* | ΔE | T280 (%) | T660 (%) |
|---|---|---|---|---|---|---|
| PC | 85.4 ± 2.6 a | 10.1 ± 3.1 a | 3.5 ± 1.9 a | 25 ± 2 a | 87 ± 3 a | |
| PC-1ZnO | 86.0 ± 2.6 a | 8.2 ± 0.6 a | 3.6 ± 2.0 a | 2.6 ± 0.6 a | 2 ± 1 b | 77 ± 4 b |
| PC-3ZnO | 85.4 ± 2.4 a | 9.8 ± 0.5 a | 3.7 ± 1.8 a | 2.8 ± 0.1 a | 2 ± 1 b | 77 ± 3 b |
| PC-5E | 80.6 ± 2.6 b | 20.0 ± 2.2 b | 3.0 ± 2.3 a | 10.3 ± 2.7 b | 3 ± 1 b | 74 ± 3 b |
| PC-5E-1ZnO | 82.4 ± 2.4 ab | 16.9 ± 0.7 c | 3.4 ± 1.8 a | 7.1 ± 0.9 ab | 1 ± 1 b | 75 ± 3 b |
| PC-5E-3ZnO | 83.9 ± 2.4 ab | 14.0 ± 0.4 d | 3.6 ± 1.8 a | 4.3 ± 0.6 ab | 1 ± 1 b | 77 ± 3 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mellinas, A.C.; Jiménez, A.; Garrigós, M.C. Pectin-Based Films with Cocoa Bean Shell Waste Extract and ZnO/Zn-NPs with Enhanced Oxygen Barrier, Ultraviolet Screen and Photocatalytic Properties. Foods 2020, 9, 1572. https://doi.org/10.3390/foods9111572
Mellinas AC, Jiménez A, Garrigós MC. Pectin-Based Films with Cocoa Bean Shell Waste Extract and ZnO/Zn-NPs with Enhanced Oxygen Barrier, Ultraviolet Screen and Photocatalytic Properties. Foods. 2020; 9(11):1572. https://doi.org/10.3390/foods9111572
Chicago/Turabian StyleMellinas, Ana Cristina, Alfonso Jiménez, and María Carmen Garrigós. 2020. "Pectin-Based Films with Cocoa Bean Shell Waste Extract and ZnO/Zn-NPs with Enhanced Oxygen Barrier, Ultraviolet Screen and Photocatalytic Properties" Foods 9, no. 11: 1572. https://doi.org/10.3390/foods9111572
APA StyleMellinas, A. C., Jiménez, A., & Garrigós, M. C. (2020). Pectin-Based Films with Cocoa Bean Shell Waste Extract and ZnO/Zn-NPs with Enhanced Oxygen Barrier, Ultraviolet Screen and Photocatalytic Properties. Foods, 9(11), 1572. https://doi.org/10.3390/foods9111572