The Effect of Roasting on the Protein Profile and Antiradical Capacity of Flaxseed Meal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Standards and Reagents
2.3. Chemical Composition Analysis
2.4. Electrophoretic Protein Separation (SDS-PAGE)
2.5. Maillard Reaction Product Analysis (Browning Index)
2.6. Antiradical Capacity—ORAC_FL Assay
2.7. Statistical Analyses
3. Results and Discussion
3.1. Effect of Flaxseed Roasting on Protein Profile
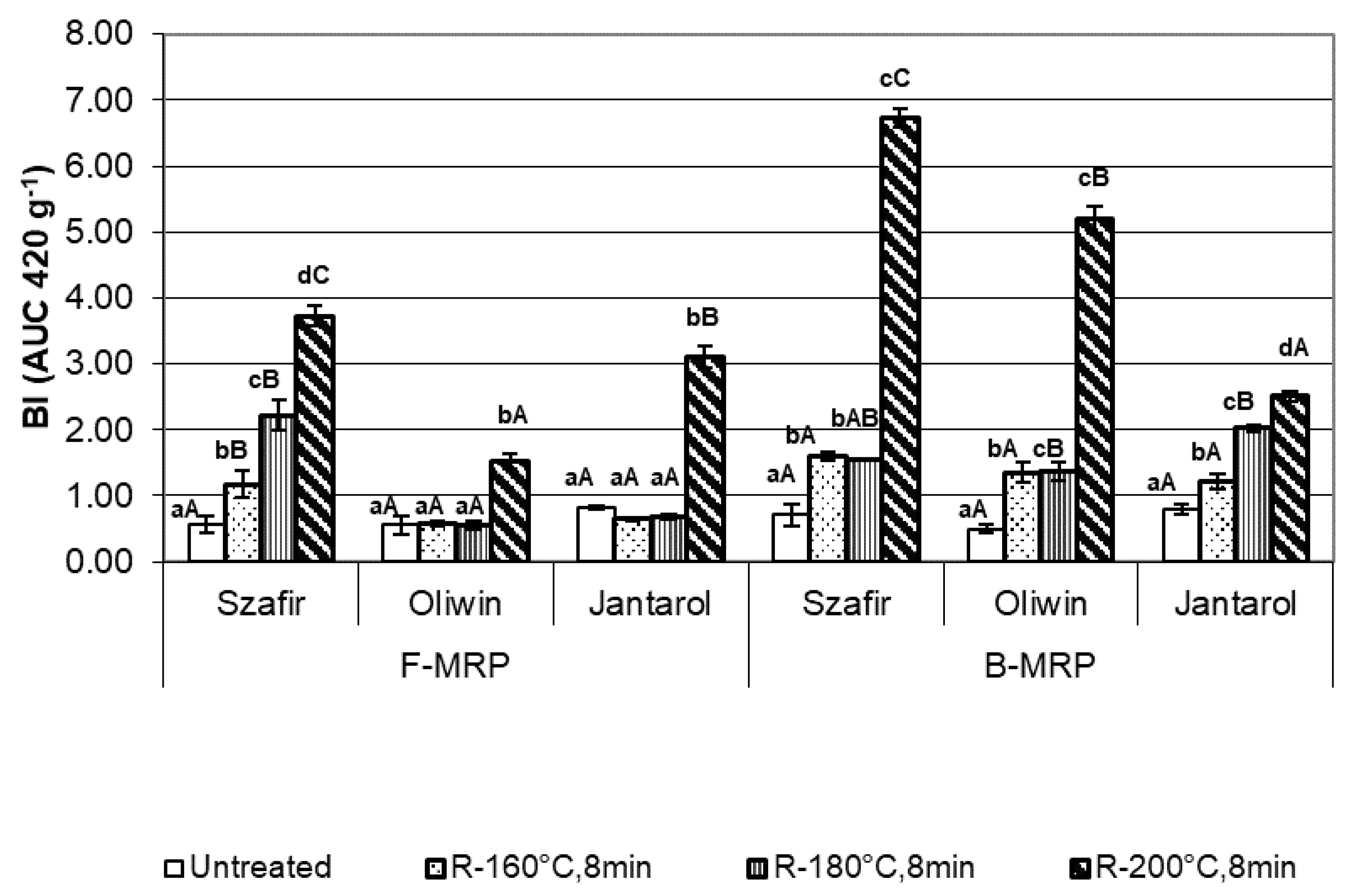
3.2. Effect of Flaxseed Roasting on the Formation of Free and Bound-To-Protein Maillard Reaction Product (MRP)
3.3. Effect of Flaxseed Roasting on Antiradical Capacity (ORAC_FL)
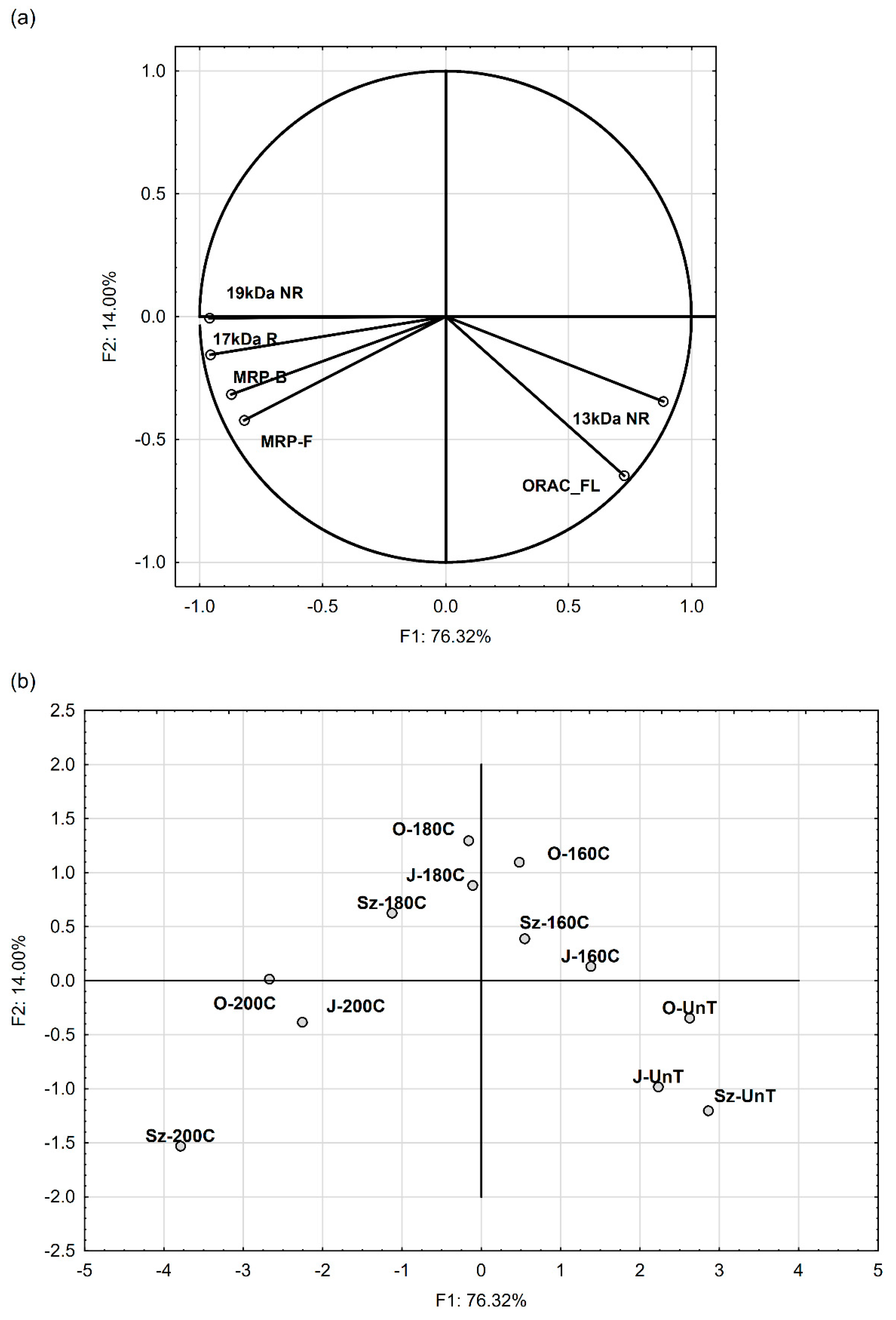
3.4. Relationship between Protein Profile, MRP, and Antiradical Capacity after Flaxseed Roasting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine and modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef] [PubMed]
- Rabetafika, H.N.; van Remoortel, V.; Danthine, S.; Paquot, M.; Blecker, C. Flaxseed proteins: Food uses and health benefits. Int. J. Food Sci. Technol. 2011, 46, 221–228. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, detoxification, utilization, and opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Marambe, H.K.; Wanasundara, J.P.D. Chapter 8—Protein from Flaxseed (Linum usitatissimum L.). In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L.B.T.-S.P.S., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 133–144. ISBN 978-0-12-802778-3. [Google Scholar]
- Oomah, B.D.; Mazza, G. Flaxseed proteins—A review. Food Chem. 1993, 48, 109–114. [Google Scholar] [CrossRef]
- Madhusudhan, K.T.; Singh, N. Isolation and characterization of a small molecular weight protein of linseed meal. Phytochemistry 1985, 24, 2507–2509. [Google Scholar] [CrossRef]
- Marcone, M.F.; Kakuda, Y.; Yada, R.Y. Salt soluble seed globulins of various dicotyledonous and monocotyledonous plants I. Isolation/purification and characterization. Food Chem. 1998, 62, 27–47. [Google Scholar] [CrossRef]
- Youle, R.J.; Huang, A.H.C. Occurrence of low molecular weight and high cysteine conteining albumin storage proteins in oilseeds of diverse species. Am. J. Bot. 1981, 68, 44–48. [Google Scholar] [CrossRef]
- Shim, Y.Y.; Gui, B.; Arnison, P.G.; Wang, Y.; Reaney, M.J.T. Flaxseed (Linum usitatissimum L.) bioactive compounds and peptide nomenclature: A review. Trends Food Sci. Technol. 2014, 38, 5–20. [Google Scholar] [CrossRef]
- Alaswad, A.A.; Krishnan, H.B. Immunological investigation for the presence of lunasin, a chemopreventive soybean peptide, in the seeds of diverse plants. J. Agric. Food Chem. 2016, 64, 2901–2909. [Google Scholar] [CrossRef]
- Borgmeyer, J.R.; Smith, C.E.; Huynh, K.Q. Isolation and characterization of a 25 kDa antifungal protein from flax seeds. Biochem. Biophys. Res. Commun. 1992, 187, 480–487. [Google Scholar] [CrossRef]
- Wu, S.; Wang, X.; Qi, W.; Guo, Q. Bioactive protein/peptides of flaxseed: A review. Trends Food Sci. Technol. 2019, 92, 184–193. [Google Scholar] [CrossRef]
- Tehrani, M.H.H.; Batal, R.; Kamalinejad, M.; Mahbubi, A. Extraction and purification of flaxseed proteins and studying their antibacterial activities. J. Plant Sci. 2014, 2, 70–76. [Google Scholar]
- Okinyo-Owiti, D.P.; Dong, Q.; Ling, B.; Jadhav, P.D.; Bauer, R.; Maley, J.M.; Reaney, M.J.T.; Yang, J.; Sammynaiken, R. Evaluating the cytotoxicity of flaxseed orbitides for potential cancer treatment. Toxicol. Rep. 2015, 2, 1014–1018. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Physicochemical and emulsification properties of flaxseed (Linum usitatissimum) albumin and globulin fractions. Food Chem. 2018, 255, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Dowling, K.; McKnight, S.; Barrow, C.J.; Wang, B.; Adhikari, B. Preparation, characterization and functional properties of flax seed protein isolate. Food Chem. 2016, 197, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Tirgar, M.; Silcock, P.; Carne, A.; Birch, E.J. Effect of extraction method on functional properties of flaxseed protein concentrates. Food Chem. 2017, 215, 417–424. [Google Scholar] [CrossRef]
- Yu, X.; Huang, S.; Nie, C.; Deng, Q.; Zhai, Y.; Shen, R. Effects of atmospheric pressure plasma jet on the physicochemical, functional, and antioxidant properties of flaxseed protein. J. Food Sci. 2020, 85, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Juodeikiene, G.; Zadeike, D.; Trakselyte-Rupsiene, K.; Gasauskaite, K.; Bartkiene, E.; Lele, V.; Viskelis, P.; Bernatoniene, J.; Ivanauskas, L.; Jakstas, V. Functionalisation of flaxseed proteins assisted by ultrasonication to produce coatings enriched with raspberries phytochemicals. LWT Food Sci. Technol. 2020, 124, 109180. [Google Scholar] [CrossRef]
- Bashir, S.; Yaseen, M.; Sharma, V.; Purohit, S.R.; Barak, S.; Mudgil, D. Rheological and textural properties of gluten free cookies based on pearl millet and flaxseed. Biointerface Res. Appl. Chem. 2020, 10, 6565–6576. [Google Scholar]
- Sanmartin, C.; Taglieri, I.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Botto, A.; Serra, A.; Conte, G.; Flamini, G.; et al. Flaxseed cake as a tool for the improvement of nutraceutical and sensorial features of sourdough bread. Foods 2020, 9, 204. [Google Scholar] [CrossRef]
- Kurt, Ş.; Ceylan, H.G. Effects of flaxseed and pH on the emulsion properties of beef by using a model system. Turk. J. Agric. Food Sci. Technol. 2018, 6, 78–83. [Google Scholar] [CrossRef][Green Version]
- Kaur, P.; Waghmare, R.; Kumar, V.; Prasad, R.; Kaur, S.; Gat, Y. Recent advances in utilization of flaxseed as potential source for value addition. OCL 2018, 25, 1–11. [Google Scholar] [CrossRef]
- Tuncel, N.B.; Uygur, A.; Yüceer, Y.K. The effects of infrared roasting on HCN content, chemical composition and storage stability of flaxseed and flaxseed oil. J. Am. Oil Chem. Soc. 2017, 94, 877–884. [Google Scholar] [CrossRef]
- Werner, C. Methods for roasting oilseed, and roasted oilseed products. U.S. Patent 2008/0274247 A1, 1–6 November 2008. [Google Scholar]
- Szydłowska-Czerniak, A.; Tymczewska, A.; Momot, M.; Włodarczyk, K. Optimization of the microwave treatment of linseed for cold-pressing linseed oil—Changes in its chemical and sensory qualities. LWT Food Sci. Technol. 2020, 126, 109317. [Google Scholar] [CrossRef]
- Wroniak, M.; Rękas, A.; Siger, A.; Janowicz, M. Microwave pretreatment effects on the changes in seeds microstructure, chemical composition and oxidative stability of rapeseed oil. LWT Food Sci. Technol. 2016, 68, 634–641. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Siger, A.; Rudzińska, M.; Bamber, W. Effect of roasting on flaxseed oil quality and stability. J. Am. Oil Chem. Soc. 2020, 97, 637–649. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Mikołajczak, B.; Kmiecik, D. Changes in oxidative stability and protein profile of flaxseeds resulting from thermal pre-treatment. J. Sci. Food Agric. 2018, 98, 5459–5469. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Mikołajczak, B.; Gliszczyńska-Świgło, A.; Niedźwiedzińska, K. Effect of thermal pre-treatment on the phenolic and protein profiles and oil oxidation dynamics of golden flaxseeds. Int. J. Food Sci. Technol. 2020, 55, 1272–1280. [Google Scholar] [CrossRef]
- Herchi, W.; Ben Ammar, K.; Bouali, I.; Bou Abdallah, I.; Guetet, A.; Boukhchina, S. Heating effects on physicochemical characteristics and antioxidant activity of flaxseed hull oil (Linum usitatissimum L). Food Sci. Technol. 2016, 36, 97–102. [Google Scholar] [CrossRef]
- AOCS Official Method 991.43 (32.1.17). Total, soluble, and insoluble dietary fiber in foods. Enzymatic-gravimetric method, MES-TRIS buffer. In AOAC Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Kahsai, O. Methods for preparing and compositions comprising plant seed-based omega-3 fatty acids. U.S. Patent 2014/0170287 A1, 19 June 2014. [Google Scholar]
- Waszkowiak, K.; Rudzińska, M. Effect of flaxseed meals and extracts on lipid stability in a stored meat product. J. Am. Oil Chem. Soc. 2014, 91, 979–987. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Gliszczyńska-Świgło, A.; Barthet, V.; Skręty, J. Effect of extraction method on the phenolic and cyanogenic glucoside profile of flaxseed extracts and their antioxidant capacity. J. Am. Oil Chem. Soc. 2015, 92, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- ISO 665:2000. Oilseeds—Determination of Moisture and Volatile Matter Content (Last Reviewed in 2012); ISO: Geneva, Switzerland, 2012. [Google Scholar]
- ISO 5983-2:2009. Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content—Part 2: Block Digestion and Steam Distillation Method; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- ISO 659:2009. Oilseeds—Determination of Oil Content (Reference method); ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Palombo, R.; Gertler, A.; Saguy, I. A simplified method for determination of browning in dairy powders. J. Food Sci. 1984, 49, 1609–1613. [Google Scholar] [CrossRef]
- Morales, F.J.; van Boekel, M.A.J.S. A study on advanced Maillard reaction in heated casein/sugar solutions: Colour formation. Int. Dairy J. 1998, 8, 907–915. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Waszkowiak, K.; Gliszczyńska-Świgło, A. Binary ethanol–water solvents affect phenolic profile and antioxidant capacity of flaxseed extracts. Eur. Food Res. Technol. 2016, 242, 777–786. [Google Scholar] [CrossRef]
- Kotecka-Majchrzak, K.; Sumara, A.; Fornal, E.; Montowska, M. Proteomic analysis of oilseed cake: A comparative study of species-species proteins and peptides extracted from ten seed species. J. Sci. Food Agric. 2020, in press. [Google Scholar] [CrossRef]
- Davis, P.J.; Williams, S.C. Protein modification by thermal processing. Allergy 1998, 53, 102–105. [Google Scholar] [CrossRef]
- Yu, P. Protein secondary structures (a-helix and b-sheet) at a cellular level and protein fractions in relation to rumen degradation behaviours of protein: A new approach. Br. J. Nutr. 2005, 94, 655–665. [Google Scholar] [CrossRef]
- Liu, J.; Shim, Y.Y.; Poth, A.G.; Reaney, M.J.T. Conlinin in flaxseed (Linum usitatissimum L.) gum and its contribution to emulsification properties. Food Hydrocoll. 2016, 52, 963–971. [Google Scholar] [CrossRef]
- Wang, H.Y.; Qian, H.; Yao, W.R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Cämmerer, B.; Kroh, L.W. Shelf life of linseeds and peanuts in relation to roasting. LWT Food Sci. Technol. 2009, 42, 545–549. [Google Scholar] [CrossRef]
- Epaminondas, P.S.; Araujo, K.L.G.V.; Nascimento, J.A.; Silva, M.C.D.; Rosenhaim, R.; Soledade, L.E.B.; Queiroz, N.; Souza, A.L.; Santos, I.M.G.; Souza, A.G. Influence of toasting and the seed variety on the physico-chemical and thermo-oxidative characteristics of the flaxseed oil. J. Therm. Anal. Calorim. 2011, 106, 545–550. [Google Scholar] [CrossRef]
- Bekedam, E.K.; Loots, M.J.; Schols, H.A.; van Boekel, M.A.J.S.; Smit, G. Roasting effects on formation mechanisms of coffee brew melanoidins. J. Agric. Food Chem. 2008, 56, 7138–7145. [Google Scholar] [CrossRef] [PubMed]
- Bastos, D.M.; Monaro, É.; Siguemoto, É.; Séfora, M. Maillard reaction products in processed food: Pros and cons. Food Ind. Process. Methods Equip. 2012, 15, 282–300. [Google Scholar]

| Band | Protein ≈ MW (kDa) | Flaxseed Cultivar | Thermal Treatment | |||
|---|---|---|---|---|---|---|
| Untreated | Roasted | |||||
| 160 °C | 180 °C | 200 °C | ||||
| Protein contribution (%) | ||||||
| 1a | 53 | Szafir | 10.10 a ± 0.25 | 9.65 b ± 0.13 | 8.16 d ± 0.12 | 8.68 c ± 0.12 |
| Oliwin | 14.82 a ± 0.28 | 13.37 b ± 0.06 | 14.25 a ± 0.38 | 10.46 c ± 0.25 | ||
| Jantarol | 13.62 c ± 0.03 | 13.71 c ± 0.04 | 15.80 a ± 0.14 | 14.39 b ± 0.13 | ||
| 2a | 45 | Szafir | 13.48 a ± 0.14 | 12.40 b ± 0.05 | 10.32 c ± 0.28 | 7.57 d ± 0.11 |
| Oliwin | 9.28 a ± 0.27 | 4.34 b ± 0.04 | 9.10 a ± 0.13 | 4.40 b ± 0.41 | ||
| Jantarol | 11.80 a ± 0.55 | 11.64 a ± 0.52 | 11.20 a ± 0.59 | 3.29 b ± 0.37 | ||
| 3a | 31 | Szafir | 8.65 d ± 0.22 | 19.29 a ± 0.10 | 18.12 b ± 0.54 | 17.23 c ± 0.05 |
| Oliwin | 9.62 c ± 0.21 | 18.81 a ± 0.04 | 11.85 b ± 0.04 | 18.96 a ± 0.31 | ||
| Jantarol | 13.48 d ± 0.22 | 15.67 c ± 0.10 | 17.56 b ± 0.40 | 23.29 a ± 0.50 | ||
| 4a | 19 | Szafir | 15.03 d ± 0.25 | 24.94 c ± 0.34 | 29.53 b ± 1.34 | 41.02 a ± 0.36 |
| Oliwin | 12.82 d ± 0.33 | 25.21 c ± 0.22 | 26.70 b ± 0.11 | 46.84 a ± 0.31 | ||
| Jantarol | 17.31 d ± 0.09 | 18.96 c ± 0.07 | 27.18 b ± 0.43 | 42.31 a ± 0.54 | ||
| 5a | 17 | Szafir | 3.58 a ± 0.09 | 2.13 b ± 0.04 | 1.40 c ± 0.43 | 0.00 d ± 0.00 |
| Oliwin | 4.04 a ± 0.18 | 2.41 b ± 0.02 | 4.13 a ± 0.20 | 0.00 c ± 0.00 | ||
| Jantarol | 4.67 a ± 0.00 | 3.21 c ± 0.02 | 4.12 b ± 0.24 | 0.00 d ± 0.00 | ||
| 6a | 13 | Szafir | 25.88 a ± 0.52 | 4.61 b ± 0.16 | 0.20 c ± 0.01 | 0.00 c ± 0.00 |
| Oliwin | 28.54 a ± 0.06 | 10.33 b ± 0.24 | 3.74 c ± 0.13 | 0.00 d ± 0.00 | ||
| Jantarol | 19.06 a ± 0.09 | 16.92 b ± 0.35 | 2.71 c ± 0.09 | 0.00 d ± 0.00 | ||
| 7a | 12 | Szafir | 4.50 c ± 0.06 | 5.04 b ± 0.11 | 6.02 a ± 0.26 | 3.86 d ± 0.13 |
| Oliwin | 3.42 b ± 0.15 | 3.99 b ± 0.03 | 5.62 a ± 0.02 | 3.55 b ± 0.41 | ||
| Jantarol | 3.48 d ± 0.02 | 4.34 b ± 0.03 | 4.73 a ± 0.03 | 4.06 c ± 0.04 | ||
| 8a | 11 | Szafir | 8.85 c ± 0.24 | 13.21 a b ± 0.39 | 12.39 b ± 0.52 | 13.57 a ± 0.18 |
| Oliwin | 7.04 a ± 0.13 | 10.38 b ± 0.01 | 12.71 a ± 0.11 | 10.41 b ± 0.31 | ||
| Jantarol | 5.32 d ± 0.09 | 6.17 c ± 0.04 | 7.31 b ± 0.17 | 8.00 a ± 0.09 | ||
| Proteins at MW range | ||||||
| Ia | >55 | Szafir | 1.54 a ± 0.02 | 1.29 b ± 0.01 | 0.00 c ± 0.00 | 0.00 c ± 0.00 |
| Oliwin | 2.44 a ± 0.05 | 2.19 b ± 0.13 | 0.00 c ± 0.00 | 0.00 c ± 0.00 | ||
| Jantarol | 2.07 a ± 0.01 | 1.32 b ± 0.13 | 0.00 c ± 0.00 | 0.00 c ± 0.00 | ||
| IIa | 54–16 | Szafir | 59.03 d ± 0.00 | 75.86 c ± 0.33 | 81.41 b ± 0.78 | 82.58 a ± 0.06 |
| Oliwin | 58.57 d ± 0.10 | 73.13 c ± 0.34 | 77.93 b ± 0.22 | 86.06 a ± 0.10 | ||
| Jantarol | 70.07 d ± 0.01 | 71.27 c ± 0.40 | 85.26 b ± 0.11 | 87.94 a ± 0.14 | ||
| IIIa | 15–10 | Szafir | 39.45 a ± 0.00 | 22.86 b ± 0.34 | 18.61 c ± 0.79 | 17.42 d ± 0.06 |
| Oliwin | 39.00 a ± 0.08 | 24.69 b ± 0.21 | 22.07 c ± 0.22 | 13.96 d ± 0.10 | ||
| Jantarol | 27.86 a ± 0.02 | 27.42 b ± 0.29 | 14.74 c ± 0.11 | 12.06 d ± 0.14 | ||
| Band | Protein ≈ MW (kDa) | Flaxseed Cultivar | Thermal Treatment | |||
|---|---|---|---|---|---|---|
| Untreated | Roasted | |||||
| 160 °C | 180 °C | 200 °C | ||||
| Protein contribution (%) | ||||||
| 1b | 32 | Szafir | 21.59 a ± 0.52 | 17.90 b ± 0.80 | 15.71 c ± 0.67 | 6.88 d ± 0.27 |
| Oliwin | 30.82 a ± 1.17 | 27.13 b ± 0.63 | 24.47 c ± 0.13 | 20.08 d ± 0.77 | ||
| Jantarol | 26.31 a ± 1.24 | 26.65 a ± 0.12 | 25.61 a ± 0.04 | 20.96 b ± 0.01 | ||
| 2b | 27 | Szafir | 9.15 b ± 0.54 | 8.86 b ± 0.24 | 10.71 a ± 0.29 | 4.99 c ± 0.14 |
| Oliwin | 10.15 c ± 1.43 | 11.59 b,c ± 0.48 | 13.09 a,b ± 0.24 | 14.19 a ± 0.93 | ||
| Jantarol | 11.61 d ± 1.30 | 13.15 b ± 0.01 | 15.01 a ± 0.07 | 15.73 a ± 0.10 | ||
| 3b | 20 | Szafir | 9.99 a ± 0.11 | 5.60 c ± 0.12 | 6.90 b ± 0.11 | 0.00 d ± 0.00 |
| Oliwin | 8.39 a ± 0.02 | 7.12 b ± 0.15 | 6.78 c ± 0.01 | 4.71 d ± 0.05 | ||
| Jantarol | 7.57 b ± 0.04 | 8.20 a ± 0.05 | 7.42 c ± 0.04 | 0.00 d ± 0.00 | ||
| 4b | 17 | Szafir | 20.04 c ± 0.02 | 20.80 c ± 0.53 | 41.33 b ± 0.58 | 63.76 a ± 0.04 |
| Oliwin | 22.14 d ± 0.04 | 28.70 c ± 0.08 | 37.85 b ± 0.00 | 52.24 a ± 0.02 | ||
| Jantarol | 23.07 d ± 0.13 | 27.68 c ± 0.10 | 33.60 b ± 0.32 | 49.48 a ± 0.25 | ||
| 5b | 15 | Szafir | 2.76 a ± 0.00 | 1.81 b ± 0.04 | 1.37 c ± 0.01 | 0.00 d ± 0.00 |
| Oliwin | 2.69 a ± 0.06 | 0.00 b ± 0.00 | 0.00 b ± 0.00 | 0.00 b ± 0.00 | ||
| Jantarol | 3.37 a ± 0.07 | 1.02 b ± 0.00 | 0.00 c ± 0.00 | 0.00 c ± 0.00 | ||
| 6b | 11 | Szafir | 20.60 b ± 0.35 | 24.96 a ± 0.06 | 10.61 d ± 0.38 | 11.8 c ± 0.08 |
| Oliwin | 10.38 b ± 0.07 | 13.96 a ± 0.41 | 8.54 c ± 0.07 | 5.58 d ± 0.06 | ||
| Jantarol | 11.56 a ± 0.02 | 11.77 a ± 0.35 | 8.78 b ± 0.08 | 4.45 c ± 0.04 | ||
| Proteins at MW range | ||||||
| Ib | >36 | Szafir | 4.99 b ± 0.03 | 3.29 d ± 0.06 | 6.49 a ± 0.21 | 4.61 c ± 0.03 |
| Oliwin | 6.84 a ± 0.06 | 4.81 c ± 0.05 | 5.91 b ± 0.14 | 2.63 d ± 0.03 | ||
| Jantarol | 6.63 a ± 0.01 | 3.69 c ± 0.01 | 5.01 b ± 0.06 | 6.11 a ± 0.06 | ||
| IIb | 35–17 | Szafir | 63.15 c ± 0.12 | 53.36 d ± 1.20 | 75.23 b ± 0.08 | 83.51 a ± 0.12 |
| Oliwin | 71.68 d ± 0.38 | 75.28 c ± 0.07 | 82.75 b ± 0.14 | 91.79 a ± 0.09 | ||
| Jantarol | 75.33 d ± 0.04 | 78.37 c ± 0.21 | 82.27 b ± 0.31 | 89.45 a ± 0.12 | ||
| IIIb | 16–10 | Szafir | 29.39 b ± 0.08 | 39.33 a ± 0.22 | 14.40 c ± 0.42 | 11.81 d ± 0.08 |
| Oliwin | 21.01 a ± 0.30 | 18.26 b ± 0.03 | 11.35 c ± 0.01 | 5.58 d ± 0.06 | ||
| Jantarol | 16.96 a ± 0.05 | 17.13 a ± 0.23 | 12.73 b ± 0.25 | 4.45 c ± 0.04 | ||
| Flaxseed Cultivar | Thermal Treatment | p | |||
|---|---|---|---|---|---|
| Untreated | Roasted | ||||
| 160 °C | 180 °C | 200 °C | |||
| Szafir | 136.05 ± 0.87 d,C | 85.99 ± 1.81 c,B | 57.94 ± 2.98 a,A | 70.17 ± 2.06 b,B | <0.0001 |
| Oliwin | 100.82 ± 1.73 c,A | 59.75 ± 5.14 a,A | 56.92 ± 2.15 a,A | 62.03 ± 2.52 a,A | <0.0001 |
| Jantarol | 130.70 ± 4.23 c,B | 88.26 ± 3.65 b,B | 69.74 ± 7.30 a,B | 71.95 ± 5.84 a,B | <0.0001 |
| p | <0.0001 | <0.0001 | 0.0069 | 0.0121 | |
| Variabilities | Correlation Coefficient r | |||||
|---|---|---|---|---|---|---|
| 17 kDa R | 13 kDa NR | 19 kDa NR | ORAC_FL | MRP-F | MRP-B | |
| 13 kDa NR | −0.746 ** | - | - | - | - | - |
| 19 kDa NR | 0.905 *** | −0.853 *** | - | - | - | - |
| ORAC_FL | −0.618 * | 0.806 ** | −0.657 * | - | - | - |
| MRP-F | 0.829 ** | −0.608 * | 0.745 ** | −0.343 NS | - | - |
| MRP-B | 0.879 *** | −0.632 * | 0.837 ** | −0.437 NS | 0.731 ** | - |
| Roasting | 0.694 * | −0.914 *** | 0.781 ** | −0.894 *** | 0.497 NS | 0.584 * |
| Cultivar | −0.091 NS | 0.080 NS | −0.046 NS | 0.041 NS | −0.235 NS | −0.235 NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waszkowiak, K.; Mikołajczak, B. The Effect of Roasting on the Protein Profile and Antiradical Capacity of Flaxseed Meal. Foods 2020, 9, 1383. https://doi.org/10.3390/foods9101383
Waszkowiak K, Mikołajczak B. The Effect of Roasting on the Protein Profile and Antiradical Capacity of Flaxseed Meal. Foods. 2020; 9(10):1383. https://doi.org/10.3390/foods9101383
Chicago/Turabian StyleWaszkowiak, Katarzyna, and Beata Mikołajczak. 2020. "The Effect of Roasting on the Protein Profile and Antiradical Capacity of Flaxseed Meal" Foods 9, no. 10: 1383. https://doi.org/10.3390/foods9101383
APA StyleWaszkowiak, K., & Mikołajczak, B. (2020). The Effect of Roasting on the Protein Profile and Antiradical Capacity of Flaxseed Meal. Foods, 9(10), 1383. https://doi.org/10.3390/foods9101383





