Analysis of Apple Candying by Microwave Spectroscopy
Abstract
:1. Introduction
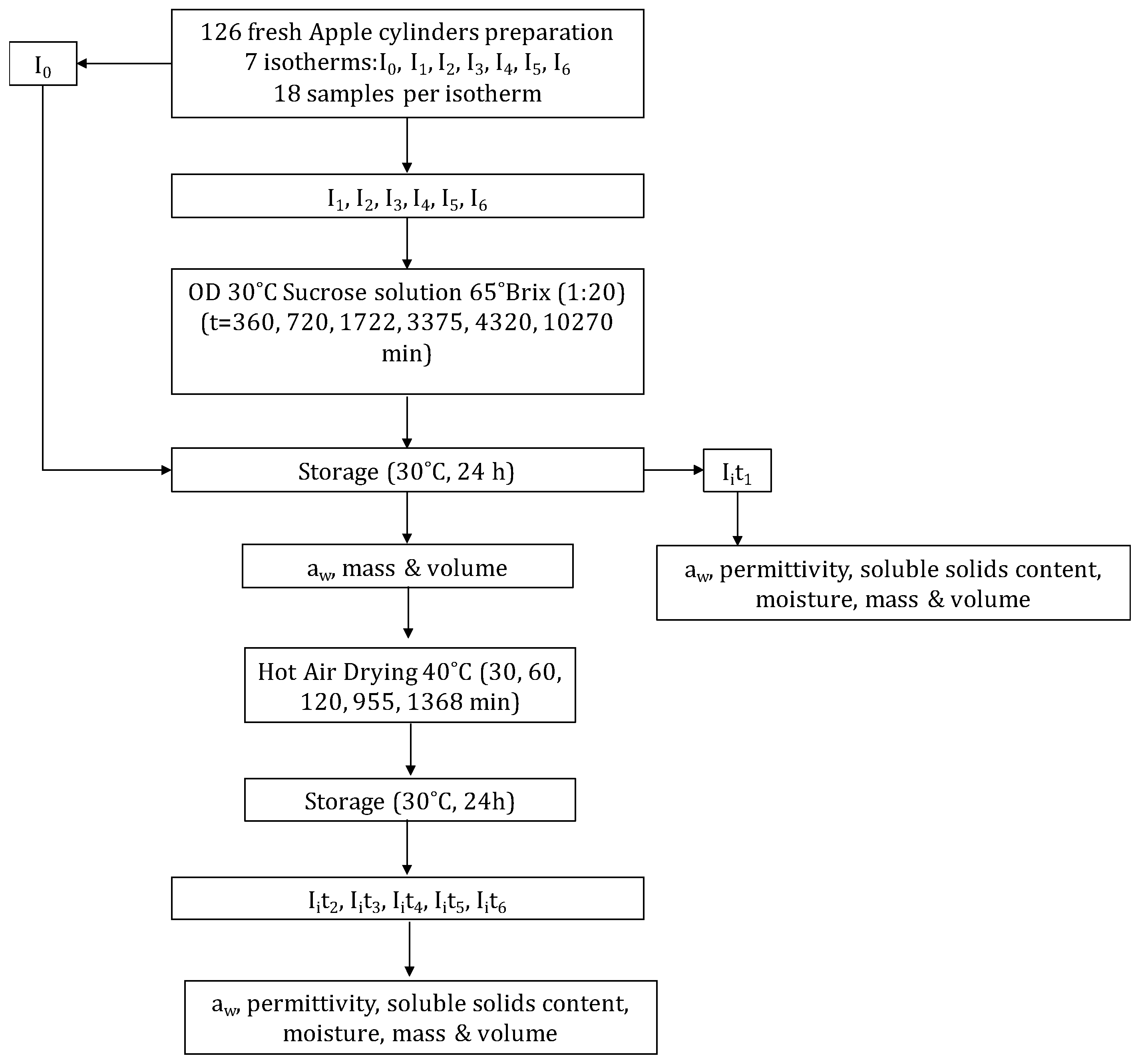
2. Materials and Methods
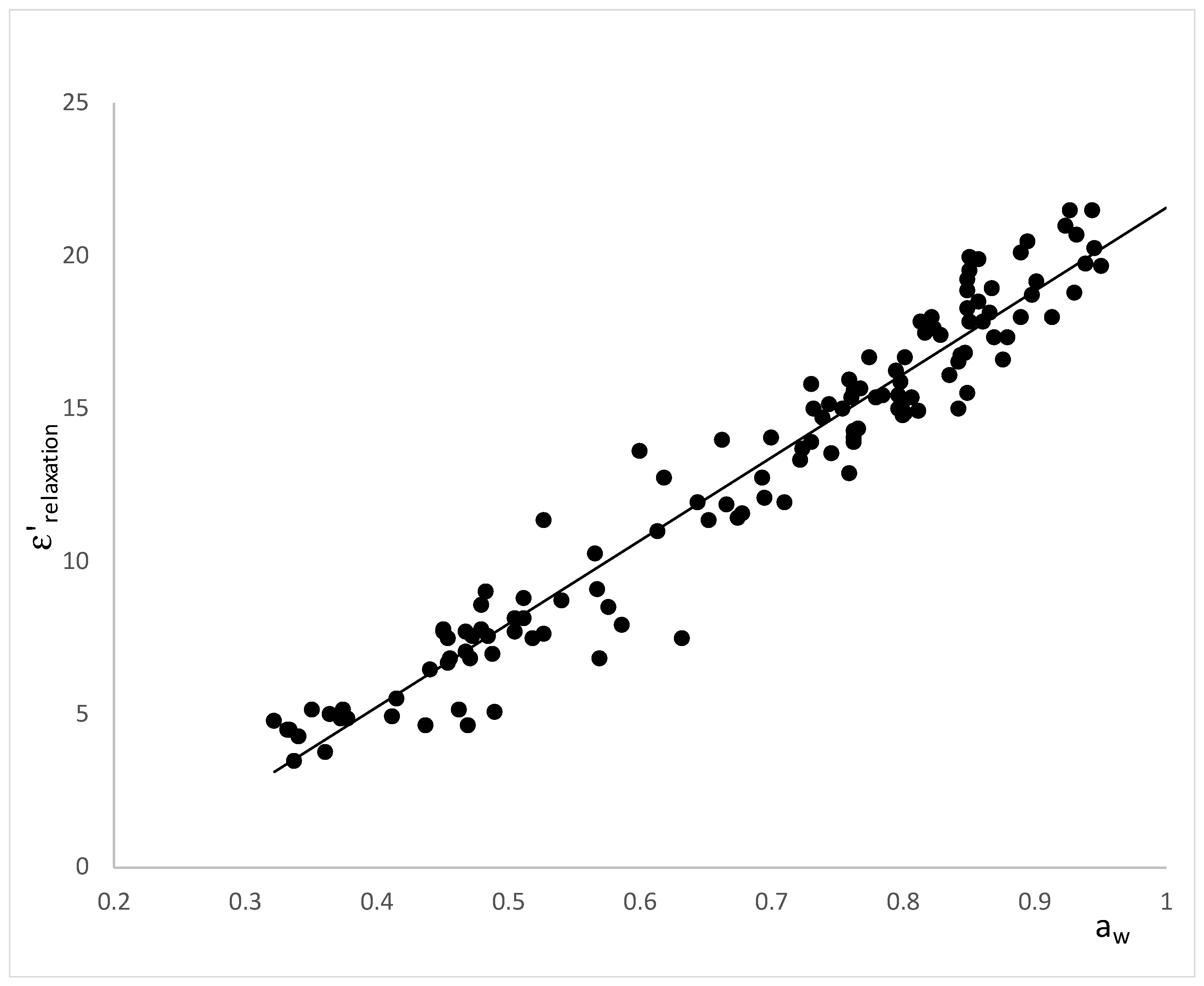
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krokida, M.K.; Marinos-Kouris, D. Rehydration kinetics of dehydrated products. J. Food Eng. 2003, 57, 1–7. [Google Scholar] [CrossRef]
- Sacilik, K.; Elicin, A.K. The thin layer drying characteristics of organic apple slices. J. Food Eng. 2006, 73, 281–289. [Google Scholar] [CrossRef]
- Ahmed, I.; Qazi, I.M.; Jamal, S. Developments in osmotic dehydration technique for the preservation of fruits and vegetables. Innov. Food Sci. Emerg. Technol. 2016, 34, 29–43. [Google Scholar] [CrossRef]
- Castro-Giraldez, M.; Tylewicz, U.; Fito, P.J.; Dalla Rosa, M.; Fito, P. Analysis of chemical and structural changes in kiwifruit (Actinidia deliciosa cv Hayward) through the osmotic dehydration. J. Food Eng. 2011, 105, 599–608. [Google Scholar] [CrossRef]
- Fito, P.; LeMaguer, M.; Betoret, N.; Fito, P.J. Advanced food process engineering to model real foods and processes: The “SAFES” methodology. J. Food Eng. 2007, 83, 173–185. [Google Scholar] [CrossRef]
- Salisbury, F.; Ross, C. Plant Physiology. In Plant Physiology and Plant Cells, 4th ed.; Salisbury, F., Ross, C., Eds.; Wadsworth Pub. Co.: Belmont, CA, USA, 1991; pp. 3–26. [Google Scholar]
- Agre, P.; Bonhivers, M.; Borgnia, M.J. The aquaporins, blueprints for cellular plumbing systems. J. Biol. Chem. 1998, 273, 14659–14662. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.B.; Vicente, S.; Hodara, K.; Castro, M.A.; Alzamora, S.M. Osmotic dehydration of apple: Influence of sugar and water activity on tissue structure, rheological properties and water mobility. J. Food Eng. 2013, 119, 104–114. [Google Scholar] [CrossRef]
- Vicente, S.; Nieto, A.B.; Hodara, K.; Castro, M.A.; Alzamora, S.M. Changes in structure, rheology, and water mobility of apple tissue induced by osmotic dehydration with glucose or trehalose. Food Bioprocess. Technol. 2012, 5, 3075–3089. [Google Scholar] [CrossRef]
- Mavroudis, N.E.; Dejmek, P.; Sjöholm, I. Osmotic-treatment-induced cell death and osmotic processing kinetics of apples with characterised raw material properties. J. Food Eng. 2004, 63, 47–56. [Google Scholar] [CrossRef]
- Alzamora, S.M.; Castro, M.A.; Nieto, A.B.; Vidales, S.L.; Salvatori, D.M. The role of tissue microstructure in the textural characteristics of minimally processed fruits. In Minimally Processed Fruits and Vegetables; Alzamora, S.M., Tapia, S., Lopez-Malo, A., Eds.; Aspen Publishers Inc.: Frederick, MD, USA, 2000; pp. 153–171. [Google Scholar]
- Mauro, M.A.; Dellarosa, N.; Tylewicz, U.; Tappi, S.; Laghi, L.; Rocculi, P.; Dalla Rosa, M. Calcium and ascorbic acid affect cellular structure and water mobility in apple tissue during osmotic dehydration in sucrose solutions. Food Chem. 2016, 195, 19–28. [Google Scholar] [CrossRef]
- Lewicki, P.P. Design of hot air drying for better foods. Trends Food Sci. Technol. 2006, 17, 153–163. [Google Scholar] [CrossRef]
- Lewicki, P.P.; Jakubczyk, E. Effect of hot air temperature on mechanical properties of dried apples. J. Food Eng. 2004, 64, 307–314. [Google Scholar] [CrossRef]
- Mathlouthi, M. X-ray diffraction study of the molecular association in aqueous solutions of d-fructose, d-glucose, and sucrose. Carbohydr Res. 1981, 91, 113–123. [Google Scholar] [CrossRef]
- Mathlouthi, M.; Genotelle, J. Role of water in sucrose crystallization. Carbohydr. Polym. 1998, 37, 335–342. [Google Scholar] [CrossRef]
- Mathlouthi, M. Amorphous sugar. In Sucrose; Mathlouthi, M., Reiser, P., Eds.; Springer: Boston, MA, USA, 1995; pp. 75–100. [Google Scholar]
- Traffano-Schiffo, M.V.; Castro-Giraldez, M.; Colom, R.J.; Fito, P.J. Innovative photonic system in radiofrequency and microwave range to determine chicken meat quality. J. Food Eng. 2018, 239, 1–7. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Castro-Giraldez, M.; Herrero, V.; Colom, R.J.; Fito, P.J. Development of a non-destructive detection system of Deep Pectoral Myopathy in poultry by dielectric spectroscopy. J. Food Eng. 2018, 237, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Traffano-Schiffo, M.V.; Castro-Giraldez, M.; Colom, R.J.; Fito, P.J. Development of a spectrophotometric system to detect white striping physiopathy in whole chicken carcasses. Sensors 2017, 17, 1024. [Google Scholar] [CrossRef]
- Trabelsi, S.; Roelvink, J. Investigating the influence of aging on radiofrequency dielectric properties of chicken meat. J. Microw. Power Electromagn. Energy 2014, 48, 215–220. [Google Scholar] [CrossRef]
- Samuel, D.; Trabelsi, S.; Karnuah, A.B.; Anthony, N.B.; Aggrey, S.E. The use of dielectric spectroscopy as a tool for predicting meat quality in poultry. Int. J. Poult. Sci. 2012, 11, 551. [Google Scholar] [CrossRef]
- Castro-Giraldez, M.; Toldrá, F.; Fito, P. Development of a dielectric spectroscopy technique for the determination of key bichemical markers of meat quality. Food Chem. 2011, 127, 228–233. [Google Scholar] [CrossRef]
- Castro-Giraldez, M.; Toldrá, F.; Fito, P. Low frequency dielectric measurements to assess post-mortem ageing of pork meat. LWT Food Sci. Technol. 2011, 44, 1465–1472. [Google Scholar] [CrossRef]
- Castro-Giraldez, M.; Toldrá, F.; Fito, P. Low-frequency dielectric spectrum to determine pork meat quality. Innov. Food Sci. Emerg. Technol. 2010, 2, 376–386. [Google Scholar] [CrossRef]
- Castro-Giráldez, M.; Aristoy, M.C.; Toldrá, F.; Fito, P. Microwave dielectric spectroscopy for the determination of pork meat quality. Food Res. Int. 2010, 1, 2369–2377. [Google Scholar] [CrossRef]
- Kent, M.; Peymann, A.; Gabriel, C.; Knight, A. Determination of added water in pork products using microwave dielectric spectroscopy. Food Control. 2002, 13, 143–149. [Google Scholar] [CrossRef]
- Brunton, N.P.; Lyng, J.; Zhang, L.; Jacquier, J.C. The use of dielectric properties and other physical analyses for assessing protein denaturation in beef biceps femoris muscle during cooking from 5 to 85 °C. Meat Sci. 2006, 72, 236–244. [Google Scholar] [CrossRef]
- Mohiri, A.; Burhanudin, Z.A.; Ismail, I. Dielectric properties of slaughtered and non-slaughtered goat meat. In Proceedings of the RF and Microwave Conference (RFM), IEEE International, Seremban, Negeri Sembilan, Malaysia, 12–14 December 2011; pp. 393–397. [Google Scholar]
- Lyng, J.G.; Zhang, L.; Brunton, N.P. A survey of the dielectric properties of meats and ingredients used in meat product manufacture. Meat Sci. 2005, 69, 589–602. [Google Scholar] [CrossRef]
- Everard, C.D.; Fagan, C.C.; O’Donell, C.P.; O’Callaghan, D.J.; Lyng, J. Dielectric properties of process cheese from 0.3 to 3GHz. J. Food Eng. 2006, 75, 415–422. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Castro-Giraldez, M.; Colom, R.J.; Fito, P.J. New Spectrophotometric System to Segregate Tissues in Mandarin Fruit. Food Bioprocess. Technol. 2018, 2, 399–406. [Google Scholar] [CrossRef]
- Cuibus, L.; Castro-Giraldez, M.; Fito, P.J.; Fabbri, A. Application of infrared thermography and dielectric spectroscopy for controlling freezing process of raw potato. Innov. Food Sci. Emerg. Technol. 2014, 1, 80–87. [Google Scholar] [CrossRef]
- Nelson, S.O.; Trabelsi, S. Dielectric spectroscopy of wheat from 10 MHz to 1.8 GHz. Meas. Sci. Technol. 2006, 17, 2294–2298. [Google Scholar] [CrossRef]
- Venkatesh, M.S.; Raghavan, G.S.V. An overview of microwave processing and dielectric properties of agri-food materials. Biosyst. Eng. 2004, 88, 1–18. [Google Scholar] [CrossRef]
- Nelson, S.O. Dielectric spectroscopy in agriculture. J. Non Cryst. Solids 2005, 351, 2940–2944. [Google Scholar] [CrossRef]
- Nelson, S.O. Dielectric properties of agricultural products and some applications. Res. Agric. Eng. 2008, 54, 104–112. [Google Scholar] [CrossRef]
- Castro-Giraldez, M.; Fito, P.J.; Ortola, M.D.; Balaguer, N. Study of pomegranate ripening by dielectric spectroscopy. Postharvest Biol. Technol. 2013, 86, 346–353. [Google Scholar] [CrossRef]
- Shang, L.; Guo, W.; Nelson, S.O. Apple variety identification based on dielectric spectra and chemometric methods. Food Anal. Methods 2015, 8, 1042–1052. [Google Scholar] [CrossRef]
- Castro-Giraldez, M.; Fito, P.J.; Chenoll, C.; Fito, P. Development of a dielectric spectroscopy technique for determining key chemical components of apple maturity. J. Agric. Food Chem. 2010, 6, 3761–3766. [Google Scholar] [CrossRef]
- Castro-Giraldez, M.; Fito, P.J.; Chenoll, C.; Fito, P. Development of a dielectric spectroscopy technique for the determination of apple (Granny Smith) maturity. J. Food Eng. 2010, 4, 749–754. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Castro-Giraldez, M.; Colom, R.J.; Fito, P.J. Study of the application of dielectric spectroscopy to predict the water activity of meat during drying process. J. Food Eng. 2015, 166, 285–290. [Google Scholar] [CrossRef]
- Castro-Giraldez, M.; Fito, P.J.; Fito, P. Application of microwaves dielectric spectroscopy for controlling pork meat (Longissimus dorsi) salting process. J. Food Eng. 2010, 4, 484–490. [Google Scholar] [CrossRef]
- Talens, C.; Castro-Giraldez, M.; Fito, P.J. Study of the effect of microwave power coupled with hot air drying on orange peel by dielectric spectroscopy. LWT Food Sci. Technol. 2016, 66, 622–628. [Google Scholar] [CrossRef]
- Velazquez-Varela, J.; Castro-Giraldez, M.; Cuibus, L.; Tomas-Egea, J.A.; Socaciu, C.; Fito, P.J. Study of the cheese salting process by dielectric properties at microwave frequencies. J. Food Eng. 2018, 224, 121–128. [Google Scholar] [CrossRef]
- Velazquez-Varela, J.; Castro-Giraldez, M.; Fito, P.J. Control of the brewing process by using microwaves dielectric spectroscopy. J. Food Eng. 2013, 119, 633–639. [Google Scholar] [CrossRef]
- Castro-Giraldez, M.; Fito, P.J.; Andrés, A.M.; Fito, P. Study of the puffing process of amaranth seeds by dielectric spectroscopy. J. Food Eng. 2012, 11, 298–304. [Google Scholar] [CrossRef]
- Castro-Giraldez, M.; Fito, P.J.; Fito, P. Application of microwaves dielectric spectroscopy for controlling osmotic dehydration of kiwifruit (Actinidia deliciosa cv Hayward). Innov. Food Sci. Emerg. Technol. 2011, 12, 623–627. [Google Scholar] [CrossRef]
- Castro-Giraldez, M.; Fito, P.J.; Fito, P. Application of microwaves dielectric spectroscopy for controlling long time osmotic dehydration of parenchymatic apple tissue. J. Food Eng. 2011, 2, 227–233. [Google Scholar] [CrossRef]
- Baker-Jarvis, J.; Kim, S. The Interaction of Radio-Frequency Fields with Dielectric Materials at Macroscopic to Mesoscopic Scales. J. Res. Natl. Inst. Stand. Technol. 2012, 117, 1–60. [Google Scholar] [CrossRef]
- Roychoudhuri, C.; Kracklauer, A.F.; Creath, K. The Nature of Light: What Is a Photon; CRC Press: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Horie, K.; Ushiki, H.; Winnik, F.M. Molecular Photonics; Wiley: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Crane, C.A.; Pantoya, M.L.; Weeks, B.L. Spatial observation and quantification of microwave heating in materials. Rev. Sci. Instrum. 2013, 84, 084705. [Google Scholar] [CrossRef]
- Castro-Giraldez, M.; Fito, P.J.; Fito, P. Nonlinear thermodynamic approach to analyze long time osmotic dehydration of parenchymatic apple tissue. J. Food Eng. 2011, 102, 34–42. [Google Scholar] [CrossRef]
- AOAC. Official Method 934.06 Moisture in Dried Fruits; AOAC: Rockville, MD, USA, 2000. [Google Scholar]
- Tylewicz, U.; Fito, P.J.; Castro-Giraldez, M.; Fito, P.; Dalla Rosa, M. Analysis of kiwifruit osmodehydration process by systematic approach systems. J. Food Eng. 2011, 104, 438–444. [Google Scholar] [CrossRef]
- Starzak, M.; Mathlouthi, M. Temperature dependence of water activity in aqueous solutions of sucrose. Food Chem. 2006, 96, 346–370. [Google Scholar] [CrossRef]
- Lewis, D.F. Structure of sugar confectionery. In Sugar Confectionery Manufacture; Jackson, E.B., Ed.; Blackie: Glasgow, UK, 1990; pp. 331–350. [Google Scholar]
- Roos, Y.; Karel, M. Water and molecular weight effects on glass transitions in amorphous carbohydrates and carbohydrate solutions. J. Food Sci. 1991, 56, 1676–1681. [Google Scholar] [CrossRef]


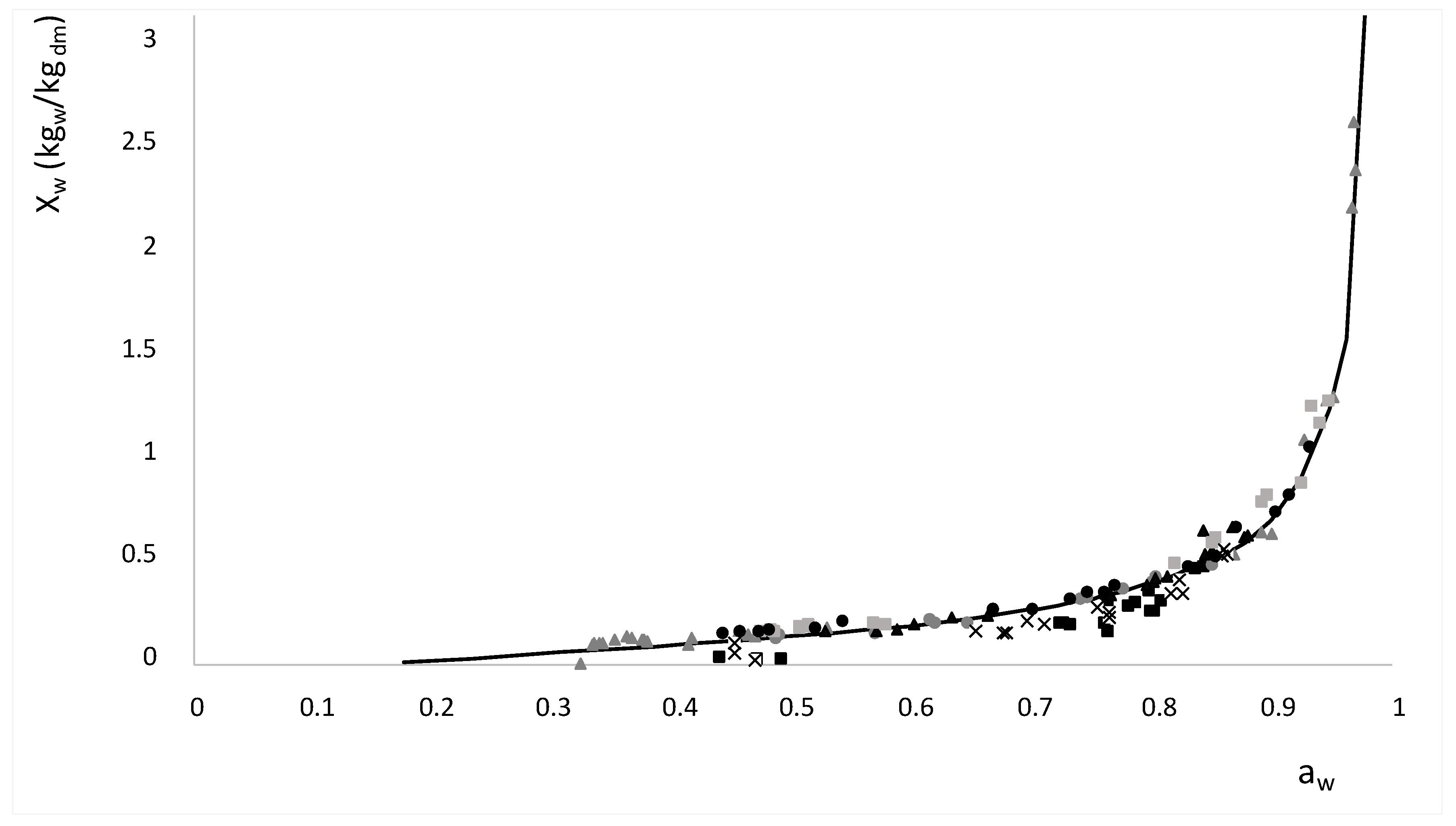
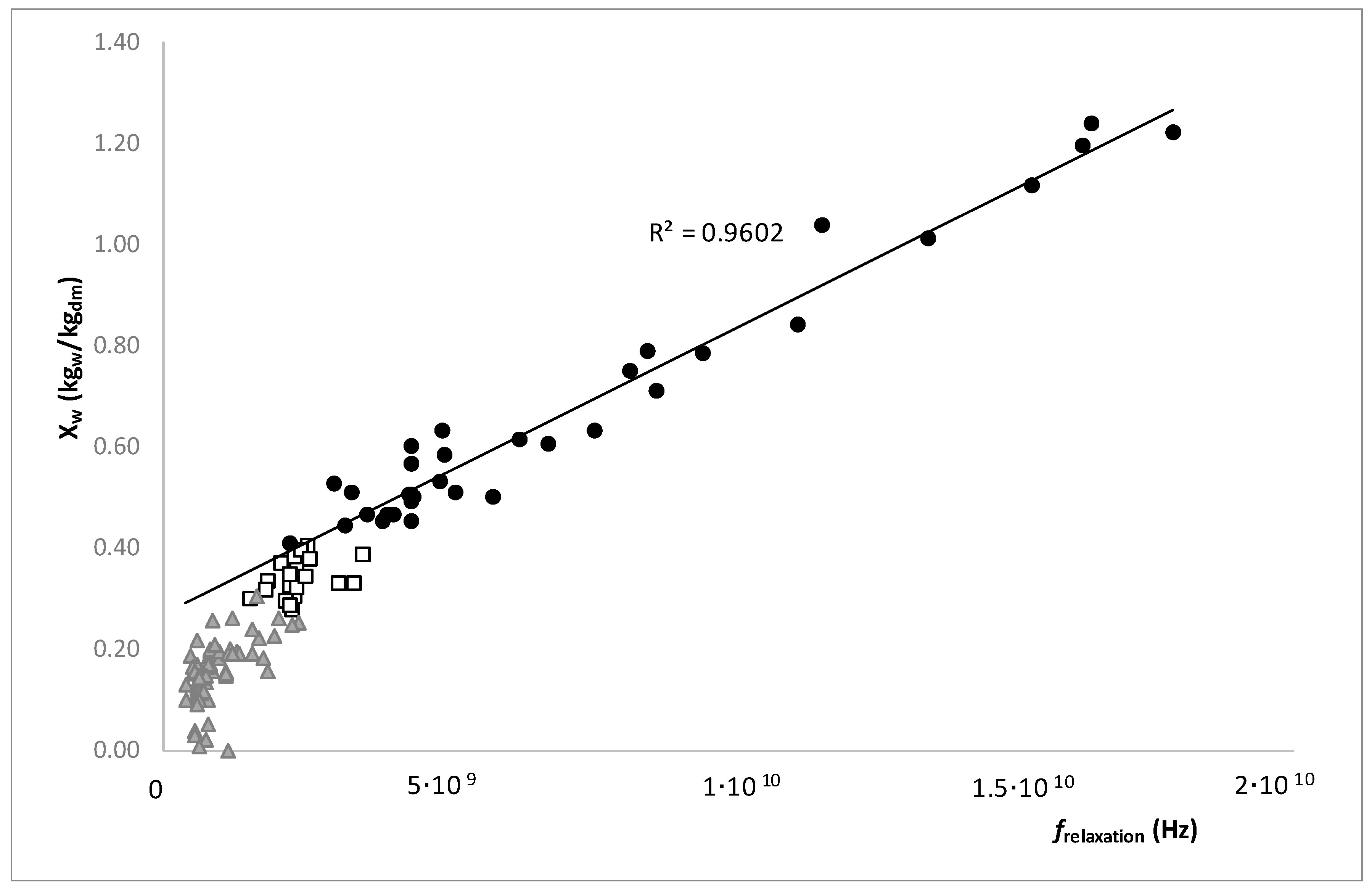
 ) and (
) and (  ) lines represent the saturation and supersaturation curves obtained from [16], (
) lines represent the saturation and supersaturation curves obtained from [16], (  ) lines represent different values of viscosity obtained from Lewis (1990) [58], and (●) represent the different experimental values of samples with OD and HAD treatments.
) lines represent different values of viscosity obtained from Lewis (1990) [58], and (●) represent the different experimental values of samples with OD and HAD treatments.
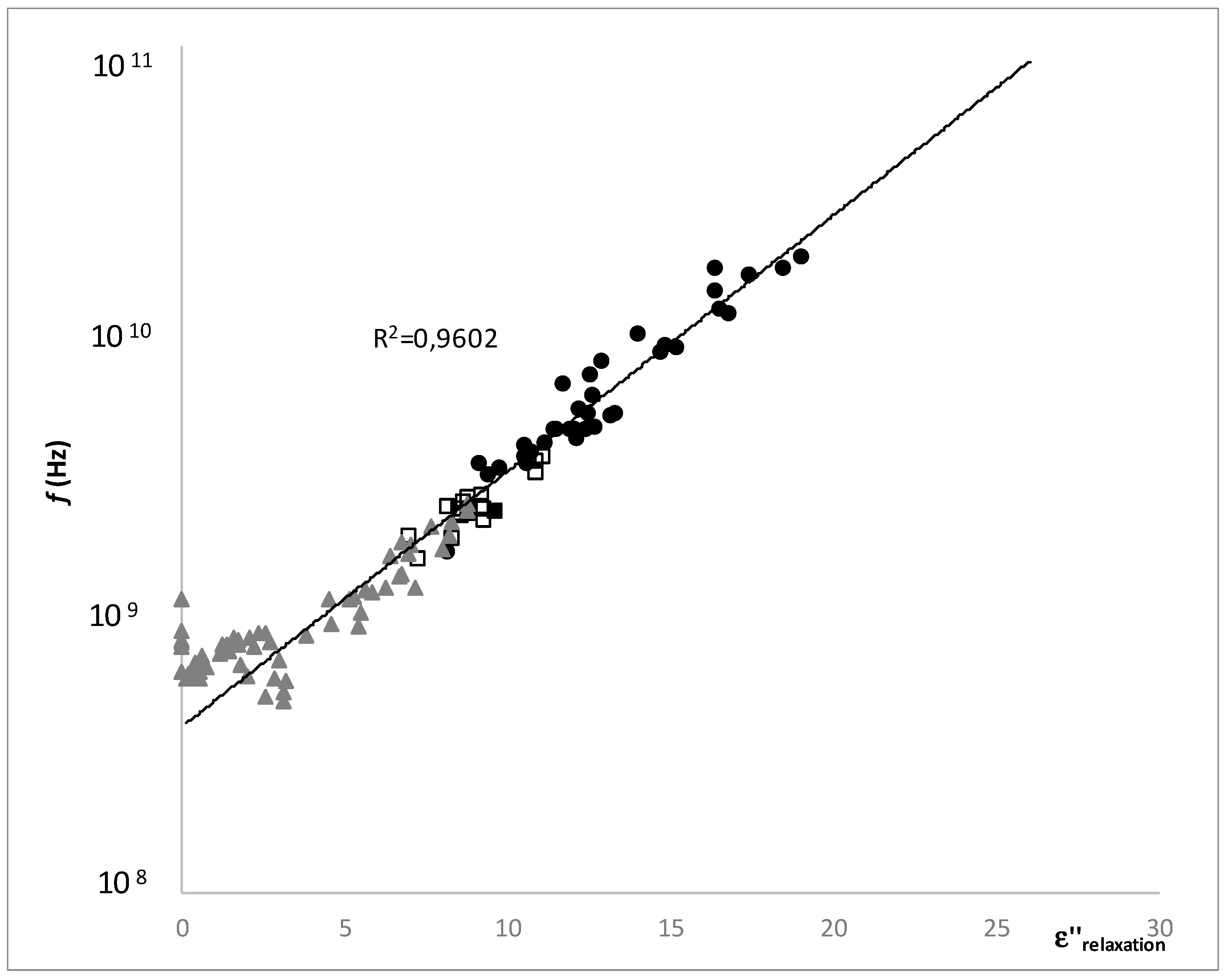
 ) and (
) and (  ) lines represent the saturation and supersaturation curves obtained from [16], (
) lines represent the saturation and supersaturation curves obtained from [16], (  ) lines represent different values of viscosity obtained from Lewis (1990) [58], and (●) represent the different experimental values of samples with OD and HAD treatments.
) lines represent different values of viscosity obtained from Lewis (1990) [58], and (●) represent the different experimental values of samples with OD and HAD treatments.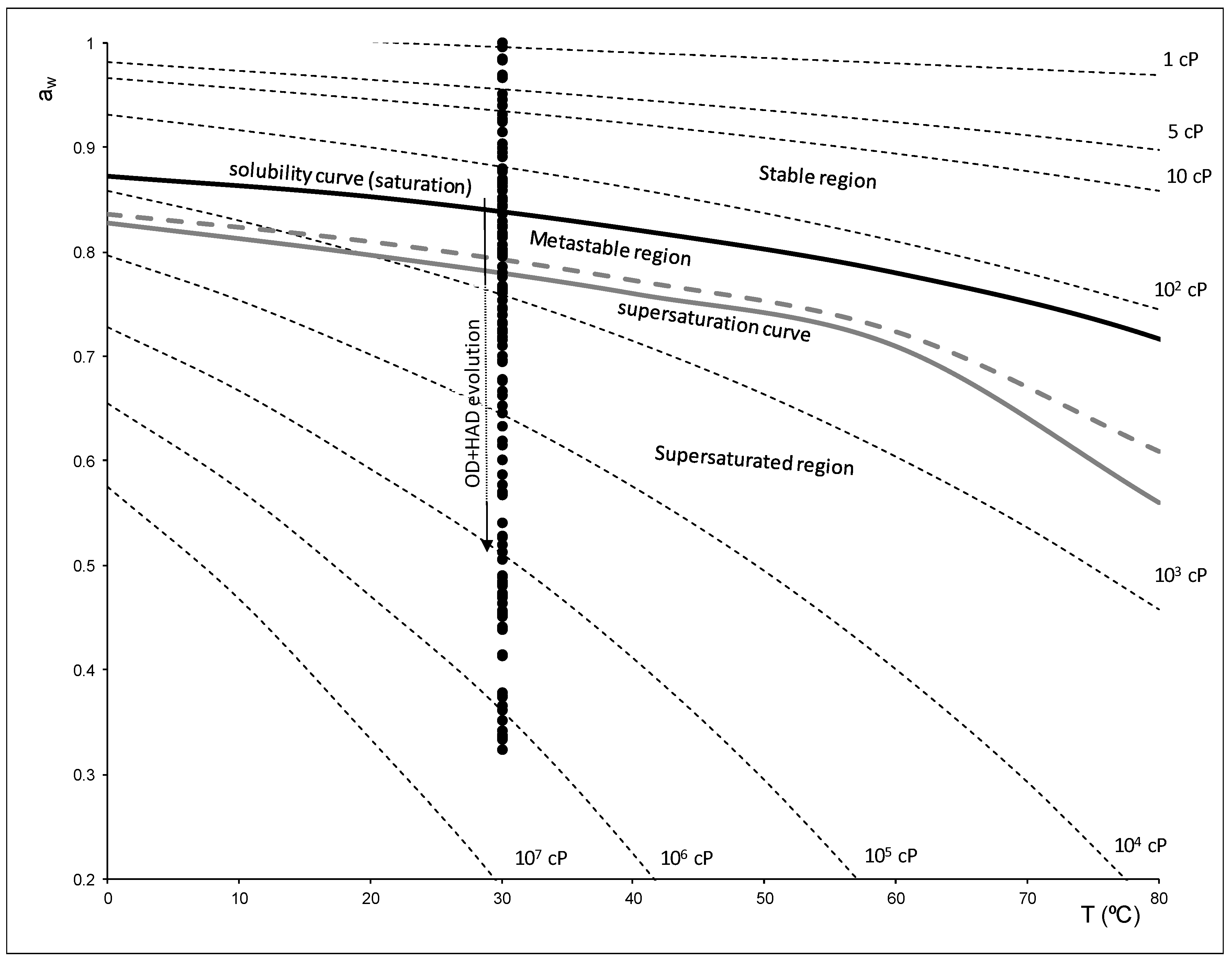

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomas-Egea, J.A.; Fito, P.J.; Castro-Giraldez, M. Analysis of Apple Candying by Microwave Spectroscopy. Foods 2019, 8, 316. https://doi.org/10.3390/foods8080316
Tomas-Egea JA, Fito PJ, Castro-Giraldez M. Analysis of Apple Candying by Microwave Spectroscopy. Foods. 2019; 8(8):316. https://doi.org/10.3390/foods8080316
Chicago/Turabian StyleTomas-Egea, Juan A., Pedro J. Fito, and Marta Castro-Giraldez. 2019. "Analysis of Apple Candying by Microwave Spectroscopy" Foods 8, no. 8: 316. https://doi.org/10.3390/foods8080316
APA StyleTomas-Egea, J. A., Fito, P. J., & Castro-Giraldez, M. (2019). Analysis of Apple Candying by Microwave Spectroscopy. Foods, 8(8), 316. https://doi.org/10.3390/foods8080316




