Feasibility of Using Seaweed (Gracilaria coronopifolia) Synbiotic as a Bioactive Material for Intestinal Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Bacterial Strains and Culture Conditions
2.3. G. coronopifolia Synbiotic Preparations
2.4. Cell Culture
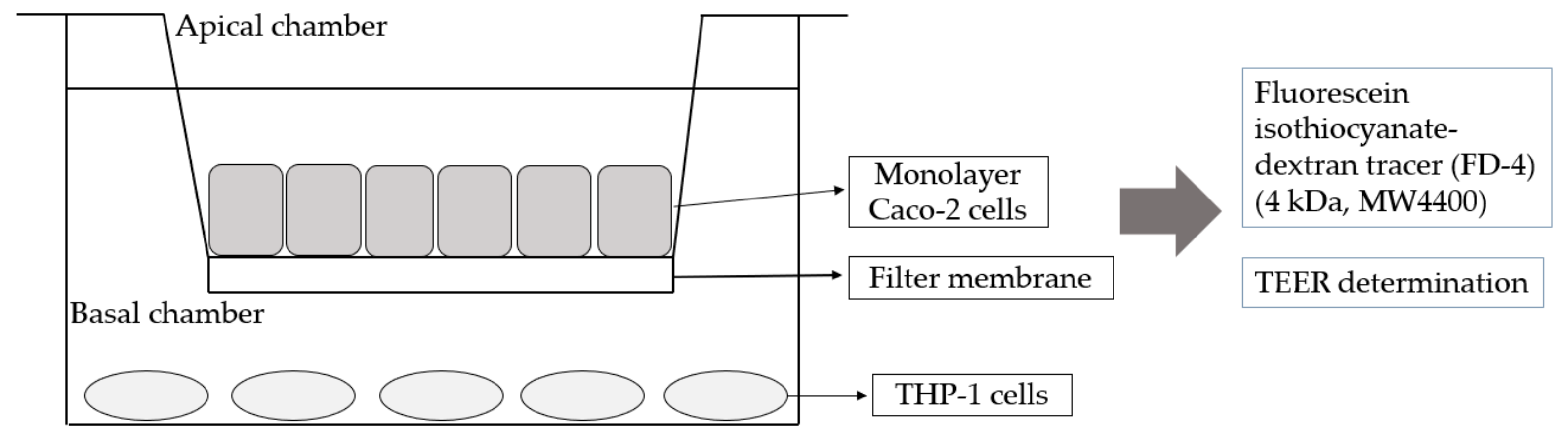
2.5. Co-Culture of Caco-2 and THP-1 Cells
2.6. Cell Viability Assay (MTT Assay)
2.7. Single-Layer Cell TEER Determination
2.8. Determining ROS Production
2.9. Interleukin-8 (IL-8) Analysis
2.10. Statistical Analysis
3. Results and Discussions
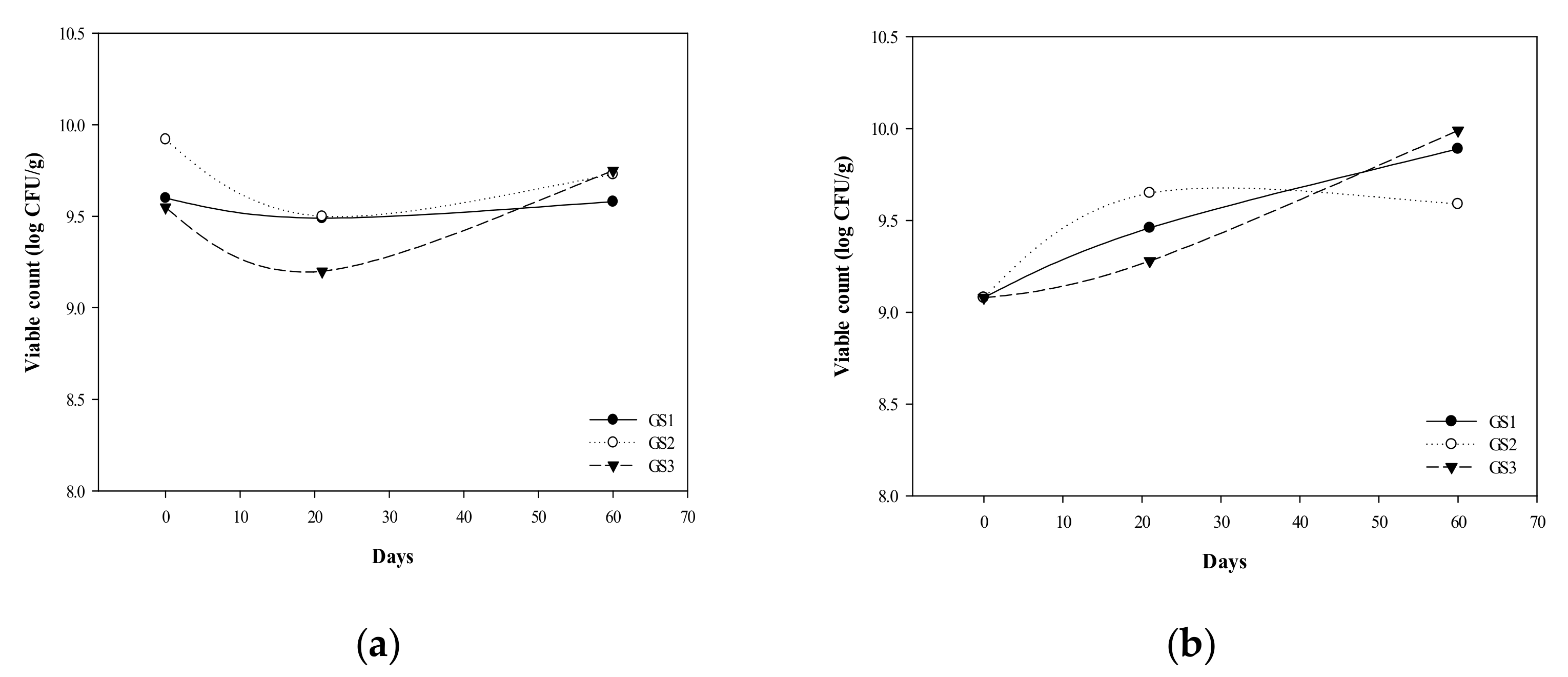
3.1. G. coronopifolia Synbiotic Synthesis and Storage Test
3.2. Survival of G. coronopifolia Synbiotics in Caco-2 Cells
3.3. Establishment of Caco-2 Cells and THP-1 Macrophage Co-Culture Mode
3.4. Effect of G. coronopifolia Synbiotics on Intestinal Monolayer Membrane
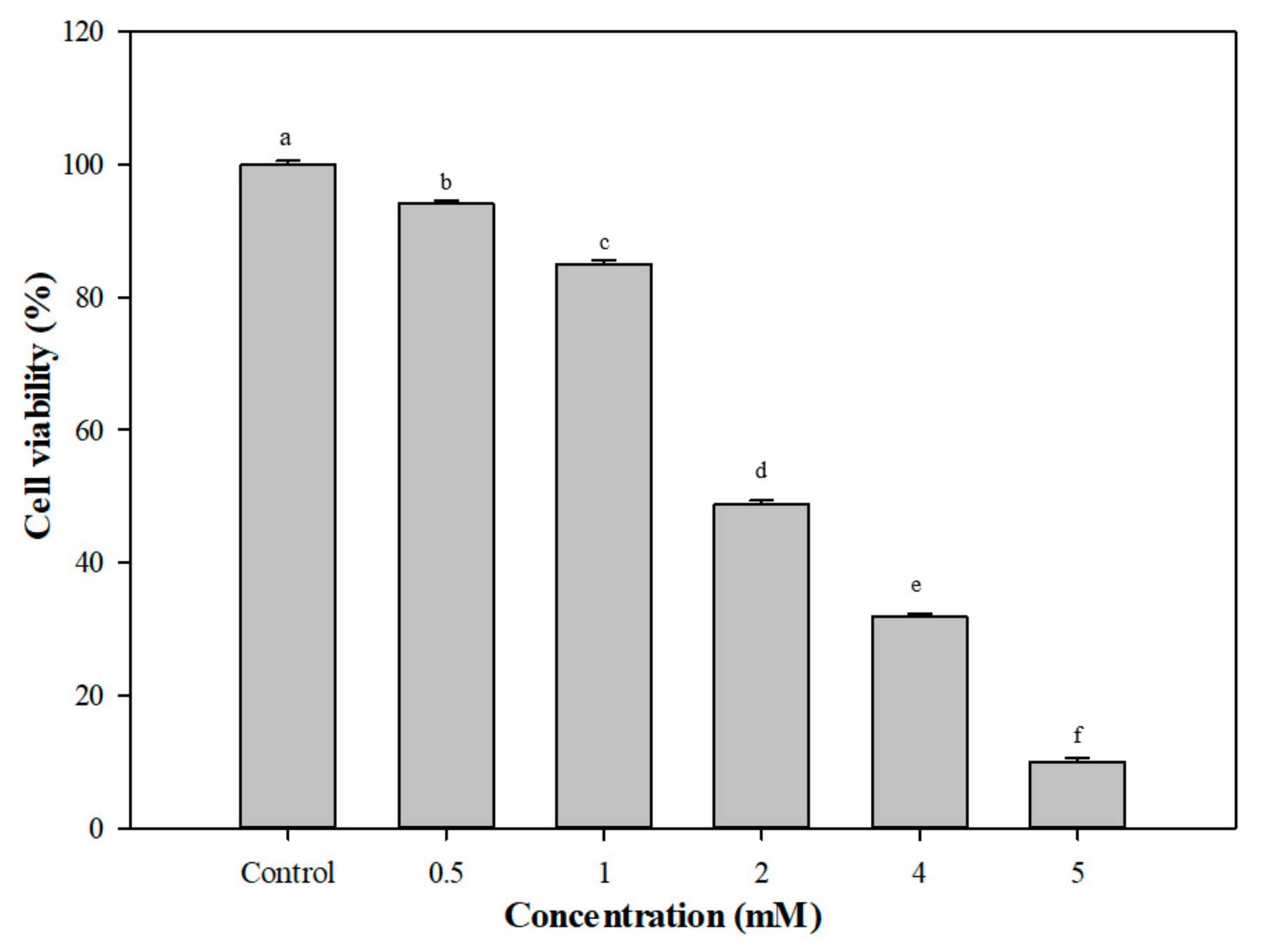
3.5. Protective Effects of G. coronopifolia Synbiotics on THP-1 and Caco-2 Cells under Oxidative Stress
3.6. Assessment of Inflammatory Factors in G. coronopifolia Synbiotic
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Al-Enazi, N.M.; Awaad, A.S.; Alqasoumi, S.I.; Alwethairi, M.F. Biological activities of the red algae Galaxaura rugosa and Liagora hawaiiana butters. Saudi Pharm. J. 2018, 26, 25–32. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, C.L.F.; Falcão, H.D.S.; Lima, G.R.D.M.; Montenegro, C.D.A.; Lira, N.S.; De Athayde-Filho, P.F.; Rodrigues, L.C.; Souza, M.D.F.V.D.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from Marine Algae of the Genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar] [CrossRef] [PubMed]
- Ratnasooriya, W.D.; Premakumara, G.A.S.; Tillekeratne, L.M.V. Post-coital contraceptive activity of crude extracts of Sri Lankan marine red algae. Contraception 1994, 50, 291–299. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chang, J.-S.; Sheu, F.; Shyu, Y.-T.; Yu, A.Y.-C.; Wong, S.-L.; Dai, C.-F.; Lee, T.-M. Seasonal growth dynamics of Laurencia papillosa and Gracilaria coronopifolia from a highly eutrophic reef in southern Taiwan: Temperature limitation and nutrient availability. J. Exp. Mar. Biol. Ecol. 2005, 315, 49–69. [Google Scholar] [CrossRef]
- Guarner, F. Enteric flora in health and disease. Digestion 2006, 73, 5–12. [Google Scholar] [CrossRef]
- Cha, K.H.; Lee, E.H.; Yoon, H.S.; Lee, J.H.; Kim, J.Y.; Kang, K.; Park, J.-S.; Jin, J.B.; Ko, G.; Pan, C.-H. Effects of fermented milk treatment on microbial population and metabolomic outcomes in a three-stage semi-continuous culture system. Food Chem. 2018, 263, 216–224. [Google Scholar] [CrossRef]
- Parvez, S.; Malik, K.; Kang, S.A.; Kim, H.-Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Agriculture Organization of the United Nations/World Health Organization. Guidelines for the Evaluation of Probiotics in Food; Report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food: London ON, Canada; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy; World Health Organization (WHO): Geneva, Switzerland, 2002. [Google Scholar]
- Levri, K.M.; Ketvertis, K.; Deramo, M.; Merenstein, J.H.; D’Amico, F. Do probiotics reduce adult lactose intolerance? A systematic review. J. Fam. Pract. 2005, 54, 613–620. [Google Scholar]
- Guandalini, S. Probiotics for children with diarrhea: An update. J. Clin. Gastroenterol. 2008, 42, S53–S57. [Google Scholar] [CrossRef]
- Forsythe, P.; Bienenstock, J. Immunomodulation by commensal and probiotic bacteria. Immunol. Investig. 2010, 39, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Chalova, V.I.; Lingbeck, J.M.; Kwon, Y.M.; Ricke, S.C. Extracellular antimutagenic activities of selected probiotic Bifidobacterium and Lactobacillus spp. as a function of growth phase. J. Environ. Sci. Health Part B 2008, 43, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.-T. Roles of Probiotics and prebiotics in colon cancer prevention: Postulated mechanisms and in-vivo evidence. Int. J. Mol. Sci. 2008, 9, 854–863. [Google Scholar] [CrossRef]
- Gustaw, W.; Kordowska-Wiater, M.; Koziol, J. The influence of selected prebiotics on the growth of lactic acid bacteria for bio-yoghurt production. Acta Sci. Pol. Technol. Aliment. 2011, 10, 455–466. [Google Scholar] [PubMed]
- Khurana, H.; Kanawjia, S. Recent trends in development of fermented milks. Curr. Nutr. Food Sci. 2007, 3, 91–108. [Google Scholar] [CrossRef]
- Shinde, T.; Perera, A.P.; Vemuri, R.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Shastri, S.; Southam, B.; Eri, R.; Stanley, R. Synbiotic Supplementation Containing Whole Plant Sugar Cane Fibre and Probiotic Spores Potentiates Protective Synergistic Effects in Mouse Model of IBD. Nutrients 2019, 11, 818. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxidative Med. Cell. Longev. 2017, 2017, 1–18. [Google Scholar] [CrossRef]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in inflammatory bowel disease: Clinical aspects and animal models. Saudi J. Gastroenterol. 2016, 22, 3–17. [Google Scholar] [CrossRef]
- Huttenhower, C.; Kostic, A.D.; Xavier, R.J. Inflammatory bowel disease as a model for translating the microbiome. Immunity 2014, 40, 843–854. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef]
- Knipp, G.T.; Ho, N.F.H.; Barsuhn, C.L.; Borchardt, R.T. Paracellular Diffusion in Caco-2 Cell Monolayers: Effect of Perturbation on the Transport of Hydrophilic Compounds That Vary in Charge and Size. J. Pharm. Sci. 1997, 86, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.S.; Chikhale, P.J.; Shah, P.K.; Borchardt, R.T. Utilization of a Human Intestinal Epithelial Cell Culture System (Caco-2) for Evaluating Cytoprotective Agents. Pharm. Res. 1993, 10, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Turgeon, S.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Quinn, E.M.; Slattery, H.; Thompson, A.P.; Kilcoyne, M.; Joshi, L.; Hickey, R.M. Mining Milk for Factors which Increase the Adherence of Bifidobacterium longum subsp. infantis to Intestinal Cells. Foods 2018, 7, 196. [Google Scholar] [CrossRef]
- Takisawa, R.; Nishitani, Y.; Mizuno, M.; Osawa, R. Anti-Inflammatory Effect of Bifidobacterium longum on Macrophage-Like THP-1 Cells via Epithelial Cell Caco-2. Biosci. Microflora 2009, 28, 45–48. [Google Scholar] [CrossRef][Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Booth, R.; Kim, H. Characterization of a microfluidic in vitro model of the blood-brain barrier (muBBB). Lab. Chip. 2012, 12, 1784–1792. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef]
- De Palencia, P.F.; López, P.; Corbi, A.L.; Peláez, C.; Requena, T. Probiotic strains: Survival under simulated gastrointestinal conditions, in vitro adhesion to Caco-2 cells and effect on cytokine secretion. Eur. Food Res. Technol. 2008, 227, 1475–1484. [Google Scholar] [CrossRef]
- Rastall, R.A.; Gibson, G.R.; Gill, H.S.; Guarner, F.; Klaenhammer, T.R.; Pot, B.; Reid, G.; Rowland, I.R.; Sanders, M.E. Modulation of the microbial ecology of the human colon by probiotics, prebiotics and synbiotics to enhance human health: An overview of enabling science and potential applications. FEMS Microbiol. Ecol. 2005, 52, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Hickman, J. Transepithelial/endothelial Electrical Resistance (TEER) theory and applications for microfluidic body-on-a-chip devices. J. Rare Dis. Res. Treat. 2016, 1, 46–52. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Pulido, R.; Saura-Calixto, F. Antioxidant activity of fresh and processed edible seaweeds. J. Sci. Food Agric. 2001, 81, 530–534. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Yoshizawa, Y.; Tsunehiro, J.; Nomura, K.; Itoh, M.; Fukui, F.; Ametani, A.; Kaminogawa, S. In Vivo Macrophage-stimulation Activity of the Enzyme-degraded Water-soluble Polysaccharide Fraction from a Marine Alga (Gracilaria verrucosa). Biosci. Biotechnol. Biochem. 1996, 60, 1667–1671. [Google Scholar] [CrossRef]
- McDermid, K.J.; Stuercke, B.; Haleakala, O.J. Total dietary fiber content in Hawaiian marine algae. Bot. Mar. 2005, 48, 437–440. [Google Scholar] [CrossRef]
- Smit, A.J. Medicianl and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Shu, M.-H.; Appleton, D.; Zandi, K.; Abubakar, S. Anti-inflammatory, gastroprotective and anti-ulcerogenic effects of red algae Gracilaria changii (Gracilariales, Rhodophyta) extract. BMC Complement. Altern. Med. 2013, 13, 61. [Google Scholar] [CrossRef]
- Williams, J.P.; Meyers, J.A. Immune-mediated inflammatory disorders (I.M.I.D.s): The economic and clinical costs. Am. J. Manag. Care 2002, 8, 664–681. [Google Scholar]

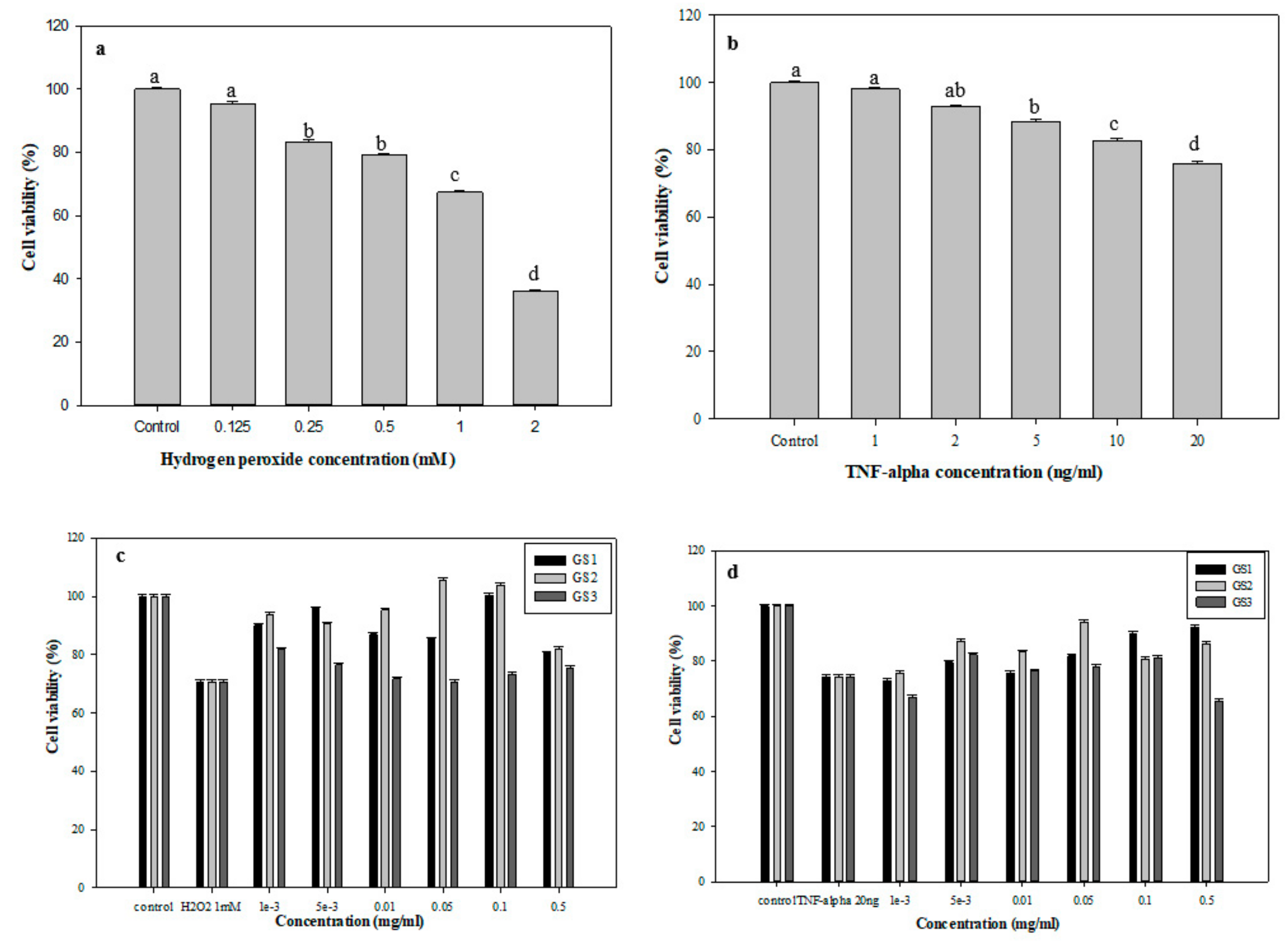
| Concentration | Control | 0.5 mM H2O2 | 0.001 | 0.005 | 0.01 | 0.05 | Control | 20 ng/mL TNF-α | 0.001 | 0.005 | 0.01 | 0.05 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GS1 | 100 | 105.4 | 84.3 | 81.1 | 67.3 | 63.3 | 100 | 124.5 | 95.2 | 103.6 | 93.0 | 106.6 |
| GS2 | 100 | 105.4 | 62.8 | 89.5 | 81.0 | 48.8 | 100 | 124.5 | 60.6 | 76.2 | 81.1 | 72.8 |
| GS3 | 100 | 105.4 | 74.1 | 66.0 | 56.2 | 68.6 | 100 | 124.5 | 78.2 | 57.7 | 63.0 | 91.3 |
| Concentration | Control | TNF-α (ng/mL) | 0.001 | 0.005 | 0.01 | 0.05 |
|---|---|---|---|---|---|---|
| GS1 | 0.34 | 1.47 | 1.13 | 1.10 | 1.77 | 1.12 |
| GS2 | 0.34 | 1.47 | 1.38 | 0.56 | 1.08 | 1.61 |
| GS3 | 0.34 | 1.47 | 1.62 | 1.62 | 1.11 | 1.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.-H.; Lu, W.-C.; Chan, Y.-J.; Zhao, Y.-P.; Nie, X.-B.; Jiang, C.-X.; Ji, Y.-X. Feasibility of Using Seaweed (Gracilaria coronopifolia) Synbiotic as a Bioactive Material for Intestinal Health. Foods 2019, 8, 623. https://doi.org/10.3390/foods8120623
Li P-H, Lu W-C, Chan Y-J, Zhao Y-P, Nie X-B, Jiang C-X, Ji Y-X. Feasibility of Using Seaweed (Gracilaria coronopifolia) Synbiotic as a Bioactive Material for Intestinal Health. Foods. 2019; 8(12):623. https://doi.org/10.3390/foods8120623
Chicago/Turabian StyleLi, Po-Hsien, Wen-Chien Lu, Yung-Jia Chan, Yu-Ping Zhao, Xiao-Bao Nie, Chang-Xing Jiang, and Yu-Xiang Ji. 2019. "Feasibility of Using Seaweed (Gracilaria coronopifolia) Synbiotic as a Bioactive Material for Intestinal Health" Foods 8, no. 12: 623. https://doi.org/10.3390/foods8120623
APA StyleLi, P.-H., Lu, W.-C., Chan, Y.-J., Zhao, Y.-P., Nie, X.-B., Jiang, C.-X., & Ji, Y.-X. (2019). Feasibility of Using Seaweed (Gracilaria coronopifolia) Synbiotic as a Bioactive Material for Intestinal Health. Foods, 8(12), 623. https://doi.org/10.3390/foods8120623






