Feasibility of Using Seaweed (Gracilaria coronopifolia) Synbiotic as a Bioactive Material for Intestinal Health
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Bacterial Strains and Culture Conditions
2.3. G. coronopifolia Synbiotic Preparations
2.4. Cell Culture
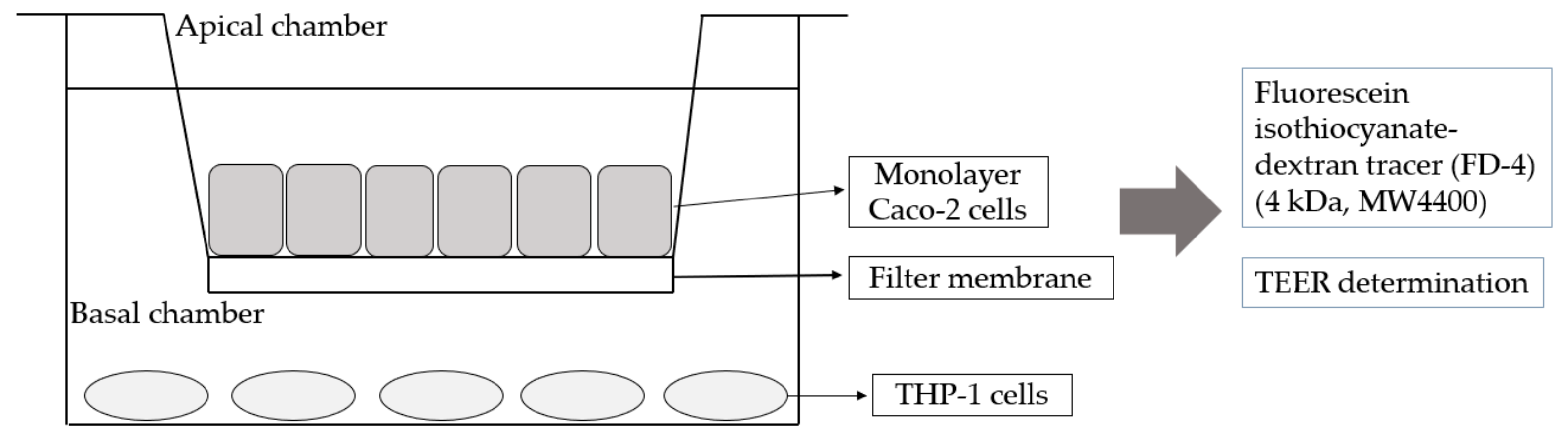
2.5. Co-Culture of Caco-2 and THP-1 Cells
2.6. Cell Viability Assay (MTT Assay)
2.7. Single-Layer Cell TEER Determination
2.8. Determining ROS Production
2.9. Interleukin-8 (IL-8) Analysis
2.10. Statistical Analysis
3. Results and Discussions
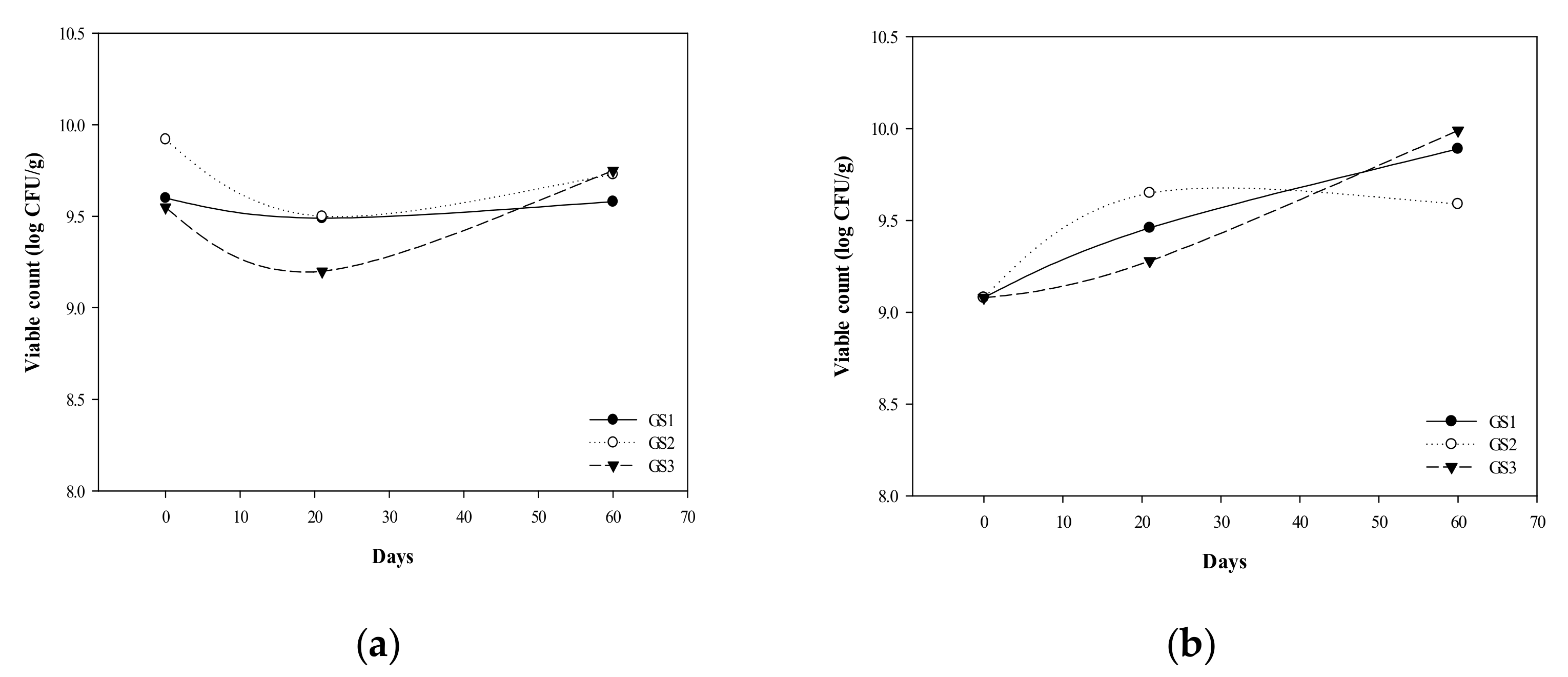
3.1. G. coronopifolia Synbiotic Synthesis and Storage Test
3.2. Survival of G. coronopifolia Synbiotics in Caco-2 Cells
3.3. Establishment of Caco-2 Cells and THP-1 Macrophage Co-Culture Mode
3.4. Effect of G. coronopifolia Synbiotics on Intestinal Monolayer Membrane
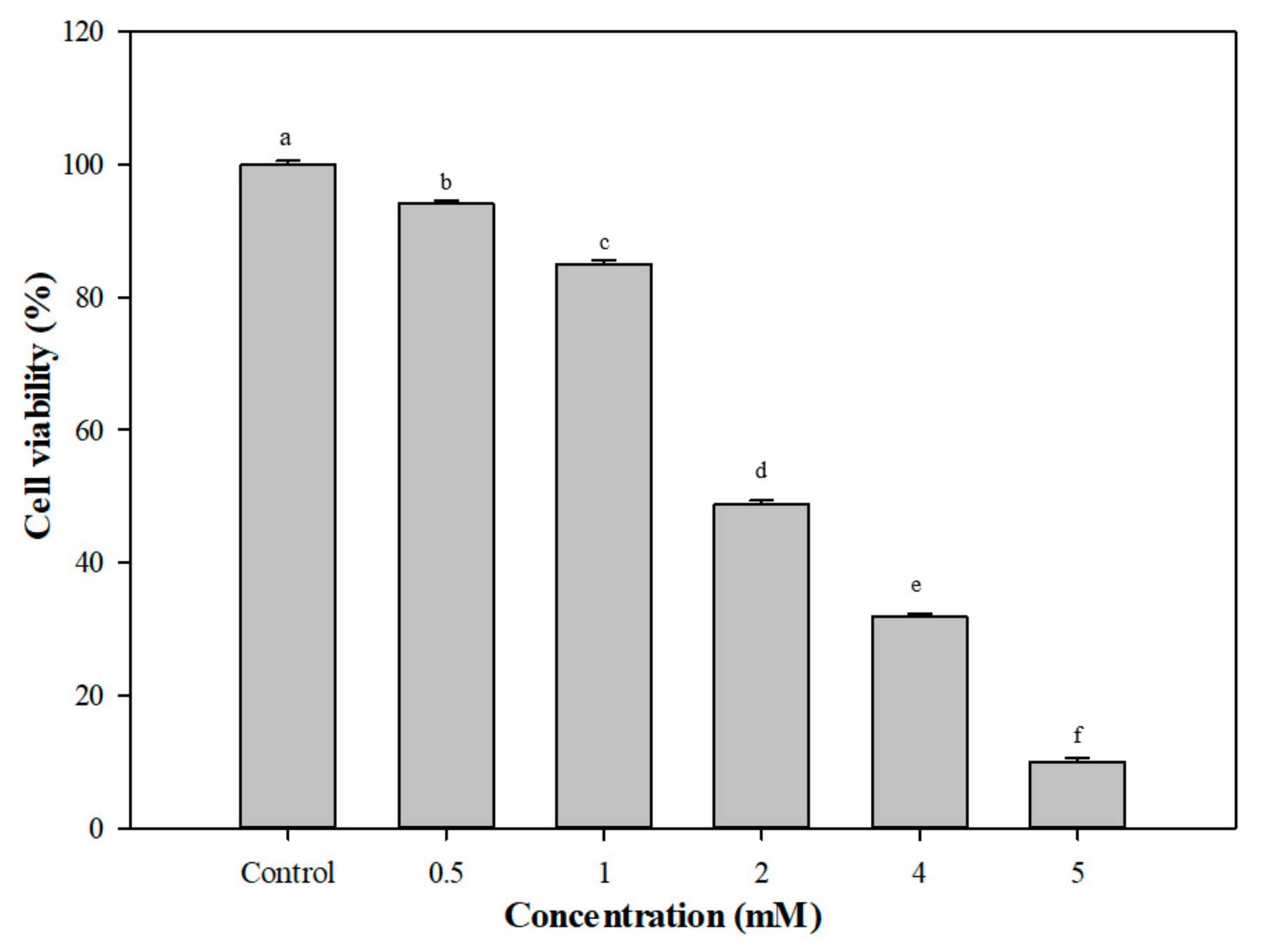
3.5. Protective Effects of G. coronopifolia Synbiotics on THP-1 and Caco-2 Cells under Oxidative Stress
3.6. Assessment of Inflammatory Factors in G. coronopifolia Synbiotic
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Al-Enazi, N.M.; Awaad, A.S.; Alqasoumi, S.I.; Alwethairi, M.F. Biological activities of the red algae Galaxaura rugosa and Liagora hawaiiana butters. Saudi Pharm. J. 2018, 26, 25–32. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, C.L.F.; Falcão, H.D.S.; Lima, G.R.D.M.; Montenegro, C.D.A.; Lira, N.S.; De Athayde-Filho, P.F.; Rodrigues, L.C.; Souza, M.D.F.V.D.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from Marine Algae of the Genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar] [CrossRef] [PubMed]
- Ratnasooriya, W.D.; Premakumara, G.A.S.; Tillekeratne, L.M.V. Post-coital contraceptive activity of crude extracts of Sri Lankan marine red algae. Contraception 1994, 50, 291–299. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chang, J.-S.; Sheu, F.; Shyu, Y.-T.; Yu, A.Y.-C.; Wong, S.-L.; Dai, C.-F.; Lee, T.-M. Seasonal growth dynamics of Laurencia papillosa and Gracilaria coronopifolia from a highly eutrophic reef in southern Taiwan: Temperature limitation and nutrient availability. J. Exp. Mar. Biol. Ecol. 2005, 315, 49–69. [Google Scholar] [CrossRef]
- Guarner, F. Enteric flora in health and disease. Digestion 2006, 73, 5–12. [Google Scholar] [CrossRef]
- Cha, K.H.; Lee, E.H.; Yoon, H.S.; Lee, J.H.; Kim, J.Y.; Kang, K.; Park, J.-S.; Jin, J.B.; Ko, G.; Pan, C.-H. Effects of fermented milk treatment on microbial population and metabolomic outcomes in a three-stage semi-continuous culture system. Food Chem. 2018, 263, 216–224. [Google Scholar] [CrossRef]
- Parvez, S.; Malik, K.; Kang, S.A.; Kim, H.-Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Agriculture Organization of the United Nations/World Health Organization. Guidelines for the Evaluation of Probiotics in Food; Report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food: London ON, Canada; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy; World Health Organization (WHO): Geneva, Switzerland, 2002. [Google Scholar]
- Levri, K.M.; Ketvertis, K.; Deramo, M.; Merenstein, J.H.; D’Amico, F. Do probiotics reduce adult lactose intolerance? A systematic review. J. Fam. Pract. 2005, 54, 613–620. [Google Scholar]
- Guandalini, S. Probiotics for children with diarrhea: An update. J. Clin. Gastroenterol. 2008, 42, S53–S57. [Google Scholar] [CrossRef]
- Forsythe, P.; Bienenstock, J. Immunomodulation by commensal and probiotic bacteria. Immunol. Investig. 2010, 39, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Chalova, V.I.; Lingbeck, J.M.; Kwon, Y.M.; Ricke, S.C. Extracellular antimutagenic activities of selected probiotic Bifidobacterium and Lactobacillus spp. as a function of growth phase. J. Environ. Sci. Health Part B 2008, 43, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.-T. Roles of Probiotics and prebiotics in colon cancer prevention: Postulated mechanisms and in-vivo evidence. Int. J. Mol. Sci. 2008, 9, 854–863. [Google Scholar] [CrossRef]
- Gustaw, W.; Kordowska-Wiater, M.; Koziol, J. The influence of selected prebiotics on the growth of lactic acid bacteria for bio-yoghurt production. Acta Sci. Pol. Technol. Aliment. 2011, 10, 455–466. [Google Scholar] [PubMed]
- Khurana, H.; Kanawjia, S. Recent trends in development of fermented milks. Curr. Nutr. Food Sci. 2007, 3, 91–108. [Google Scholar] [CrossRef]
- Shinde, T.; Perera, A.P.; Vemuri, R.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Shastri, S.; Southam, B.; Eri, R.; Stanley, R. Synbiotic Supplementation Containing Whole Plant Sugar Cane Fibre and Probiotic Spores Potentiates Protective Synergistic Effects in Mouse Model of IBD. Nutrients 2019, 11, 818. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxidative Med. Cell. Longev. 2017, 2017, 1–18. [Google Scholar] [CrossRef]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in inflammatory bowel disease: Clinical aspects and animal models. Saudi J. Gastroenterol. 2016, 22, 3–17. [Google Scholar] [CrossRef]
- Huttenhower, C.; Kostic, A.D.; Xavier, R.J. Inflammatory bowel disease as a model for translating the microbiome. Immunity 2014, 40, 843–854. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef]
- Knipp, G.T.; Ho, N.F.H.; Barsuhn, C.L.; Borchardt, R.T. Paracellular Diffusion in Caco-2 Cell Monolayers: Effect of Perturbation on the Transport of Hydrophilic Compounds That Vary in Charge and Size. J. Pharm. Sci. 1997, 86, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.S.; Chikhale, P.J.; Shah, P.K.; Borchardt, R.T. Utilization of a Human Intestinal Epithelial Cell Culture System (Caco-2) for Evaluating Cytoprotective Agents. Pharm. Res. 1993, 10, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Turgeon, S.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Quinn, E.M.; Slattery, H.; Thompson, A.P.; Kilcoyne, M.; Joshi, L.; Hickey, R.M. Mining Milk for Factors which Increase the Adherence of Bifidobacterium longum subsp. infantis to Intestinal Cells. Foods 2018, 7, 196. [Google Scholar] [CrossRef]
- Takisawa, R.; Nishitani, Y.; Mizuno, M.; Osawa, R. Anti-Inflammatory Effect of Bifidobacterium longum on Macrophage-Like THP-1 Cells via Epithelial Cell Caco-2. Biosci. Microflora 2009, 28, 45–48. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Booth, R.; Kim, H. Characterization of a microfluidic in vitro model of the blood-brain barrier (muBBB). Lab. Chip. 2012, 12, 1784–1792. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef]
- De Palencia, P.F.; López, P.; Corbi, A.L.; Peláez, C.; Requena, T. Probiotic strains: Survival under simulated gastrointestinal conditions, in vitro adhesion to Caco-2 cells and effect on cytokine secretion. Eur. Food Res. Technol. 2008, 227, 1475–1484. [Google Scholar] [CrossRef]
- Rastall, R.A.; Gibson, G.R.; Gill, H.S.; Guarner, F.; Klaenhammer, T.R.; Pot, B.; Reid, G.; Rowland, I.R.; Sanders, M.E. Modulation of the microbial ecology of the human colon by probiotics, prebiotics and synbiotics to enhance human health: An overview of enabling science and potential applications. FEMS Microbiol. Ecol. 2005, 52, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Hickman, J. Transepithelial/endothelial Electrical Resistance (TEER) theory and applications for microfluidic body-on-a-chip devices. J. Rare Dis. Res. Treat. 2016, 1, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Pulido, R.; Saura-Calixto, F. Antioxidant activity of fresh and processed edible seaweeds. J. Sci. Food Agric. 2001, 81, 530–534. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Yoshizawa, Y.; Tsunehiro, J.; Nomura, K.; Itoh, M.; Fukui, F.; Ametani, A.; Kaminogawa, S. In Vivo Macrophage-stimulation Activity of the Enzyme-degraded Water-soluble Polysaccharide Fraction from a Marine Alga (Gracilaria verrucosa). Biosci. Biotechnol. Biochem. 1996, 60, 1667–1671. [Google Scholar] [CrossRef] [Green Version]
- McDermid, K.J.; Stuercke, B.; Haleakala, O.J. Total dietary fiber content in Hawaiian marine algae. Bot. Mar. 2005, 48, 437–440. [Google Scholar] [CrossRef] [Green Version]
- Smit, A.J. Medicianl and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Shu, M.-H.; Appleton, D.; Zandi, K.; Abubakar, S. Anti-inflammatory, gastroprotective and anti-ulcerogenic effects of red algae Gracilaria changii (Gracilariales, Rhodophyta) extract. BMC Complement. Altern. Med. 2013, 13, 61. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.P.; Meyers, J.A. Immune-mediated inflammatory disorders (I.M.I.D.s): The economic and clinical costs. Am. J. Manag. Care 2002, 8, 664–681. [Google Scholar]

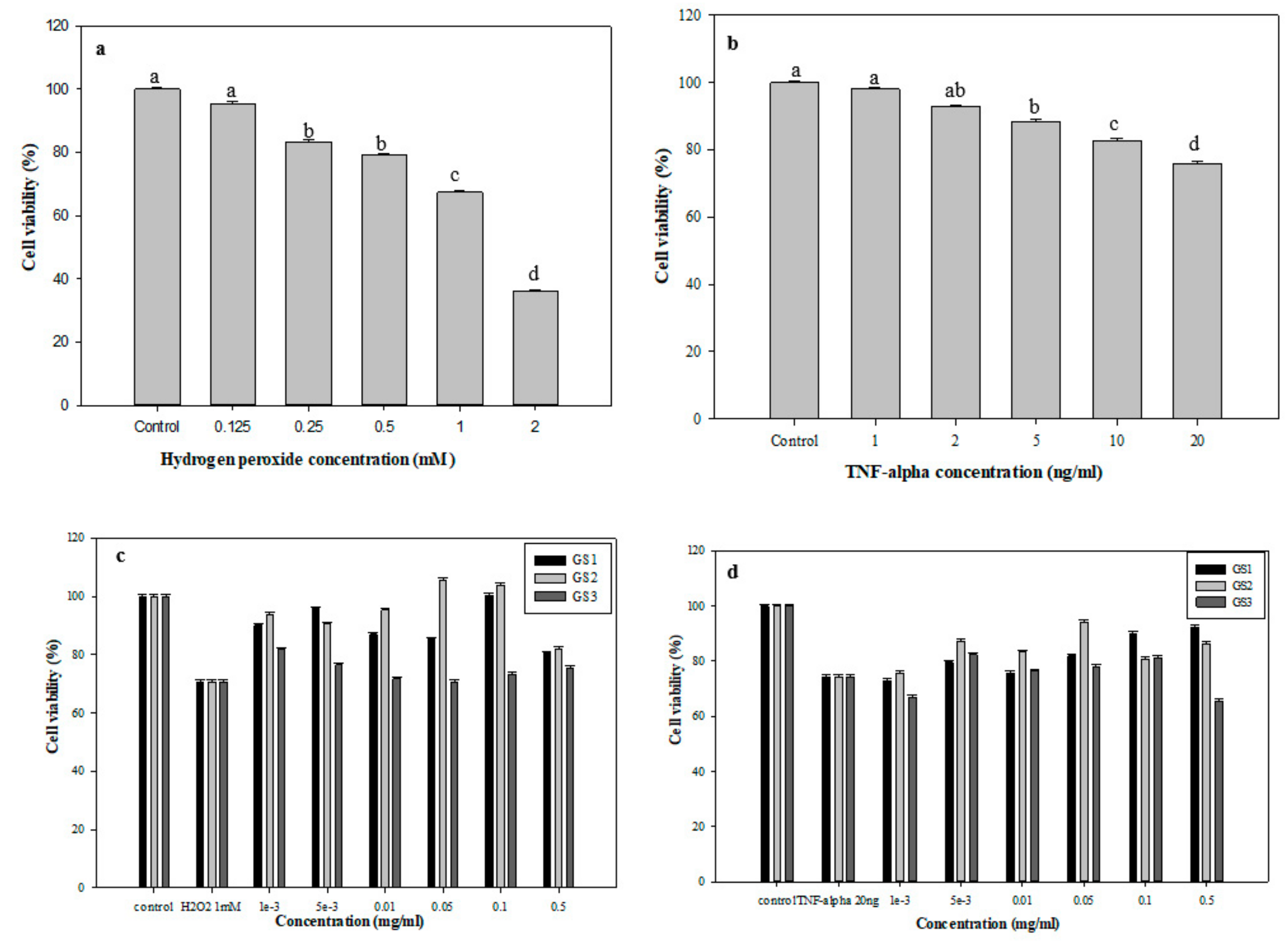
| Concentration | Control | 0.5 mM H2O2 | 0.001 | 0.005 | 0.01 | 0.05 | Control | 20 ng/mL TNF-α | 0.001 | 0.005 | 0.01 | 0.05 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GS1 | 100 | 105.4 | 84.3 | 81.1 | 67.3 | 63.3 | 100 | 124.5 | 95.2 | 103.6 | 93.0 | 106.6 |
| GS2 | 100 | 105.4 | 62.8 | 89.5 | 81.0 | 48.8 | 100 | 124.5 | 60.6 | 76.2 | 81.1 | 72.8 |
| GS3 | 100 | 105.4 | 74.1 | 66.0 | 56.2 | 68.6 | 100 | 124.5 | 78.2 | 57.7 | 63.0 | 91.3 |
| Concentration | Control | TNF-α (ng/mL) | 0.001 | 0.005 | 0.01 | 0.05 |
|---|---|---|---|---|---|---|
| GS1 | 0.34 | 1.47 | 1.13 | 1.10 | 1.77 | 1.12 |
| GS2 | 0.34 | 1.47 | 1.38 | 0.56 | 1.08 | 1.61 |
| GS3 | 0.34 | 1.47 | 1.62 | 1.62 | 1.11 | 1.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.-H.; Lu, W.-C.; Chan, Y.-J.; Zhao, Y.-P.; Nie, X.-B.; Jiang, C.-X.; Ji, Y.-X. Feasibility of Using Seaweed (Gracilaria coronopifolia) Synbiotic as a Bioactive Material for Intestinal Health. Foods 2019, 8, 623. https://doi.org/10.3390/foods8120623
Li P-H, Lu W-C, Chan Y-J, Zhao Y-P, Nie X-B, Jiang C-X, Ji Y-X. Feasibility of Using Seaweed (Gracilaria coronopifolia) Synbiotic as a Bioactive Material for Intestinal Health. Foods. 2019; 8(12):623. https://doi.org/10.3390/foods8120623
Chicago/Turabian StyleLi, Po-Hsien, Wen-Chien Lu, Yung-Jia Chan, Yu-Ping Zhao, Xiao-Bao Nie, Chang-Xing Jiang, and Yu-Xiang Ji. 2019. "Feasibility of Using Seaweed (Gracilaria coronopifolia) Synbiotic as a Bioactive Material for Intestinal Health" Foods 8, no. 12: 623. https://doi.org/10.3390/foods8120623
APA StyleLi, P.-H., Lu, W.-C., Chan, Y.-J., Zhao, Y.-P., Nie, X.-B., Jiang, C.-X., & Ji, Y.-X. (2019). Feasibility of Using Seaweed (Gracilaria coronopifolia) Synbiotic as a Bioactive Material for Intestinal Health. Foods, 8(12), 623. https://doi.org/10.3390/foods8120623






