Effects of Proteolytic and Lipolytic Enzyme Supplementations on Lipolysis and Proteolysis Characteristics of White Cheeses
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Manufacture of Experimental Turkish White Cheeses
2.2.2. Methods of Analysis
Gross Composition of Milk, Whey and Cheese Samples
Total Free Amino Acid Ratios
Total Free Fatty Acid Ratios
Free Amino Acid Composition
Mineral Analyses with Atomic Absorption Spectrophotometer
Statistical Analyses
3. Results and Discussion
3.1. Raw Milk and Whey Characteristics and Cheese Yield Performance
3.2. Gross Composition of Cheeses
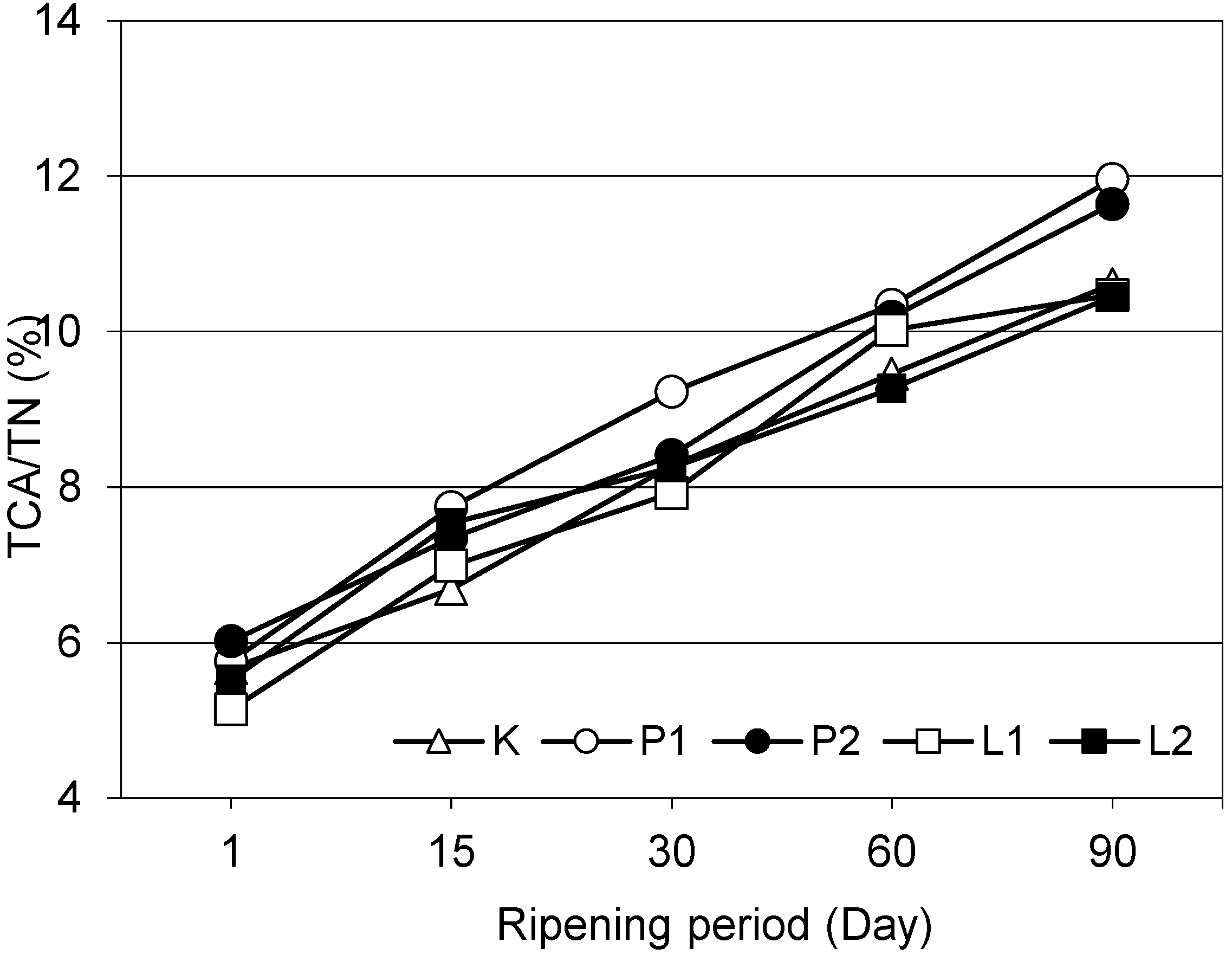
3.3. Nitrogen Fractions and Ripening Index of the Cheeses
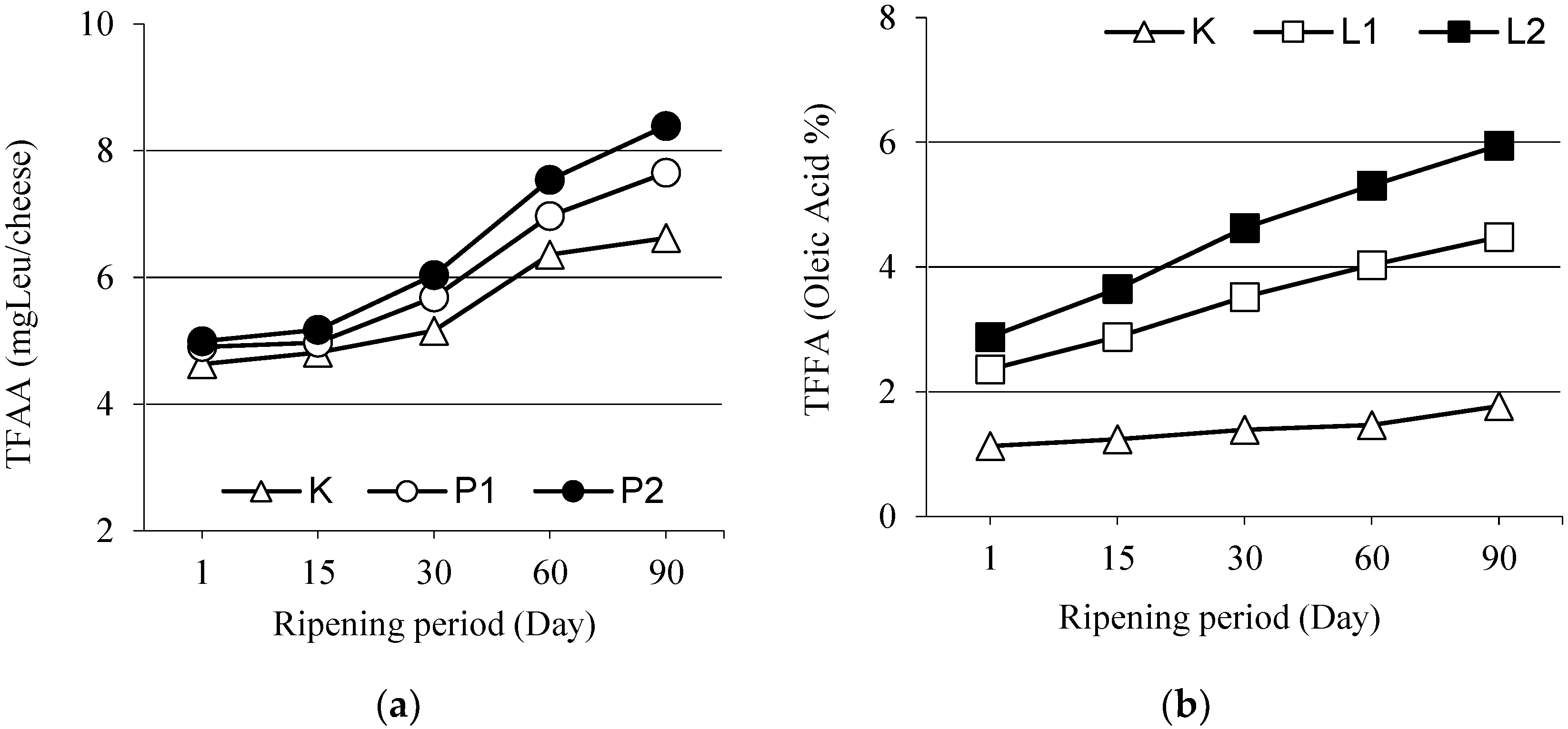
3.4. Total Free Amino Acids
3.5. Individual Free Amino Acid Composition
3.6. Total Free Fatty Acids (TFFA)
3.7. Mineral Composition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pachlová, V.; Bunka, F.; Flasarová, R.; Válková, P.; Bunková, L. The effect of elevated temperature on ripening of Dutch type cheese. Food Chem. 2012, 132, 1846–1854. [Google Scholar] [CrossRef]
- Fox, P.F. Proteolysis during cheese manufacture and ripening. J. Dairy Sci. 1989, 72, 1379–1400. [Google Scholar] [CrossRef]
- Mcsweeney, P.L.H. Biochemistry of cheese ripening. Int. J. Dairy Technol. 2004, 57, 127–144. [Google Scholar] [CrossRef]
- Poveda, J.M.; Molina, G.M.; Gómez-Alonso, S. Variability of biogenic amine and free amino acid concentrations in regionally produced goat milk cheeses. J. Food Compost. Anal. 2016, 51, 85–92. [Google Scholar] [CrossRef]
- Ceruti, R.J.; Zorrilla, S.E.; Sihufe, G.A. The influence of elevated initial ripening temperature on the proteolysis in Reggianito cheese. Food Res. Int. 2012, 48, 34–40. [Google Scholar] [CrossRef]
- McCarthy, C.M.; Kelly, P.M.; Wilkinson, M.G.; Guinee, T.P. Effect of fat and salt reduction on the changes in the concentrations of free amino acids and free fatty acids in Cheddar-style cheeses during maturation. J. Food Compost. Anal. 2017, 59, 132–140. [Google Scholar] [CrossRef]
- Giannoglou, M.; Karra, Z.; Platakou, E.; Katsaros, G.; Moatsou, G.; Taoukis, P. Effect of high pressure treatment applied on starter culture or on semi-ripened cheese in the quality and ripening of cheese in brine. Innov. Food Sci. Emerg. Technol. 2016, 38, 312–320. [Google Scholar] [CrossRef]
- Saldo, J.; McSweeney, P.L.H.; Sendra, E.; Kelly, A.L.; Guamis, B. Proteolysis in caprine milk cheese treated by high pressure to accelerate cheese ripening. Int. Dairy J. 2002, 12, 35–44. [Google Scholar] [CrossRef]
- Karami, M. Enhancing the lipolysis of feta-type cheese made from ultrafiltered cow’s milk. LWT Food Sci. Technol. 2017, 80, 386–393. [Google Scholar] [CrossRef]
- Ferrazza, R.E.; Fresno, J.M.; Ribeiro, J.I.; Tornadijo, M.E.; Furtado, M.M. Changes in the microbial flora of Zamorano cheese (P.D.O.) by accelerated ripening process. Food Res. Int. 2004, 37, 149–155. [Google Scholar] [CrossRef]
- Scolari, G.; Vescovo, M.; Sarra, P.G.; Bottazzi, V. Proteolysis in cheese made with liposome-entrapped proteolytic enzymes. Le Lait 1993, 73, 281–292. [Google Scholar] [CrossRef]
- Folkertsma, B.; Fox, P.F.; Mcsweeney, P.L.H. Accelerated ripening of cheddar cheese at elevated temperatures. Int. Dairy J. 1996, 6, 1117–1134. [Google Scholar] [CrossRef]
- Fox, P.F.; Wallace, J.M.; Morgan, S.; Lynch, C.M.; Niland, E.J.; Tobin, J. Acceleration of cheese ripening. Antonie van Leeuwenhoek 1996, 70, 271–297. [Google Scholar] [CrossRef] [PubMed]
- Law, B.A. Controlled and accelerated cheese ripening: The research base for new technologies. Int. Dairy J. 2001, 11, 383–398. [Google Scholar] [CrossRef]
- Kilcawley, K.N.; Nongonierma, A.B.; Hannon, J.A.; Doolan, I.A.; Wilkinson, M.G. Evaluation of commercial enzyme systems to accelerate Cheddar cheese ripening. Int. Dairy J. 2012, 26, 50–57. [Google Scholar] [CrossRef]
- Corsetti, A.; Gobbetti, M.; Smacchi, E.; Angelis, M.; Rossi, J. Accelerated ripening of Pecorino Umbro cheese. J. Dairy Res. 1998, 65, 631–642. [Google Scholar] [CrossRef]
- Lin, J.C.C.; Jeon, I.J. Effects of commercial food grade enzymes on free fatty acid profiles in granular cheddar cheese. J. Food Sci. 1987, 52, 78–83. [Google Scholar] [CrossRef]
- Mcsweeney, P.L.H.; Sousa, M.J. Biochemical pathways for the production of flavour compounds in cheeses during ripening: A review. Le Lait 2000, 80, 293–324. [Google Scholar] [CrossRef]
- Fernandez-Garcia, E.; Lopez-Fandino, R.; Alonso, I.; Ramos, M. The use of lipolytic and proteolytic enzymes in the manufacture of Manchego type cheese from ovine and bovine milk. J. Dairy Sci. 1994, 77, 2139–2149. [Google Scholar] [CrossRef]
- Fernandez-Garcia, E.; Lopez-Fandino, R.; Alonso, I. Effect of a food-grade enzyme preparation from aspergillus oryzae on free fatty acid release in Manchego-type cheese from ovine and bovine milk. Zeitschrift für Lebensmittel-Untersuchung und Forschung 1994, 199, 262–264. Available online: https://link.springer.com/content/pdf/10.1007%2FBF01193308.pdf (accessed on 7 May 2018). [CrossRef]
- Jarmul, J.; Reps, A.; Leman, H. Application of microbial preparation for acceleration of cheese ripening in the manufacture of tilsit type whey protein ıncorporated-cheese. Arta Academiae Agriculturae ac Technicae Olstenensis Technologia Alimentorum 1995, 28, 41–48. [Google Scholar]
- Koçak, C.; Avşar, Y.K.; Gürsel, A.; Semiz, A. Effect of lipase enzyme (Palatase A 750 L) on the ripening of Tulum cheese. Turk. J. Agric. For. 1995, 19, 171–177. [Google Scholar]
- Koçak, C.; Gürsel, A.A.; Avşar, Y.K.; Semiz, A. Effect of lipase enzyme (Palatase M 200 L) on the ripening of skin cheese. Egypt. J. Dairy Sci. 1995, 23, 43–52. [Google Scholar]
- IDF. Determination of the Total Solid Content (Cheese and Processed Cheese); IDF Standard 4A; International Dairy Federation: Brussels, Belgium, 1982. [Google Scholar]
- Anonymous. TS-1018 Çiğ İnek Sütü Standardı; Türk Standartları Enstitüsü: Ankara, Turkey, 1994; 15p. [Google Scholar]
- IDF. Milk Determination of Nitrogen Content; International Dairy Federation: Brussels, Belgium, 1993; p. 12. [Google Scholar]
- Anonymous. Gıda Maddeleri Muayene ve Analiz Yöntemleri; Genel Yayın No: 65; T. C. Tarım Orman Köy İşleri Bakanlığı, Gıda İşleri Genel Müdürlüğü: Ankara, Turkey, 1983; 796p. [Google Scholar]
- Kotterer, R.; Munch, S. Untersuchungsverfahren fur das Milchwirtschaftliche Laboratorium; Volkswirtschaftliche Verlag GmbH: Munchen, Germany, 1978; 201p. [Google Scholar]
- Anonymous. TS 591 Beyaz Peynir Standardı; Türk Standartları Enstitüsü: Ankara, Turkey, 1995; 9p. [Google Scholar]
- Kuchroo, C.N.; Fox, P.F. Soluble nitrogen in Cheddar cheese: Comparison of extraction procedures. Milchwissenschaft 1982, 37, 331–335. [Google Scholar]
- Jarrett, W.D.; Aston, J.W.; Dulley, J.R. A simple method for estimating free amino acids in Cheddar cheese. Aust. J. Dairy Technol. 1982, 37, 55–58. [Google Scholar]
- Polychroniadou, A.; Michaelidou, A.; Paschaloudis, N. Effect of time, temperature and extraction method on the trichloroacetic acid-soluble nitrogen of cheese. Int. Dairy J. 1999, 9, 559–568. [Google Scholar] [CrossRef]
- Argumosa, O.B.; Carballo, J.; Bernardo, A.; Martin, R. Chemical characterization of a Spanish artisanal goat cheese (Babai-Laciana Variety). Microbiol. Aliments Nutr. 1992, 10, 69–79. [Google Scholar]
- Folkertsma, B.; Fox, P.F. Use of cd-ninhydrin reagent to assess proteolysis in cheese during ripening. J. Dairy Res. 1992, 59, 217–224. [Google Scholar] [CrossRef]
- Nunez, M.; Garcia-Aser, C.; Rodriguez-Martin, M.A.; Medina, M.; Gaya, P. The effect of ripening and cooking temperatures on proteolysis and lipolysis in Manchego cheese. Food Chem. 1986, 21, 115–123. [Google Scholar] [CrossRef]
- Badawy, A.A.-B. The EZ: Faast family of amino acid analysis kits: Application of the GC-FID kit for rapid determination of plasma tryptophan and other amino acids. In Amino Acid Analysis. Methods in Molecular Biology (Methods and Protocols); Alterman, M., Hunziker, P., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 828, ISBN 978-1-61779-445-2. [Google Scholar]
- American Organization of Analytical Chemists (AOAC). Atomic Absorption Spectrophotometric Method; American Organization of Analytical Chemists (AOAC): Arlington, VA, USA, 1990; Volume 68, p. 514. [Google Scholar]
- Nasr, M. Acceleration of Romi cheese ripening by addition of fungal esteraz lipase powder. Egypt. J. Dairy Sci. 1983, 11, 309–315. [Google Scholar]
- El-Soda, M. Accelerated Cheese Ripening. In Encyclopedia of Dairy Science; Fuquay, J.W., Fox, P.F., Eds.; Academic Press: Paris, France, 2003; Volume 1, pp. 327–329. [Google Scholar]
- Abd El-Salam, M.H.; El-Shibiny, S.; Moneib, A.F.; Abo El-Heiba, A.; El-Khamy, A.F. Addition of lipase in the manufacture of pickled soft cheese fromwhole and skim milk powder. Egypt. J. Dairy Sci. 1981, 9, 143–149. [Google Scholar]
- Aydemir, S.; Akın, N.; Koçak, C. Effect of lipase enzyme on the ripening of white pickled cheese. J. Food Lipids 2001, 8, 205–213. [Google Scholar] [CrossRef]
- Hagrass, A.E.A.; El-Ghandour, M.A.; Hammad, Y.A.; Hofi, A.A. Production of Ras cheese from recombined milk. III. Effect of some ripening agents. Egypt. J. Dairy Sci. 1983, 11, 271–279. [Google Scholar]
- El-Soda, M.; Ezzat, N.; Hassaneın, S.; El-Abassy, F.; Wahba, A. Acceleration of Ras cheese ripening. 1. Addition of commercial enzymes preparation. Egypt. J. Dairy Sci. 1990, 18, 171–178. [Google Scholar]
- El-Shazly, A.; El-Tahra, M.A.; El-Din, M.M.Z.; Guıoda, M.S. Effect of Using some lipolytic enzymes on the chemical composition and sensory evaluation of Roquefort cheese. Egypt. J. Food Sci. 1995, 22, 129–137. [Google Scholar]
- Koçak, C.; Bitlis, A.; Gürsel, A.; Avşar, Y.K. Effect of added fungal lipase on the ripening of Kasar cheese. Milchwissenschaft 1996, 51, 13–17. [Google Scholar]
- Ezzat, N. Accelerated ripening of Ras cheese with a commercial proteinase and intracelluler enzymes from Lactobacillus delbrueckii subsp bulgaricus, Propionibacterium freudenreichii and Brevibacterium linens. Le Lait 1990, 70, 459–466. [Google Scholar] [CrossRef][Green Version]
- Bitlis, A. Lipaz Enziminin (Palatase M 200 L) Kaşar Peynirinin Olgunlaşması Üzerine Etkisi. Yüksek Lisans Tezi, Fen Bilimleri Enstitüsü, Ankara Üniversitesi, Ankara, Turkey, 1992; 90p. [Google Scholar]
- Nunez, M.; Guillen, A.M.; Rodriguez-Martin, M.A.; Marcilla, A.M.; Gaya, P.; Medina, M. Accelerated ripening of ewes’ milk Manchego cheese: The effect of neutral proteinases. J. Dairy Sci. 1991, 74, 4108–4118. [Google Scholar] [CrossRef]
- Kheadr, E.E.; Vuıllemard, J.C.; El-Deeb, S.A. Acceleration of Cheddar cheese lipolysis by using liposome-entrapped lipases. J. Food Sci. 2002, 67, 485–491. [Google Scholar] [CrossRef]
- Hannon, J.A.; Wilkinson, M.G.; Delahunty, C.M.; Wallace, J.M.; Morrissey, P.A.; Beresford, T.P. Use of autolytic starter systems to accelerate the ripening of Cheddar cheese. Int. Dairy J. 2003, 13, 313–323. [Google Scholar] [CrossRef]
- Karaca, O.B.; Güven, M. Effect of Ripening Temperatures and Microbial Enzymes on Proteolysis in White Pickled Cheese. In Proceedings of the 3rd International Symposium on “Traditional Foods from Adriatic to Caucasus”, Sarajevo, Bosnia and Herzegovina, 1–4 October 2015; 183p. [Google Scholar]
- Valsamaki, K.; Michaelidou, A.; Polychroniadou, A. Biogenic amine production in Feta cheese. Food Chem. 2000, 71, 259–266. [Google Scholar] [CrossRef]
- Gaya, P.; Medina, M.; Rodriguez-Marin, M.A.; Nunez, M. Accelerated ripening of ewes’ milk Manchego cheese: The effect of elevated ripening temperatures. J. Dairy Sci. 1990, 73, 26–32. [Google Scholar] [CrossRef]
- Güven, M.; Karaca, O.B. Proteolysis levels of White cheeses salted and ripened in brines prepared from various salts. Int. J. Dairy Technol. 2001, 54, 29–33. [Google Scholar] [CrossRef]
- Cinbaş, T.; Kılıç, M. Proteolysis and lipolysis in White cheeses manufactured by two different production methods. Int. J. Food Sci. Technol. 2006, 41, 530–537. [Google Scholar] [CrossRef]
- Picon, A.; Gaya, P.; Medina, M.; Nunez, M. The effect of liposome-encapsulated bacillus subtilis neutral proteinase on Manchego cheese ripening. J. Dairy Sci. 1995, 78, 1238–1247. [Google Scholar] [CrossRef]
- Hayashi, K.; Revell, D.F.; Law, B.A. Effect of partially purified extracellular serine proteinases produced by Brevibacterium Linens on the accelerated ripening of Cheddar cheese. J. Dairy Sci. 1990, 73, 579–583. [Google Scholar] [CrossRef]
- Law, B.A.; Wigmore, A.S. Accelerated cheese ripening with food grade proteinases. J. Dairy Res. 1982, 49, 137–146. [Google Scholar] [CrossRef]
- Lin, J.C.C.; Jeon, I.J.; Roberts, H.A.; Milliken, G.A. Effect of commercial food grade enzymes on proteolysis and textural changes in granular Cheddar cheese. J. Food Sci. 1987, 52, 620–625. [Google Scholar] [CrossRef]
- Kandarakis, I.; Moatsou, G.; Georgala, A.I.K.; Kaminarides, S.; Anifantakis, E. Effect of draining temperature on the biochemical characteristics of Feta cheese. Food Chem. 2001, 72, 369–378. [Google Scholar] [CrossRef]
- Awad, S. Texture and flavour development in Ras cheese made from raw and pasteurised milk. Food Chem. 2006, 97, 394–400. [Google Scholar] [CrossRef]
- Skeie, S.; Feten, G.; Almøy, T.; Østlie, H.; Isaksson, T. The use of near infrared spectroscopy to predict selected free amino acids during cheese ripening. Int. Dairy J. 2006, 16, 236–242. [Google Scholar] [CrossRef]
- Diana, M.; Rafecas, M.; Arco, C.; Quilez, J. Free amino acid profile of Spanish artisanal cheeses: Importance of gamma-aminobutyric acid (GABA) and ornithine content. J. Food Compos. Anal. 2014, 35, 94–100. [Google Scholar] [CrossRef]
- Dinkçi, N. Mucor miehei’den Elde Edilen Lipaz (Piccantase A) Enziminin Beyaz Peynirin Olgunlaşmasında Kullanılması Üzerine Araştırmalar. Yüksek Lisans Tezi, Fen Bilimleri Enstitüsü, Ege Üniversitesi, İzmir, Turkey, 1999; 119p. [Google Scholar]
- Gambelli, L.; Belloni, P.; Ingrao, G.; Pizzoferrato, L.; Santaroni, G.P. Minerals and Trace Elements in Some Italian Dairy Products. J. Food Compos. Anal. 1999, 12, 27–35. [Google Scholar] [CrossRef]
| Properties | Raw Cow Milk | Whey | ||||
|---|---|---|---|---|---|---|
| C * | P1 | P2 | L1 | L2 | ||
| pH | 6.61 ± 0.01 | 5.63 ± 0.02 a | 5.62 ± 0.03 a | 5.62 ± 0.02 a | 5.67 ± 0.10 a | 5.69 ± 0.10 a |
| TA ** | 6.69 ± 0.02 | 8.74 ± 0.12 a | 8.71 ± 0.08 a | 8.75 ± 0.09 a | 8.32 ± 0.81 a | 8.26 ± 0.67 a |
| DM ** (%) | 11.23 ± 0.10 | 6.52 ± 0.08 a | 6.55 ± 0.09 a | 6.64 ± 0.08 a | 6.56 ± 0.10 a | 6.64 ± 0.08 a |
| Fat (%) | 3.20 ± 0.00 | 0.25 ± 0.03 b | 0.30 ± 0.00 a | 0.30 ± 0.00 a | 0.26 ± 0.05 b | 0.30 ± 0.00 a |
| Protein (%) | 3.13 ± 0.02 | 0.91 ± 0.01 a | 0.91 ± 0.02 a | 0.90 ± 0.02 a | 0.91 ± 0.01 a | 0.91 ± 0.01 a |
| Lactose (%) | 4.37 ± 0.06 | 4.27 ± 0.21 a | 4.34 ± 0.28 a | 4.49 ± 0.14 a | 4.25 ± 0.13 a | 4.32 ± 0.10 a |
| C * | P1 | P2 | L1 | L2 | |
|---|---|---|---|---|---|
| Yield (%) | 14.02 ± 0.31 a | 13.26 ± 0.32 b | 12.97 ± 0.22 b | 13.24 ± 0.63 b | 12.99 ± 0.42 b |
| Yield (%40) | 15.53 ± 0.36 a | 14.79 ± 0.14 ab | 14.20 ± 0.21 b | 14.49 ± 0.68 b | 14.33 ± 0.45 b |
| Day | 1 | 30 | 60 | 90 | |
|---|---|---|---|---|---|
| Dry matter (%) | C * | 44.28 ± 0.101C a | 45.43 ± 0.498B b | 45.94 ± 0.635B a | 46.93 ± 0.257A a |
| P1 | 44.63 ± 0.666B a | 46.27 ± 0.342A ab | 46.75 ± 0.723A a | 47.19 ± 0.732A a | |
| P2 | 43.80 ± 0.810B a | 46.90 ± 0.580A a | 46.49 ± 0.897A a | 47.48 ± 0.622A a | |
| L1 | 43.76 ± 0.042C a | 45.42 ± 0.605B b | 47.21 ± 1.257A a | 45.62 ± 0.222B b | |
| L2 | 44.12 ± 0.055B a | 46.69 ± 0.298A a | 46.26 ± 0.573A a | 46.71 ± 0.361A a | |
| Fat (%) | C | 22.00 ± 0.000C a | 22.92 ± 0.144BC a | 23.75 ± 0.661AB a | 24.08 ± 0.144A a |
| P1 | 22.58 ± 0.520B a | 23.33 ± 0.382B a | 24.58 ± 0.804A a | 24.67 ± 0.577A a | |
| P2 | 21.50 ± 0.500Ca | 23.42 ± 0.382B a | 24.08 ± 0.629AB a | 24.83 ± 0.520A a | |
| L1 | 21.83 ± 0.289B a | 22.83 ± 0.289AB a | 23.83 ± 0.764A a | 23.42 ± 0.382A a | |
| L2 | 22.00 ± 0.000B a | 23.08 ± 0.144A a | 23.25 ± 0.250A a | 23.58 ± 0.382A a | |
| Protein (%) | C | 17.39 ± 0.280A a | 18.11 ± 0.604A b | 18.11 ± 0.436A a | 18.39 ± 0.111A ab |
| P1 | 17.32 ± 0.346B a | 18.35 ± 0.066A b | 18.19 ± 0.086A a | 18.11 ± 0.053A bc | |
| P2 | 17.38 ± 0.108C a | 19.37 ± 0.217A a | 18.31 ± 0.065B a | 17.71 ± 0.456C c | |
| L1 | 17.53 ± 0.260B a | 18.26 ± 0.120B b | 19.06 ± 0.702A a | 17.88 ± 0.188B bc | |
| L2 | 17.42 ± 0.163C a | 19.23 ± 0.423A a | 18.34 ± 0.038B a | 18.70 ± 0.401AB a | |
| Salt (%) | C | 2.24 ± 0.025A b | 2.13 ± 0.066AB a | 1.87 ± 0.179BC ab | 1.67 ± 0.299C a |
| P1 | 2.15 ± 0.010A c | 1.94 ± 0.017AB c | 1.69 ± 0.144B b | 1.89 ± 0.221B a | |
| P2 | 2.53 ± 0.047A a | 2.09 ± 0.100B ab | 1.65 ± 0.112C b | 1.96 ± 0.291B a | |
| L1 | 1.79 ± 0.068BC e | 2.08 ± 0.012A ab | 1.74 ± 0.105C b | 1.95 ± 0.166AB a | |
| L2 | 1.93 ± 0.050A d | 2.02 ± 0.032A bc | 2.08 ± 0.085A a | 2.06 ± 0.125A a | |
| PV ** | C | 68.83 ± 0.289A a | 58.00 ± 5.196BC a | 61.67 ± 0.577B a | 63.00 ± 0.500AB c |
| P1 | 55.50 ± 0.866C d | 53.17 ± 0.289D bc | 58.67 ± 0.289B b | 63.83 ± 0.289A c | |
| P2 | 58.83 ± 1.756B c | 49.17 ± 0.764C c | 58.17 ± 0.577B b | 68.33 ± 0.577A b | |
| L1 | 60.00 ± 0.000B c | 54.00 ± 0.866D ab | 58.33 ± 1.527C b | 70.17 ± 0.289A a | |
| L2 | 63.17 ± 0.289B b | 50.67 ± 0.764D bc | 59.33 ± 0.764C b | 70.17 ± 0.577A a | |
| TA ** (LA%) | C | 2.02 ± 0.035C a | 2.26 ± 0.040B a | 2.38 ± 0.097AB a | 2.51 ± 0.076A a |
| P1 | 2.06 ± 0.104C a | 2.23 ± 0.040BC a | 2.39 ± 0.147AB a | 2.52 ± 0.102A a | |
| P2 | 2.02 ± 0.015C a | 2.24 ± 0.031B a | 2.28 ± 0.065B a | 2.41 ± 0.000A a | |
| L1 | 2.03 ± 0.064D a | 2.30 ± 0.096BC a | 2.44 ± 0.006AB a | 2.47 ± 0.127A a | |
| L2 | 2.05 ± 0.146B a | 2.34 ± 0.038A a | 2.40 ± 0.029A a | 2.46 ± 0.031A a |
| Day | 1 | 15 | 30 | 60 | 90 | |
|---|---|---|---|---|---|---|
| WSN ** (%) | C * | 0.193 ± 0.003E c | 0.268 ± 0.005D b | 0.306 ± 0.004C c | 0.362 ± 0.009B b | 0.428 ± 0.013A b |
| P1 | 0.216 ± 0.005E ab | 0.288 ± 0.003D a | 0.329 ± 0.004C b | 0.404 ± 0.005B a | 0.462 ± 0.003A a | |
| P2 | 0.229 ± 0.009E a | 0.289 ± 0.012D a | 0.350 ± 0.011C a | 0.391 ± 0.015B a | 0.451 ± 0.014A a | |
| L1 | 0.196 ± 0.011D c | 0.268 ± 0.001C b | 0.304 ± 0.018B c | 0.395 ± 0.006A a | 0.393 ± 0.010A c | |
| L2 | 0.209 ± 0.014E bc | 0.272 ± 0.001D b | 0.335 ± 0.003C ab | 0.364 ± 0.017B b | 0.405 ± 0.001A c | |
| TCA-SN (%) | C | 0.154 ± 0.005E ab | 0.188 ± 0.013D a | 0.235 ± 0.003C bc | 0.269 ± 0.001B b | 0.306 ± 0.011A b |
| P1 | 0.156 ± 0.002E ab | 0.205 ± 0.009D a | 0.240 ± 0.005C bc | 0.295 ± 0.005B a | 0.339 ± 0.002A a | |
| P2 | 0.164 ± 0.012E a | 0.202 ± 0.010D a | 0.255 ± 0.003C a | 0.293 ± 0.007B a | 0.323 ± 0.003A a | |
| L1 | 0.141 ± 0.006D c | 0.184 ± 0.003C a | 0.227 ± 0.001B c | 0.299 ± 0.010A a | 0.294 ± 0.012A b | |
| L2 | 0.151 ± 0.004D bc | 0.205 ± 0.012C a | 0.249 ± 0.017B ab | 0.267 ± 0.001B b | 0.306 ± 0.010A b | |
| PTA-SN (%) | C | 0.051 ± 0.001E a | 0.054 ± 0.001D bc | 0.062 ± 0.001C a | 0.078 ± 0.002B bc | 0.083 ± 0.001A ab |
| P1 | 0.047 ± 0.001E b | 0.054 ± 0.001D bc | 0.064 ± 0.004C a | 0.080 ± 0.002B ab | 0.086 ± 0.002A a | |
| P2 | 0.044 ± 0.001D c | 0.058 ± 0.002C a | 0.060 ± 0.002C a | 0.076 ± 0.001B c | 0.086 ± 0.001A a | |
| L1 | 0.042 ± 0.001D d | 0.053 ± 0.001C cd | 0.056 ± 0.001B b | 0.081 ± 0.003A a | 0.080 ± 0.002A b | |
| L2 | 0.046 ± 0.001C bc | 0.055 ± 0.001B b | 0.055 ± 0.001B b | 0.077 ± 0.002A c | 0.079 ± 0.004A b | |
| CN (%) | C | 2.53 ± 0.050A a | 2.53 ± 0.021A a | 2.53 ± 0.098A b | 2.48 ± 0.061A a | 2.45 ± 0.023A ab |
| P1 | 2.50 ± 0.056A a | 2.36 ± 0.020C c | 2.55 ± 0.012A b | 2.45 ± 0.015B a | 2.38 ± 0.010C bc | |
| P2 | 2.49 ± 0.021B a | 2.46 ± 0.036B ab | 2.69 ± 0.032A a | 2.48 ± 0.021B a | 2.33 ± 0.070C c | |
| L1 | 2.55 ± 0.042A a | 2.36 ± 0.010B c | 2.56 ± 0.035A b | 2.59 ± 0.110A a | 2.41 ± 0.030B bc | |
| L2 | 2.52 ± 0.036B a | 2.44 ± 0.087B bc | 2.68 ± 0.065A a | 2.51 ± 0.026B a | 2.53 ± 0.064B a | |
| CN/TN (%) | C | 92.92 ± 0.050A a | 90.42 ± 0.121B a | 89.22 ± 0.474C a | 87.25 ± 0.065D a | 85.14 ± 0.471E b |
| P1 | 92.06 ± 0.321A bc | 89.13 ± 0.191B c | 88.57 ± 0.115C a | 85.85 ± 0.220D c | 83.74 ± 0.128E c | |
| P2 | 91.59 ± 0.356A c | 89.47 ± 0.472B bc | 88.49 ± 0.311C a | 86.39 ± 0.525D bc | 83.76 ± 0.616E c | |
| L1 | 92.86 ± 0.411A a | 89.79 ± 0.040B b | 89.38 ± 0.642B a | 86.78 ± 0.447C ab | 85.97 ± 0.375D a | |
| L2 | 92.34 ± 0.578A ab | 89.98 ± 0.341B ab | 88.87 ± 0.321C a | 87.33 ± 0.624D a | 86.19 ± 0.250E a | |
| PPN (%) | C | 0.038 ± 0.003C a | 0.081 ± 0.015B a | 0.071 ± 0.008B a | 0.094 ± 0.009B a | 0.123 ± 0.020A ab |
| P1 | 0.059 ± 0.003D a | 0.083 ± 0.006C a | 0.063 ± 0.006D a | 0.109 ± 0.006B a | 0.122 ± 0.002A ab | |
| P2 | 0.065 ± 0.020C a | 0.088 ± 0.013BC a | 0.094 ± 0.009B a | 0.098 ± 0.017B a | 0.128 ± 0.010A a | |
| L1 | 0.055 ± 0.010B a | 0.084 ± 0.004A a | 0.077 ± 0.019A a | 0.096 ± 0.009A a | 0.100 ± 0.014A b | |
| L2 | 0.058 ± 0.015C a | 0.067 ± 0.011BC a | 0.087 ± 0.014AB a | 0.098 ± 0.016A a | 0.099 ± 0.009Ab | |
| PPN/TN (%) | C | 1.40 ± 0.123C a | 2.89 ± 0.530B a | 2.50 ± 0.325B a | 3.29 ± 0.235B a | 4.25 ± 0.709A ab |
| P1 | 2.19 ± 0.146D a | 3.13 ± 0.244C a | 2.20 ± 0.195D a | 3.82 ± 0.214B a | 4.30 ± 0.081A ab | |
| P2 | 2.39 ± 0.760E a | 3.18 ± 0.447C a | 3.10 ± 0.271D a | 3.42 ± 0.621B a | 4.60 ± 0.392A a | |
| L1 | 2.00 ± 0.351C a | 3.21 ± 0.139AB a | 2.70 ± 0.649BC a | 3.20 ± 0.190AB a | 3.56 ± 0.517A bc | |
| L2 | 2.14 ± 0.594B a | 2.48 ± 0.437AB a | 2.88 ± 0.471AB a | 3.40 ± 0.572A a | 3.36 ± 0.368A c |
| Amino Acids | 30 Days | 90 Days | ||||
|---|---|---|---|---|---|---|
| C * | P1 | P2 | C | P1 | P2 | |
| Alanine (Ala) | 59 ± 1 a | 60 ± 5 a | 72 ± 6 a | 70 ± 3 a | 76 ± 9 a | 75 ± 3 a |
| Glycine (Gly) | 38 ± 5 a | 37 ± 4 a | 44 ± 3 a | 42 ± 3 a | 42 ± 9 a | 42 ± 4 a |
| Valine (Val) | 137 ± 19 a | 128 ± 13 a | 143 ± 21 a | 150 ± 11 a | 182 ± 26 a | 176 ± 12 a |
| Leucine (Leu) | 224 ± 24 a | 219 ± 23 a | 250 ± 9 a | 245 ± 11 a | 282 ± 39 a | 271 ± 6 a |
| Isoleucine (Ile) | 116 ± 12 a | 98 ± 6 a | 108 ± 14 a | 122 ± 6 a | 149 ± 25 a | 145 ± 1 a |
| Threonine (Thr) | 78 ± 9 a | 85 ± 6 a | 97 ± 6 a | 89 ± 13 a | 102 ± 20 a | 95 ± 1 a |
| Serine (Ser) | 86 ± 17 a | 104 ± 8 a | 128 ± 12 a | 112 ± 4 a | 107 ± 10 a | 110 ± 2 a |
| Proline (Pro) | 217 ± 19 b | 238 ± 9 a | 252 ± 26 a | 234 ± 9 b | 269 ± 10 a | 273 ± 9 a |
| Aspartate (Asp) | 180 ± 40 a | 170 ± 6 a | 253 ± 29 b | 192 ± 13 b | 239 ± 49 b | 349 ± 28 a |
| Methionine (Met) | 29 ± 3 a | 29 ± 2 a | 25 ± 1 a | 29 ± 1 b | 51 ± 8 a | 55 ± 12 a |
| Glutamate (Glu) | 279 ± 11 b | 271 ± 22 b | 369 ± 32 a | 327 ± 21 b | 369 ± 27 ab | 374 ± 28 a |
| Phenylalanine (Phe) | 118 ± 13 a | 130 ± 8 a | 133 ± 5 a | 140 ± 9 a | 153 ± 27 a | 151 ± 1 a |
| Lysine (Lys) | 141 ± 12 a | 142 ± 10 a | 169 ± 15 a | 152 ± 8 a | 152 ± 9 a | 162 ± 2 a |
| Histidine (His) | 41 ± 2 a | 45 ± 2 a | 44 ± 6 a | 39 ± 0 c | 45 ± 2 b | 50 ± 2 a |
| Tyrosine (Tyr) | 101 ± 5 a | 100 ± 6 a | 107 ± 9 a | 104 ± 2 a | 111 ± 4 a | 115 ± 2 a |
| Mineral | Day | C * | P1 | P2 | L1 | L2 |
|---|---|---|---|---|---|---|
| Ca | 30 | 726 ± 01 ab | 687 ± 19 b | 699 ± 40 b | 723 ± 37 ab | 764 ± 17 a |
| 90 | 669 ± 16 bc | 646 ± 33 cd | 631 ± 13 d | 698 ± 10 ab | 718 ± 18 a | |
| P | 30 | 698 ± 11 a | 645 ± 09 a | 677 ± 16 a | 706 ± 18 a | 713 ± 08 a |
| 90 | 671 ± 03 ab | 641 ± 05 c | 631 ± 12 bc | 646 ± 28 bc | 704 ± 10 a | |
| Na | 30 | 1968 ± 02 a | 1728 ± 26 b | 1933 ± 40 a | 1928 ± 89 a | 1665 ± 14 b |
| 90 | 1860 ± 25 a | 1560 ± 36 d | 1724 ± 83 b | 1704 ± 30 bc | 1612 ± 61 cd | |
| K | 30 | 253 ± 05 ab | 253 ± 16 c | 231 ± 06 b | 233 ± 09 a | 226 ± 21 a |
| 90 | 188 ± 17 b | 161 ± 04 c | 183 ± 10 c | 173 ± 13 bc | 209 ± 15 a | |
| Mg | 30 | 210 ± 08 a | 211 ± 07 a | 204 ± 07 a | 209 ± 06 a | 209 ± 02 a |
| 90 | 179 ± 03 a | 179 ± 09 a | 175 ± 04 a | 185 ± 07 a | 189 ± 05 a | |
| Zn | 30 | 4.63 ± 0.070 a | 4.02 ± 0.030 d | 4.43 ± 0.060 b | 4.09 ± 0.069 d | 4.33 ± 0.023 c |
| 90 | 3.95 ± 0.040 a | 4.00 ± 0.047 a | 3.80 ± 0.072 b | 3.83 ± 0.017 b | 4.00 ± 0.055 a | |
| Fe | 30 | 0.407 ± 0.038 a | 0.389 ± 0.017 a | 0.385 ± 0.011 a | 0.391 ± 0.002 a | 0.365 ± 0.028 a |
| 90 | 0.379 ± 0.015 ab | 0.361 ± 0.017 b | 0.393 ± 0.009 a | 0.390 ± 0.015 a | 0.363 ± 0.004 b | |
| Cu | 30 | 0.107 ± 0.011 a | 0.065 ± 0.005 b | 0.070 ± 0.009 b | 0.078 ± 0.014 b | 0.073 ± 0.001 b |
| 90 | 0.110 ± 0.001 a | 0.067 ± 0.002 b | 0.069 ± 0.007 b | 0.071 ± 0.010 b | 0.069 ± 0.005 b | |
| Mn | 30 | 0.058 ± 0.001 a | 0.056 ± 0.005 a | 0.055 ± 0.004 a | 0.055 ± 0.001 a | 0.057 ± 0.002 a |
| 90 | 0.053 ± 0.006 a | 0.045 ± 0.002 a | 0.047 ± 0.000 a | 0.044 ± 0.004 a | 0.048 ± 0.001 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaca, O.B.; Güven, M. Effects of Proteolytic and Lipolytic Enzyme Supplementations on Lipolysis and Proteolysis Characteristics of White Cheeses. Foods 2018, 7, 125. https://doi.org/10.3390/foods7080125
Karaca OB, Güven M. Effects of Proteolytic and Lipolytic Enzyme Supplementations on Lipolysis and Proteolysis Characteristics of White Cheeses. Foods. 2018; 7(8):125. https://doi.org/10.3390/foods7080125
Chicago/Turabian StyleKaraca, Oya Berkay, and Mehmet Güven. 2018. "Effects of Proteolytic and Lipolytic Enzyme Supplementations on Lipolysis and Proteolysis Characteristics of White Cheeses" Foods 7, no. 8: 125. https://doi.org/10.3390/foods7080125
APA StyleKaraca, O. B., & Güven, M. (2018). Effects of Proteolytic and Lipolytic Enzyme Supplementations on Lipolysis and Proteolysis Characteristics of White Cheeses. Foods, 7(8), 125. https://doi.org/10.3390/foods7080125




