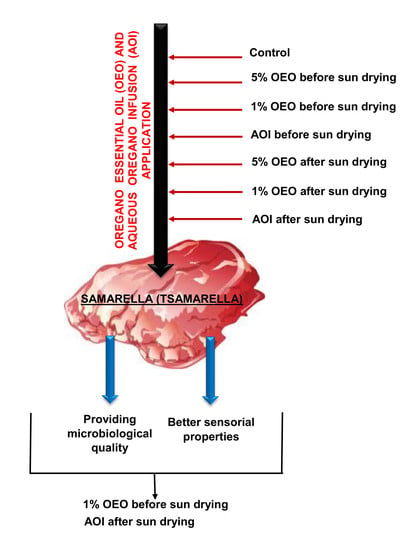
Effect of Oregano Essential Oil and Aqueous Oregano Infusion Application on Microbiological Properties of Samarella (Tsamarella), a Traditional Meat Product of Cyprus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Oregano Infusion and Oregano Essential Oil
2.2. Experimental Production of Samarella
2.3. Microbiological Analysis
2.4. Physicochemical Analysis
2.5. Sensorial Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Temelli, S. Geleneksel yöntemlerle üretilen kurutulmuş et ürünleri. J. Fac. Vet. Med./Vet. Fak. Derg. 2011, 30, 61–66. [Google Scholar] [CrossRef]
- Purriños, L.; GarcíaFontán, M.C.; Carballo, J.; Lorenzo, J. Study of the counts, species and characteristics of the yeast population during the manufacture of dry-cured “lacón” effect of salt level. Food Microbiol. 2013, 34, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Cocolin, L.; Urso, R.; Rantsiou, K.; Cantoni, C.; Comi, G. Dynamics and characterization of yeasts during natural fermentation of Italian sausages. FEMS Yeast Res. 2006, 6, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, L.M.; Jacobsen, T.; Nielsen, P.V.; Frisvad, J.C.; Koch, A.G. Mycobiota in the processing areas of two different meat products. Int. J. Food Microbiol. 2008, 124, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Sonjak, S.; Licen, M.; Frisvad, J.C.; Gunde-Cimerman, N.G. The mycobiota of three dry-cured meat products from Slovenia. Food Microbiol. 2011, 28, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Hakeri, B.H. Kıbrıs Türkçesi Sözlüğü; SAMTAY VakfıYayınları; KKTC: Gazimağusa, Northern Cyprus, 2003. [Google Scholar]
- Kabataş, O. Kıbrıs Türkçesinin Etimilojik Sözlüğü; Öncü Basımevi: Ankara, Turkey, 2007. [Google Scholar]
- Yorgancıoğlu, O.M. Kıbrıs Türk Folkloru “Duydum, Gördüm, Yazdım”; Cambulat Basımevi: Lefkoşa, Cyprus, 2000. [Google Scholar]
- Slow Food Foundation for Biodiversity. Available online: http://www.fondazioneslowfood.com/en/slow-food-presidia/tsamarella/ (accessed on 6 May 2016).
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Khanjari, A.; Karabagias, I.K.; Kontominas, M.G. Combined effect of N,O-carboxymethyl chitosan and oregano essential oil to extend shelf life and control Listeria monocytogenes in raw chicken meat fillets. Food Sci. Technol. 2013, 53, 94–99. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific opinion of the panel on the use of oregano and lemon balm extracts as a food additive. EFSA J. 2010, 8, 1514. [Google Scholar] [CrossRef]
- García-Díez, J.; Alheiro, J.; Pinto, A.L.; Soares, L.; Falco, V.; Fraquez, M.J.; Patarata, L. Behaviour of food-borne pathogens on dry cured sausage manufactured with herbs and spices essential oils and their sensorial acceptability. Food Control 2016, 59, 262–270. [Google Scholar] [CrossRef]
- Govaris, A.; Solomakos, N.; Pexara, A.; Chatzopoulou, P.S. The antimicrobial effect of oregano essential oil, nisin and their combination against Salmonella enteritidis in minced sheep meat during refrigerated storage. Int. J. Food Microbiol. 2010, 137, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Tsigarida, E.; Skandamis, P.; Nychas, G.J.E. Behaviour of Listeria monocytogenes and autochthonous flora on meat stored under aerobic, vacuum and modified atmosphere-packaging conditions with or without the presence of oregano essential oil at 5 °C. J. Appl. Microbiol. 2000, 89, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, A.; Güven, A.; Gülmez, M. Bazı bitki infüzyonları ve hidrodistilatlarının piliç etlerininde kontaminasyonu ve raf ömrüne etkisi. Kafkas Univ. Vet. Fak. Derg. 2011, 17, 137–143. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists (AOAC): Washington, DC, USA, 2000. [Google Scholar]
- Hecer, C.; Ulusoy, B. Gıda Analizleri; Dora Basım Yayın Dağıtım: Bursa, Turkey, 2015. [Google Scholar]
- Petrou, S.; Tsiraki, M.V.; Giatrakou, V.; Savvaidis, I.N. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int. J. Food Microbiol. 2012, 156, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.P.P.; Trindade, M.A.; Lorenzo, J.M.; Munekata, P.E.S.; Melo, M.P. Effects of oregano extract on oxidative, microbiological and sensory stability of sheep burgers packed in modified atmosphere. Food Control 2016, 63, 65–75. [Google Scholar] [CrossRef]
- Pothakos, V.; Devlieghere, F.; Villani, F.; Björkroth, J.; Ercolini, D. Lactic acid bacteria and their controversial role in fresh meat spoilage. Meat Sci. 2015, 109, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, A.; Nasi, A.; Ferrocino, I.; Di Monaco, R.; Mauriello, G.; Villani, F.; Ercolini, D. Spoilage-related activity of Carnobacterium maltaromaticum strains in air-stored and vacuum-packed meat. Appl. Environ. Microbiol. 2011, 77, 7382–7393. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, F.S. Microorganisms in Foods 2: Sampling for Microbiological Analysis, Principles and Specific Applications; University of Toronto Press: Buffalo, NY, USA, 1974; Volume 2, p. 141. [Google Scholar]
- Asefa, D.T.; Gjerde, R.O.; Sidhu, M.S.; Langsrud, S.; Kure, C.F.; Nesbakken, T.; Skaar, I. Moulds contaminants on Norwegian dry-cured meat products. Int. J. Food Microbiol. 2009, 128, 435–439. [Google Scholar] [CrossRef] [PubMed]
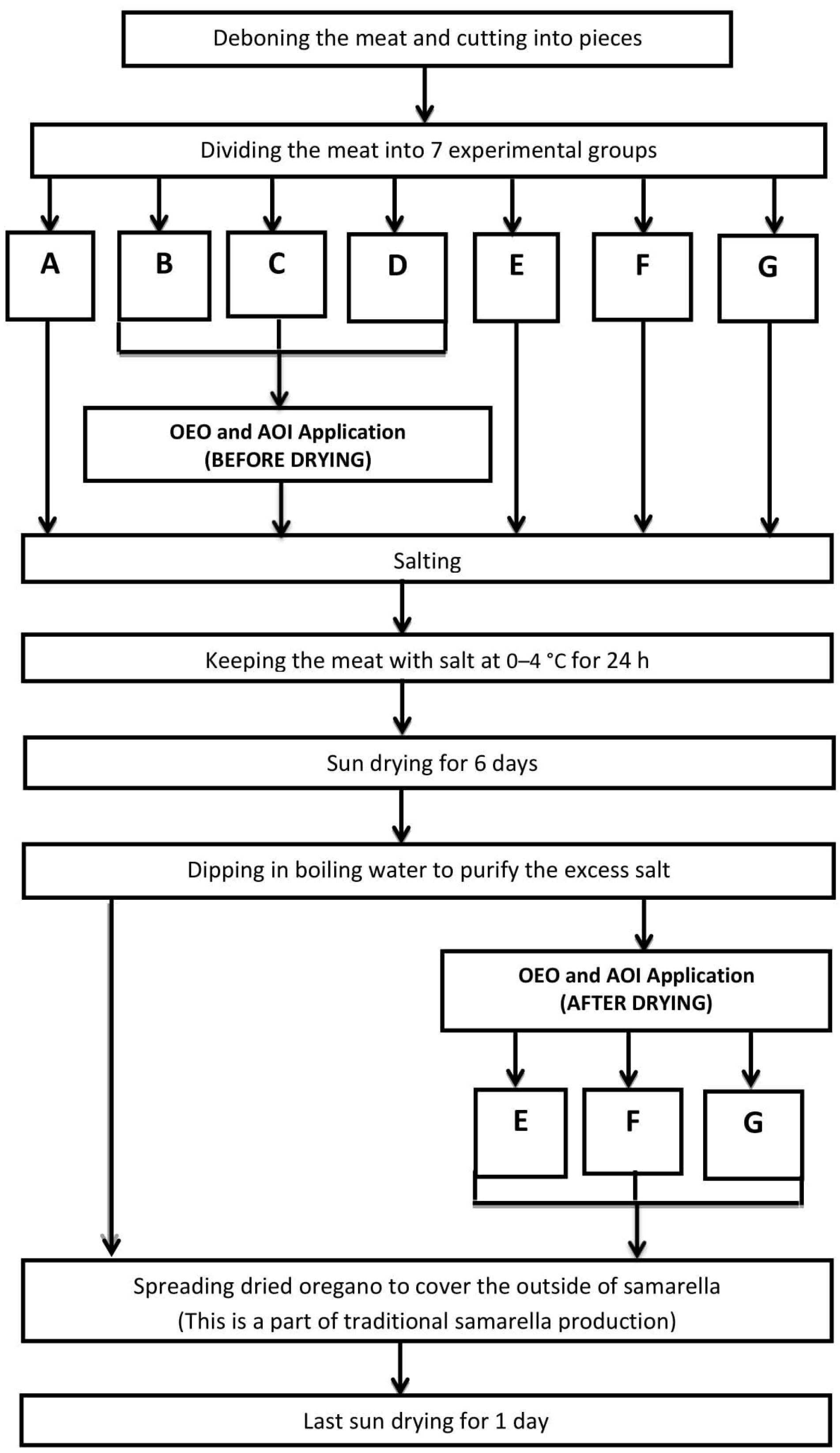
| Group of Samples | Application |
|---|---|
| SAMPLE A | No Application |
| SAMPLE B | Immersion to 5% OEO before sun drying stage |
| SAMPLE C | Immersion to 1% OEO before sun drying stage |
| SAMPLE D | Immersion to AOI before sun drying stage |
| SAMPLE E | Immersion to 5% OEO after sun drying stage |
| SAMPLE F | Immersion to 1% OEO after sun drying stage |
| SAMPLE G | Immersion to AOI after sun drying stage |
| Analysed Microorganisms | Selective Medium | Incubation Parameters |
|---|---|---|
| Micrococcaceae | Baird Parker Agar (BPA) | 37 °C/48 h |
| Enterobacteriaceae | Violet Red Bile Glucose (VRBG) Agar | 37 °C/24 h |
| Moulds | Sabouraud Dextrose Agar (SDA) | 25 °C/72–96 h |
| Total aerobic mesophilic bacteria (TAMB) | Standard Plate Count Agar (PCA) | 37 °C/48 h |
| Anaerobic sulphite-reducing Clostridia | Sulfite Polymyxin Sulfadiazine (SPS) Agar | 37 °C/48 h |
| Lactic acid bacteria (LAB) | De Man, Rogosa, Sharpe (MRS) Agar | 30 °C/48–72 h |
| pH | Dry Matter (%) | Salt (%) | ||||
|---|---|---|---|---|---|---|
| Mean ± SEM | Min–Max | Mean ± SEM | Min–Max | Mean ± SEM | Min–Max | |
| Sample A | 5.98 ± 0.04 | 5.90–6.10 | 65.70 ± 0.07 | 65.50–65.20 | 17.60 ± 0.02 | 17.50–17.70 |
| Sample B | 6.08 ± 0.02 | 6.00–6.10 | 65.17 ± 0.02 a | 65.10–65.20 | 14.00 ± 0.02 a | 13.90–14.10 |
| Sample C | 5.90 ± 0.04 b | 5.80–6.00 | 54.72 ± 0.06 a,b | 54.50–54.90 | 12.90 ± 0.03 a,b | 12.80–13.00 |
| Sample D | 5.98 ± 0.03 b | 5.90–6.10 | 66.75 ± 0.07 a,b,c | 66.50–67.00 | 8.83 ± 0.05 a,b,c | 8.70–9.00 |
| Sample E | 5.95 ± 0.02 b | 5.90–6.00 | 69.08 ± 0.40 a,b,c,d | 69.00–69.20 | 14.58 ± 0.30 a,b,c,d | 14.50–14.70 |
| Sample F | 5.98 ± 0.01 | 5.90–6.00 | 64.43 ± 0.42 a,b,c,d,e | 64.30–64.60 | 13.52 ± 0.47 a,b,c,d,e | 13.30–13.60 |
| Sample G | 5.93 ± 0.04 | 5.80–6.10 | 68.43 ± 0.42 a,b,c,d,e,f | 68.30–68.60 | 8.87 ± 0.03 a,b,c,d,e,f | 8.80–9.00 |
| Micrococcaceae | TAMB | LAB | Moulds | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | Min–Max | Mean ± SEM | Min–Max | Mean ± SEM | Min–Max | Mean ± SEM | Min–Max | |
| Sample A | 2.47 ± 0.01 | 2.46–2.48 | 3.85 ± 0.03 | 3.84–3.86 | 2.69 ± 0.01 | 2.69–2.70 | 3.69 ± 0.01 | 3.69–3.70 |
| Sample B | udl * | -- | 2.99 ± 0.01 a | 2.99–3.00 | 2.48 ± 0.01 a | 2.46–2.48 | 1.99 ± 0.01 a | 1.98–2.00 |
| Sample C | 2.32 ± 0.01 a | 2.31–2.33 | 3.32 ± 0.01 a,b | 3.31–3.32 | 2.60 ± 0.01 a,b | 2.69–2.70 | 1.99 ± 0.01 a | 1.97–2.01 |
| Sample D | 2.58 ± 0.01 a,c | 2.57–2.58 | 3.52 ± 0.01 | 3.51–3.53 | 2.62 ± 0.01 a,b,c | 2.61–2.62 | 3.40 ± 0.07 a,b,c | 3.05–3.47 |
| Sample E | udl * | -- | 2.95 ± 0.01 a,b,c,d | 2.94–2.95 | 2.17 ± 0.03 a,b,c,d | 2.16–2.18 | udl * | -- |
| Sample F | 2.16 ± 0.16 | 1.99–2.98 | 3.17 ± 0.16 a,d,e | 3.00–3.99 | 2.60 ± 0.01 a,b,d,e | 2.59–2.60 | 2.25 ± 0.01 a,b,c,d | 2.24–2.26 |
| Sample G | 2.30 ± 0.01 c,d,f | 2.29–2.30 | 3.39 ± 0.01 | 3.39–3.41 | 2.62 ± 0.01 | 2.61–2.63 | 3.55 ± 0.10 | 3.53–3.60 |
| External | Internal | Texture | Taste | Odour | General Acceptance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | Min–Max | Mean ± SEM | Min–Max | Mean ± SEM | Min–Max | Mean ± SEM | Min–Max | Mean ± SEM | Min–Max | Mean ± SEM | Min–Max | |
| Sample A | 3.77 ± 0.16 | 2.00–5.00 | 3.86 ± 0.15 | 2.00–5.00 | 3.59 ± 0.10 | 3.00–4.00 | 3.23 ± 0.09 | 3.00–4.00 | 3.77 ± 0.11 | 3.00-5.00 | 3.50 ± 0.12 | 3.00–5.00 |
| Sample B | 2.77 ± 0.14 | 2.00–4.00 | 2.73 ± 0.11 | 2.00–4.00 | 2.14 ± 0.07 | 2.00–3.00 | 1.86 ± 0.17 | 1.00–4.00 | 2.18 ± 0.10 | 1.00–3.00 | 2.14 ± 0.07 | 2.00–3.00 |
| Sample C | 4.14 ± 0.11 | 3.00–5.00 | 4.14 ± 0.07 | 4.00–5.00 | 4.00 ± 0.06 a | 3.00–5.00 | 4.09 ± 0.13 a | 3.00–5.00 | 3.59 ± 0.10 | 3.00–4.00 | 4.09 ± 0.11 a | 3.00–5.00 |
| Sample D | 3.18 ± 0.08 a,b | 3.00–4.00 | 3.23 ± 0.11 a,b | 2.00–4.00 | 2.23 ± 0.09 a | 2.00–3.00 | 2.18 ± 0.08 a | 2.00–3.00 | 2.45 ± 0.12 a | 2.00–4.00 | 2.14 ± 0.07 a | 2.00–3.00 |
| Sample E | 3.86 ± 0.11 | 3.00–5.00 | 3.45 ± 0.10 a | 3.00–4.00 | 2.64 ± 0.12 a,b | 1.00–3.00 | 2.23 ± 0.11 a | 1.00–3.00 | 2.50 ± 0.12 a,b | 1.00–3.00 | 2.50 ± 0.14 a,b,d | 1.00–4.00 |
| Sample F | 3.73 ± 0.13 c,d | 3.00–5.00 | 3.41 ± 0.14 a,b | 3.00–5.00 | 3.50 ± 0.15 c | 2.00–5.00 | 3.18 ± 0.12 | 2.00–4.00 | 3.09 ± 0.09 a,c,e | 2.00–4.00 | 3.09 ± 0.11 a,e | 2.00–4.00 |
| Sample G | 3.68 ± 0.12 c,d | 3.00–5.00 | 3.68 ± 0.13 c,d | 2.00–5.00 | 4.41 ± 0.15 c | 3.00–5.00 | 4.23 ± 0.11 | 3.00–5.00 | 3.77 ± 0.16 f | 3.00–5.00 | 3.80 ± 0.10 a | 3.00–5.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulusoy, B.; Hecer, C.; Kaynarca, D.; Berkan, Ş. Effect of Oregano Essential Oil and Aqueous Oregano Infusion Application on Microbiological Properties of Samarella (Tsamarella), a Traditional Meat Product of Cyprus. Foods 2018, 7, 43. https://doi.org/10.3390/foods7040043
Ulusoy B, Hecer C, Kaynarca D, Berkan Ş. Effect of Oregano Essential Oil and Aqueous Oregano Infusion Application on Microbiological Properties of Samarella (Tsamarella), a Traditional Meat Product of Cyprus. Foods. 2018; 7(4):43. https://doi.org/10.3390/foods7040043
Chicago/Turabian StyleUlusoy, Beyza, Canan Hecer, Doruk Kaynarca, and Şifa Berkan. 2018. "Effect of Oregano Essential Oil and Aqueous Oregano Infusion Application on Microbiological Properties of Samarella (Tsamarella), a Traditional Meat Product of Cyprus" Foods 7, no. 4: 43. https://doi.org/10.3390/foods7040043
APA StyleUlusoy, B., Hecer, C., Kaynarca, D., & Berkan, Ş. (2018). Effect of Oregano Essential Oil and Aqueous Oregano Infusion Application on Microbiological Properties of Samarella (Tsamarella), a Traditional Meat Product of Cyprus. Foods, 7(4), 43. https://doi.org/10.3390/foods7040043