Optimization of Polyphenol Extraction from Allium ampeloprasum var. porrum through Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Plant Material Preparation
2.3. Extraction Procedure
2.4. Determination of Total Phenolic Content (TPC)
2.5. Scavenging Activity on 2,2’-Azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) Radical (ABTS+)
2.6. Ferric Reducing/Antioxidant Power Assay (FRAP)
2.7. Experimental Design and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Block, E. Garlic and Other Alliums. In The Lore and the Science; Royal Society of Chemistry Publishers: Cambridge, UK, 2010; ISBN 0-85404-190-7. [Google Scholar]
- Eurostat. Agricultural Statistics—Fruits and Vegetables (Annual Data); Publications Office of the European Community: Luxembourg, 2012. [Google Scholar]
- Bernaert, N.; De Clercq, H.; Van Bockstaele, E.; De Loose, M.; Van Droogenbroeck, B. Antioxidant changes during postharvest processing and storage of leek (Allium ampeloprasum var. porrum). Postharvest Biol. Technol. 2013, 86, 8–16. [Google Scholar] [CrossRef]
- Augusti, K.T. Therapeutic and medicinal values of onions and garlic. In Onions and Allied Crops Biochemistry, Food Science and Minor Crops; Brewster, J.L., Rabinowitch, H.D., Eds.; CRC Press: Boca Raton, FL, USA, 1990; Volume III, pp. 93–108. ISBN 0-8493-6302-4. [Google Scholar]
- Shon, M.Y.; Choi, S.D.; Kahng, G.G.; Nam, S.H.; Sung, N.J. Antimutagenic, antioxidant and free radical scavenging activity of ethyl acetate extracts from white, yellow and red onions. Food Chem. Toxicol. 2004, 42, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Singh, D.P.; Sarma, B.K.; Upadhyay, G.; Singh, H.B. Polyphenolics from various extracts/fractions of red onion (Allium cepa) peel with potent antioxidant and antimutagenic activities. Food Chem. Toxicol. 2009, 47, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, P.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Flavonol glucosides in Allium species. A comparative study by means of HPLC-DAD-ESI-MS-MS. Food Chem. 2008, 107, 1668–1673. [Google Scholar] [CrossRef]
- Lee, J.Y.; Mitchell, A.E. Quercetin and isorhamnetin glycosides in onion (Allium cepa L.) Varietal comparison, physical distribution, coproduct evaluation, and long-term storage stability. J. Agric. Food Chem. 2011, 59, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in The Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Fattorusso, E.; Lanzotti, V.; Taglialatela-Scafati, O.; Cicala, C. The flavonoids of leek, Allium porrum. Phytochemistry 2001, 57, 565–569. [Google Scholar] [CrossRef]
- Lim, T. Allium Ampeloprasum. In Edible Medicinal and Non Medicinal Plants; Springer: Berlin, Germany, 2015; pp. 103–123. ISBN 978-94-017-9511-1. [Google Scholar]
- Hughes, B.G.; Lawson, L.D. Antimicrobial effects of Allium sativum L. (garlic), Allium ampeloprasum L. (elephant garlic), and Allium cepa L. (onion), garlic compounds and commercial garlic supplement products. Phytother. Res. 1991, 5, 154–158. [Google Scholar] [CrossRef]
- García-Herrera, P.; Morales, P.; Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Cámara, M.; Carvalho, A.M.; Ferreira, I.C.F.R.; Pardo-de-Santayana, M.; Molina, M.; Tardío, J. Nutrients phytochemicals and antioxidant activity in wild populations of Allium ampeloprasum L., a valuable underutilized vegetable. Food Res. Int. 2014, 62, 272–279. [Google Scholar] [CrossRef]
- Pyun, M.-S.; Shin, S. Antifungal effects of the volatile oils from Allium plants against Trichophyton species and synergism of the oils with ketoconazole. Phytomedicine 2006, 13, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Nguansangiam, S.; Angsubhakorn, S.; Bhamarapravati, S.; Suksamrarn, A. Effects of elephant garlic volatile oil (Allium ampeloprasum) and T-2 toxin on murine skin. Southeast Asian J. Trop. Med. Public Health 2003, 34, 899–905. [Google Scholar] [PubMed]
- Sedighi-Hafshejani, M.; Noori-Ahmadabadi, M.; Nasri, H.; Hadi, M.; Rafieian-Kopaei, M. In-Vitro evaluation of hydroalcoholic extract of Allium ampeloprasum on rat ileum contractions. J. Isfahan Med. Sch. 2014, 31, 2238–2249. [Google Scholar]
- Gonzalez-Montelongo, R.; Lobo, M.G.; Gonzalez, M. Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds. Food Chem. 2010, 119, 1030–1039. [Google Scholar] [CrossRef]
- Gonzalez-Montelongo, R.; Lobo, M.G.; Gonzalez, M. The effect of extraction temperature, time and number of steps on the antioxidant capacity of methanolic banana peel extracts. Sep. Purif. Technol. 2010, 71, 347–355. [Google Scholar] [CrossRef]
- Al-Farsi, M.A.; Lee, C.Y. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008, 108, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Tsaoa, R.; Deng, Z. Separation procedures for naturally occurring antioxidant phytochemicals-a review. J. Chromatogr. B 2004, 812, 85–99. [Google Scholar] [CrossRef]
- Andreou, V.; Strati, I.F.; Fotakis, C.; Liouni, M.; Zoumpoulakis, P.; Sinanoglou, V.J. Herbal distillates: A new era of grape marc distillates with enriched antioxidant profile. Food Chem. 2018, 253, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Zoumpoulakis, P.G.; Glamočlija, J.; Ćirić, A.; Soković, M.; Heropoulos, G.; Proestos, C. Antiradical–antimicrobial activity and phenolic profile of pomegranate (Punica granatum L.) juices from different cultivars: A comparative study. RSC Adv. 2015, 5, 2602–2614. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Zoumpoulakis, P.; Proestos, C. Comparison of the antioxidant and antiradical activity of pomegranate (Punica granatum L.) by ultrasound-assisted and classical extraction. Anal. Lett. 2016, 49, 969–978. [Google Scholar] [CrossRef]
- Kavalcová, P.; Bystrická, J.; Tomáš, J.; Karovičová, J.; Kuchtová, V. Evaluation and comparison of the content of total polyphenols and antioxidant activity in onion, garlic and leek. Potravinarstvo 2014, 8, 272–276. [Google Scholar] [CrossRef]
- Mladenović, J.D.; Mašković, P.Z.; Pavlović, R.M.; Radovanović, B.C.; Aćamović-Đoković, G.; Cvijović, M.S. Antioxidant activity of ultrasonic extracts of leek Allium porrum L. Hem. Ind. 2011, 65, 473–477. [Google Scholar] [CrossRef]
- Bernaert, N.; De Paepe, D.; Bouten, C.; De Clercq, H.; Stewart, D.; Van Bockstaele, E.; De Loose, M.; Van Droogenbroeck, B. Antioxidant capacity, total phenolic and ascorbate content as a function of the genetic diversity of leek (Allium ampeloprasum var. porrum). Food Chem. 2012, 134, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Tsouvaltzis, P.; Gerasopoulos, D.; Siomos, A.S. Effects of base removal and heat treatment on visual and nutritional quality of minimally processed leeks. Postharvest Biol. Technol. 2007, 43, 158–164. [Google Scholar] [CrossRef]
- Haytowitz, D.B.; Bhagwat, S. USDA Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2; U.S. Department of Agriculture: Beltsville, MA, USA, 2010.
- Germ, M.; Stibilj, V.; Kreft, S.; Gaberscik, A.; Kreft, I. Flavonoid, tannin and hypericin concentrations in the leaves of St. John’s wort (Hypericum perforatum L.) are affected by UV-B radiation levels. Food Chem. 2010, 122, 471–474. [Google Scholar] [CrossRef]
- Kaspar, S.; Matros, A.; Mock, H.P. Proteome and flavonoid analysis reveals distinct responses of epidermal tissue and whole leaves upon UV-B radiation of barley (Hordeum vulgare L.) seedlings. J. Proteome Res. 2010, 9, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
- Bernaert, N.; Wouters, D.; De Vuyst, L.; De Paepe, D.; De Clercq, H.; Van Bockstaele, E.; De Loose, M.; Van Droogenbroeck, B. Antioxidant changes of leek (Allium ampeloprasum var. porrum) during spontaneous fermentation of the white shaft and green leaves. J. Sci. Food Agric. 2013, 93, 2146–2153. [Google Scholar] [CrossRef] [PubMed]
- Soininen, T.H.; Jukarainen, N.; Soininen, P.; Auriola, S.O.; Julkunen-Tiitto, R.; Oleszek, W.; Stochmal, A.; Karjalainen, R.O.; Vepsäläinen, J.J. Metabolite Profiling of Leek (Allium porrum L) Cultivars by 1H NMR and HPLC–MS. Phytochem. Anal. 2014, 25, 220–228. [Google Scholar] [CrossRef] [PubMed]
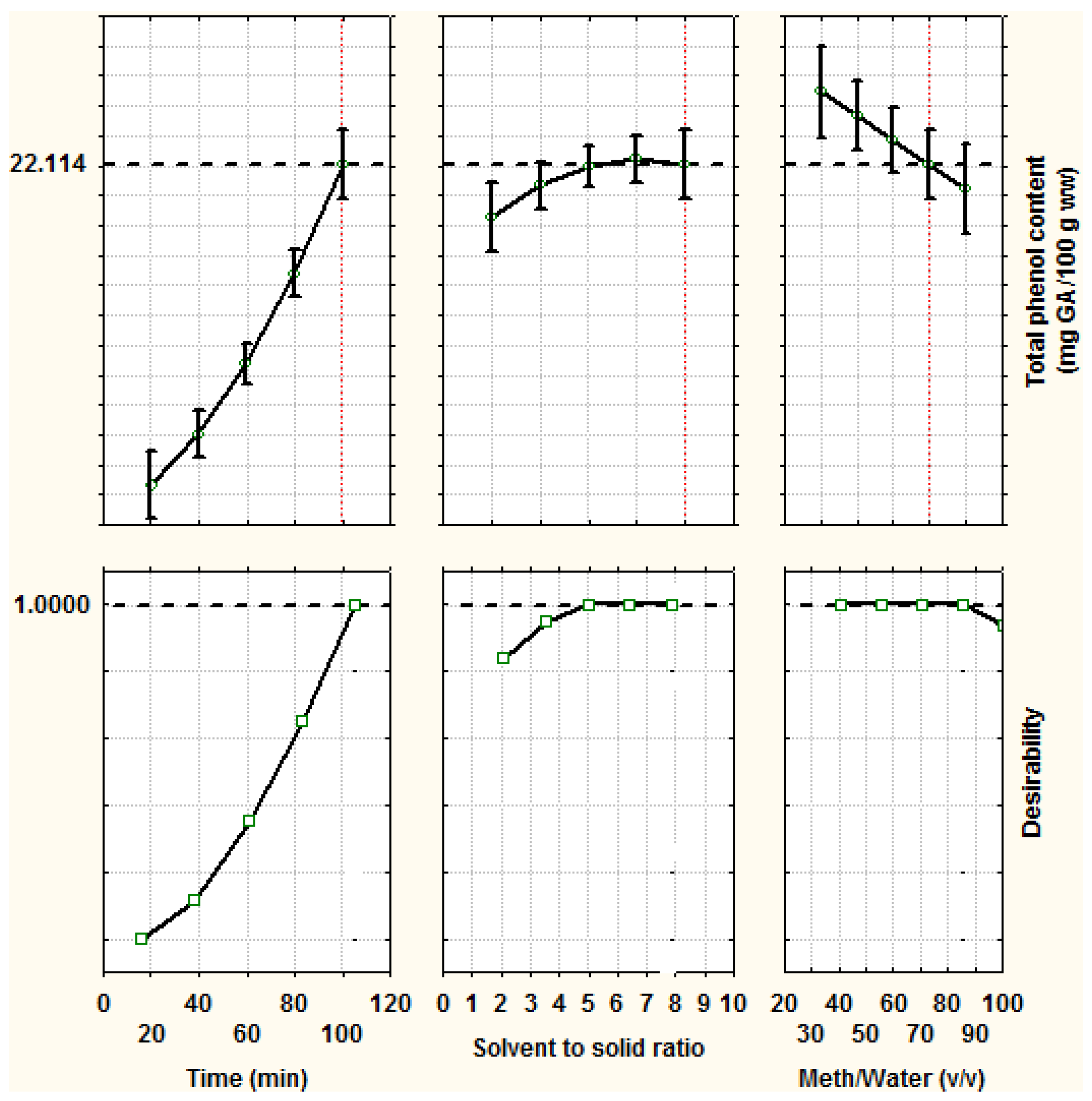
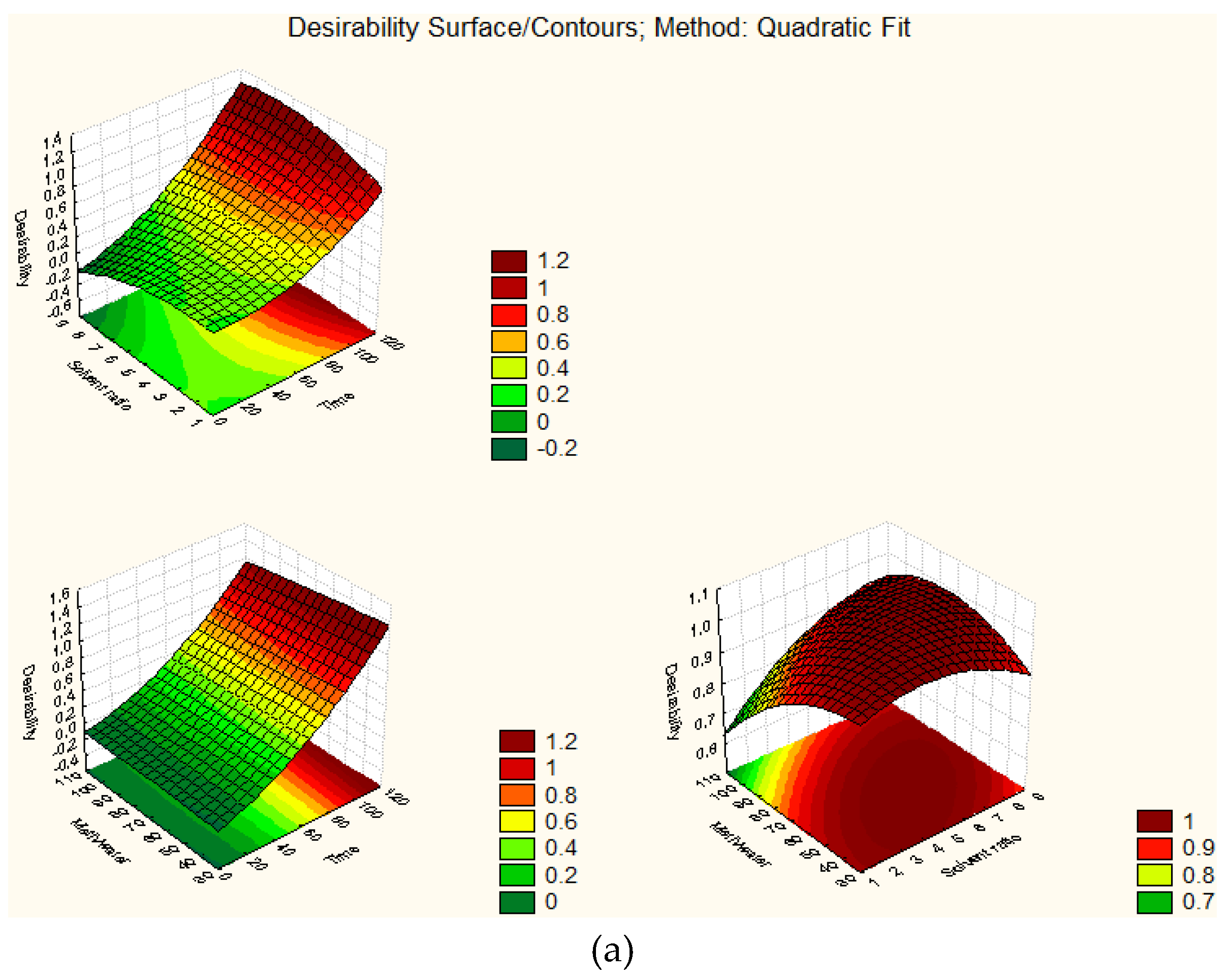
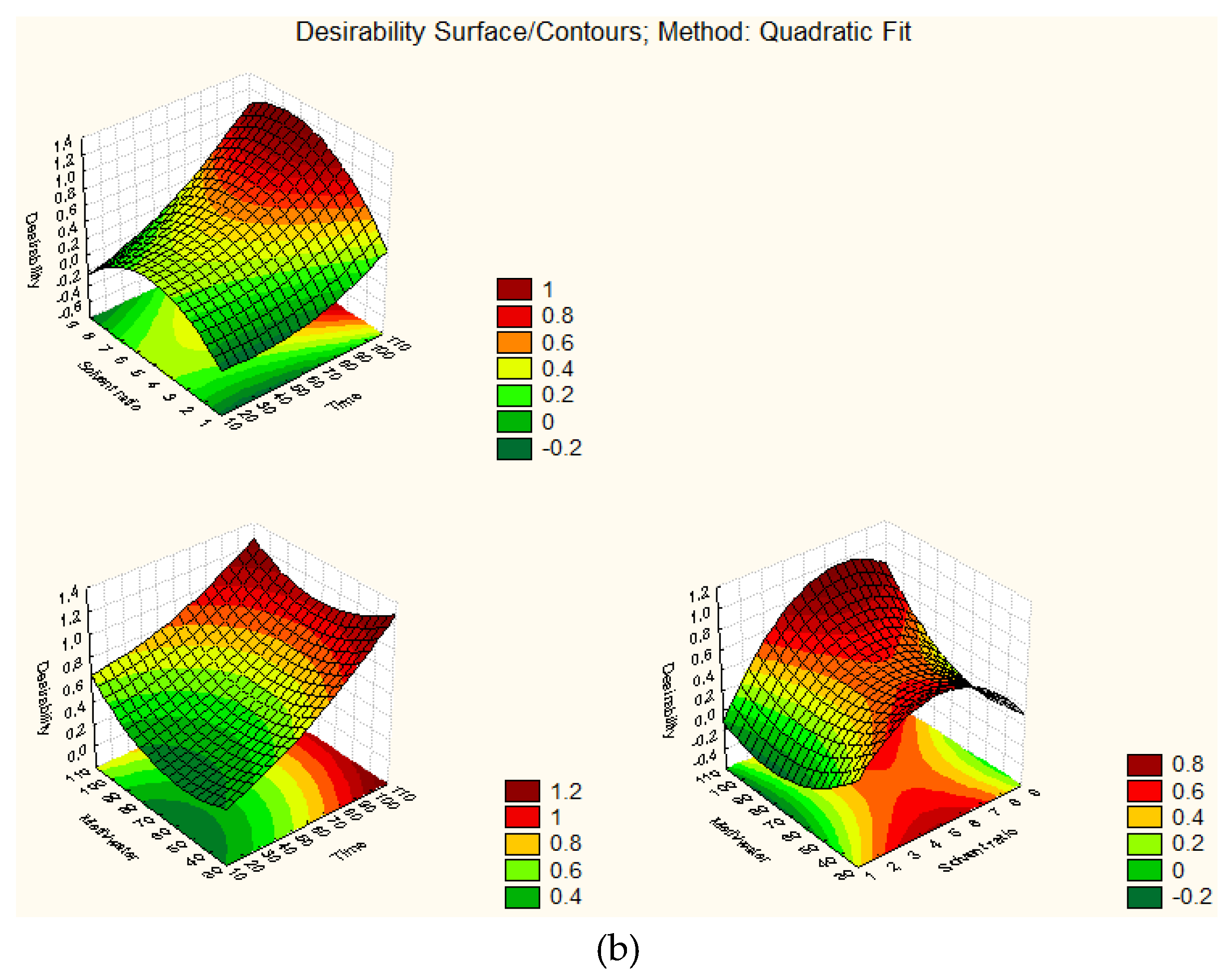
| Run No. 1 | X1 | X2 | X3 | Green Leaf | White Stem | ||
|---|---|---|---|---|---|---|---|
| Y-Observed | Y-Predicted | Y-Observed | Y-Predicted | ||||
| 1 | −1 (30) | −1 (3) | 0 (70) | 6.886 | 6.893 | 5.906 | 5.535 |
| 2 | +1 (90) | −1 (3) | 0 (70) | 17.173 | 17.538 | 9.974 | 9.771 |
| 3 | −1 (30) | +1 (7) | 0 (70) | 4.311 | 3.946 | 6.073 | 6.275 |
| 4 | +1 (90) | +1 (7) | 0 (70) | 18.762 | 18.754 | 12.606 | 12.977 |
| 5 | −1 (30) | 0 (5) | −1 (50) | 6.456 | 6.086 | 6.688 | 7.339 |
| 6 | +1 (90) | 0 (5) | −1 (50) | 21.456 | 20.729 | 12.863 | 13.344 |
| 7 | −1 (30) | 0 (5) | +1 (90) | 5.579 | 9.909 | 8.911 | 8.590 |
| 8 | +1 (90) | 0 (5) | +1 (90) | 16.867 | 15.403 | 14.391 | 10.753 |
| 9 | 0 (60) | −1 (3) | −1 (50) | 11.619 | 11.451 | 8.261 | 8.498 |
| 10 | 0 (60) | +1 (7) | −1 (50) | 10.186 | 10.505 | 9.523 | 7.727 |
| 11 | 0 (60) | −1 (3) | +1 (90) | 10.825 | 9.754 | 6.559 | 9.855 |
| 12 | 0 (60) | +1 (7) | +1 (90) | 9.782 | 10.751 | 10.484 | 9.946 |
| 13 | 0 (60) | 0 (5) | 0 (70) | 10.995 | 11.417 | 9.365 | 9.223 |
| 14 | 0 (60) | 0 (5) | 0 (70) | 11.272 | 11.417 | 9.028 | 9.223 |
| 15 | 0 (60) | 0 (5) | 0 (70) | 11.98468 | 11.417 | 9.275 | 9.223 |
| Response Variable (Total Phenolic Content, mg GAE/100 g) | 2nd Order Polynomial Equations | R2 | p |
|---|---|---|---|
| Leek green leaf | 1.53 + 0.08X1 + 0.00X12 + 0.56X2 − 0.20X22 + 0.05X3 − 0.00X32 + 0.02X1X2 − 0.00X1X3 + 0.00X2X3 | 0.98 | 0.00 |
| Leek white stem | 10.92 − 0.02X1 + 0.00X12 + 2.00X22 − 0.32X22 − 0.30X3 + 0.00X32 + 0.01X1X2 − 0.00X1X3 + 0.02X2X3 | 0.92 | 0.00 |
| Source | Sum of Squares | Degrees of Freedom | Mean Sum of Squares | F Value | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| G 1 | W 2 | G 1 | W 2 | G 1 | W 2 | G 1 | W 2 | G 1 | W 2 | |
| Model | 1447.13 | 319.68 | 9 | 9 | 160.79 | 35.52 | 120.44 | 30.38 | 0.00 | 0.00 |
| Intercept | 0.10 | 5.16 | 1 | 1 | 0.10 | 5.16 | 0.08 | 4.41 | 0.78 | 0.04 |
| Extraction time (X1) | 2.87 | 0.23 | 1 | 1 | 2.87 | 0.23 | 2.15 | 0.19 | 0.15 | 0.66 |
| Solvent to plant material ratio (X2) | 0.46 | 6.25 | 1 | 1 | 0.46 | 6.25 | 0.35 | 5.35 | 0.56 | 0.02 |
| Methanol:water ratio (X3) | 0.24 | 9.28 | 1 | 1 | 0.24 | 9.28 | 0.18 | 7.94 | 0.67 | 0.01 |
| X12 | 20.33 | 7.77 | 1 | 1 | 20.33 | 7.77 | 15.23 | 6.64 | 0.00 | 0.01 |
| X22 | 9.54 | 25.56 | 1 | 1 | 9.54 | 25.56 | 7.15 | 21.86 | 0.01 | 0.00 |
| X32 | 0.01 | 8.74 | 1 | 1 | 0.00 | 8.74 | 0.00 | 7.48 | 0.95 | 0.01 |
| X1 × X2 | 17.33 | 6.07 | 1 | 1 | 17.33 | 6.07 | 12.98 | 5.20 | 0.00 | 0.03 |
| X1 × X3 | 16.18 | 1.16 | 1 | 1 | 16.18 | 1.16 | 12.12 | 1.00 | 0.00 | 0.32 |
| X2 × X3 | 0.15 | 7.26 | 1 | 1 | 0.15 | 7.26 | 0.11 | 6.21 | 0.74 | 0.02 |
| Residual | 69.42 | 61.96 | 52 | 53 | 1.34 | 1.17 | ||||
| Total | 1516.56 | 381.64 | 61 | 62 | ||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strati, I.F.; Kostomitsopoulos, G.; Lytras, F.; Zoumpoulakis, P.; Proestos, C.; Sinanoglou, V.J. Optimization of Polyphenol Extraction from Allium ampeloprasum var. porrum through Response Surface Methodology. Foods 2018, 7, 162. https://doi.org/10.3390/foods7100162
Strati IF, Kostomitsopoulos G, Lytras F, Zoumpoulakis P, Proestos C, Sinanoglou VJ. Optimization of Polyphenol Extraction from Allium ampeloprasum var. porrum through Response Surface Methodology. Foods. 2018; 7(10):162. https://doi.org/10.3390/foods7100162
Chicago/Turabian StyleStrati, Irini F., George Kostomitsopoulos, Fotios Lytras, Panagiotis Zoumpoulakis, Charalampos Proestos, and Vassilia J. Sinanoglou. 2018. "Optimization of Polyphenol Extraction from Allium ampeloprasum var. porrum through Response Surface Methodology" Foods 7, no. 10: 162. https://doi.org/10.3390/foods7100162
APA StyleStrati, I. F., Kostomitsopoulos, G., Lytras, F., Zoumpoulakis, P., Proestos, C., & Sinanoglou, V. J. (2018). Optimization of Polyphenol Extraction from Allium ampeloprasum var. porrum through Response Surface Methodology. Foods, 7(10), 162. https://doi.org/10.3390/foods7100162








