Spatial Variation in the Mercury Concentration of Muscle Myomeres in Steaks of Farmed Southern Bluefin Tuna
Abstract
:1. Introduction

2. Methods
2.1. Experimental Design
2.2. Spatial Variation in Mercury Concentration within Marketed Tissue Cuts
2.3. Sample Preparation
2.4. Mercury Analysis
2.5. Statistical Analysis
3. Results and Discussion
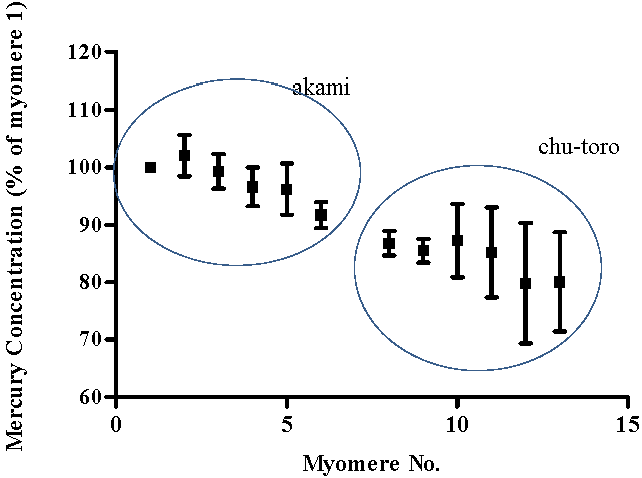
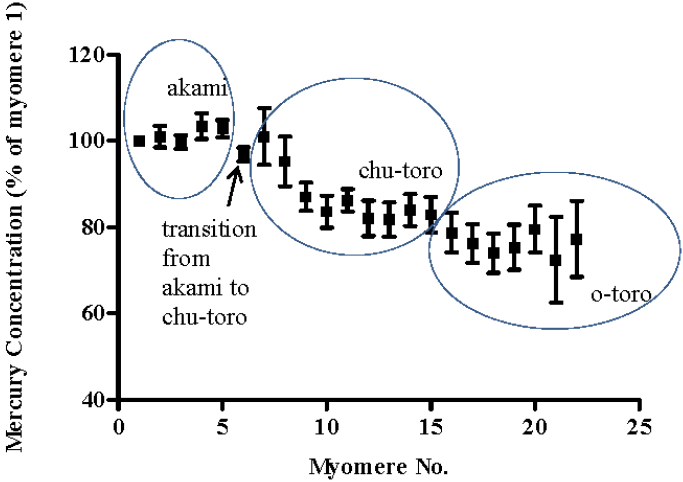
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harris, H.H.; Pickering, I.J.; George, G.N. The chemical form of mercury in fish. Science 2003, 301, 1203–1203. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Seoka, M.; Tsukamasa, Y.; Kawasaki, K.I.; Ando, M. Possibility for decreasing of mercury content in bluefin tuna Thunnus orientalis by fish culture. Fish. Sci. 2007, 73, 724–731. [Google Scholar] [CrossRef]
- Suzuki, T.; Miyama, T.; Toyama, C. The chemical form and bodily distribution of mercury in marine fish. Bull. Environ. Contam. Toxicol. 1973, 10, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.C.; Horne, D.A. Sampling the edible muscle of the swordfish (Xiphias gladius) for total mercury analysis. J. Fish. Board Can. 1973, 30, 1251–1252. [Google Scholar] [CrossRef]
- Adams, D.H. Total mercury levels in tunas from offshore waters of the Florida Atlantic coast. Mar. Pollut. Bull. 2004, 49, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Hellou, J.; Fancey, L.L.; Payne, J.F. Concentrations of twenty-four elements in bluefin tuna, Thunnus thynnus from the Northwest Atlantic. Chemosphere 1992, 24, 211–218. [Google Scholar] [CrossRef]
- Kojadinovic, J.; Potier, M.; le Corre, M.; Cosson, R.P.; Bustamante, P. Mercury content in commercial pelagic fish and its risk assessment in the Western Indian Ocean. Sci. Total Environ. 2006, 366, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Menasveta, P.; Siriyong, R. Mercury content of several predacious fish in the Andaman Sea. Mar. Pollut. Bull. 1977, 8, 200–204. [Google Scholar] [CrossRef]
- Storelli, M.M.; Stuffler, R.G.; Marcotrigiano, G.O. Total and methylmercury residues in tuna-fish from the Mediterranean sea. Food Addit. Contam. 2002, 19, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Balshaw, S.; Edwards, J.W.; Ross, K.E.; Daughtry, B.J. Mercury distribution in the muscular tissue of farmed southern bluefin tuna (Thunnus maccoyii) is inversely related to the lipid content of tissues. Food Chem. 2008, 111, 616–621. [Google Scholar] [CrossRef]
- Balshaw, S.; Edwards, J.W.; Ross, K.E.; Ellis, D.; Padula, D.J.; Daughtry, B.J. Empirical models to identify mechanisms driving reductions in tissue mercury concentration during culture of farmed southern bluefin tuna Thunnus maccoyii. Mar. Pollut. Bull. 2008, 56, 2009–2017. [Google Scholar]
- Balshaw, S.; Edwards, J.W.; Daughtry, B.J.; Ross, K.E. Risk-benefit analysis of fish consumption: Fatty acid and mercury composition of farmed southern bluefin tuna, Thunnus maccoyii. Food Chem. 2012, 131, 977–984. [Google Scholar] [CrossRef]
- Koriyama, T.; Kohata, T.; Watanabe, K.; Abe, H. Chemical components of bigeye tuna muscle and the effects of lipid on the taste (Abstract). Nippon Suisan Gakkaishi 2000, 66, 462–468. [Google Scholar] [CrossRef]
- Nakamura, Y.N.; Ando, M.; Seoka, M.; Kawasaki, K.I.; Tsukamasa, Y. Changes of proximate and fatty acid compositions of the dorsal and ventral ordinary muscles of the full-cycle cultured Pacific bluefin tuna Thunnus orientalis with the growth. Food Chem. 2007, 103, 234–241. [Google Scholar] [CrossRef]
- Nakamura, Y.N.; Ando, M.; Seoka, M.; Kawasaki, K.I.; Tsukamasa, Y. Comparison of the proximate compositions, breaking strength and histological structure by the muscle positions of the full-cycle cultured Pacific bluefin tuna Thunnus orientalis. Fish. Sci. 2005, 71, 605–611. [Google Scholar] [CrossRef]
- Roy, B.C.; Miyake, Y.; Ando, M.; Kawasaki, K.-I.; Tsukamasa, Y. Proximate and fatty acid compositions in different flesh cuts of cultured, cultured fasted, and wild Pacific bluefin tuna (Thunnus orientalis). J. Aquat. Food Prod. Technol. 2010, 19, 284–297. [Google Scholar] [CrossRef]
- Hisamichi, Y.; Haraguchi, K.; Endo, T. Levels of mercury and organohalogen compounds in Pacific bluefin tuna (Thunnus orientalis) cultured in different regions of Japan. Arch. Environ. Contam. Toxicol. 2012, 62, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Hayward, C.J.; Aiken, H.M.; Nowak, B.F. Metazoan parasites on gills of Southern Bluefin Tuna (Thunnus maccoyii) do not rapidly proliferate after transfer to sea cages. Aquaculture 2007, 262, 10–16. [Google Scholar] [CrossRef]
- Coughlin, D.J. Aerobic muscle function during steady swimming in fish. Fish Fish. 2002, 3, 63–78. [Google Scholar] [CrossRef]
- Kim, J.P. Methylmercury in rainbow trout (Oncorhynchus mykiss) from Lakes Okareka, Okaro, Rotomahana, Rotorua and Tarawera, North Island, New Zealand. Sci. Total Environ. 1995, 164, 209–219. [Google Scholar] [CrossRef]
- Rayner, M.D.; Keenan, M.J. Role of red and white muscles in the swimming of the skipjack tuna. Nature 1967, 214, 392–393. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W. The three modern faces of mercury. Environ. Health Perspect. 2002, 110 (Suppl. 1), 11–23. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ross, K.; Edwards, J. Spatial Variation in the Mercury Concentration of Muscle Myomeres in Steaks of Farmed Southern Bluefin Tuna. Foods 2015, 4, 254-262. https://doi.org/10.3390/foods4020254
Ross K, Edwards J. Spatial Variation in the Mercury Concentration of Muscle Myomeres in Steaks of Farmed Southern Bluefin Tuna. Foods. 2015; 4(2):254-262. https://doi.org/10.3390/foods4020254
Chicago/Turabian StyleRoss, Kirstin, and John Edwards. 2015. "Spatial Variation in the Mercury Concentration of Muscle Myomeres in Steaks of Farmed Southern Bluefin Tuna" Foods 4, no. 2: 254-262. https://doi.org/10.3390/foods4020254
APA StyleRoss, K., & Edwards, J. (2015). Spatial Variation in the Mercury Concentration of Muscle Myomeres in Steaks of Farmed Southern Bluefin Tuna. Foods, 4(2), 254-262. https://doi.org/10.3390/foods4020254





