Antioxidant Generation during Coffee Roasting: A Comparison and Interpretation from Three Complementary Assays
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Coffee Roasting and Preparation
| Roast profile | Final roast temperature °C | Roast time min:sec | Color of R & G coffee Colortest II | Mass loss % | Humidity % |
|---|---|---|---|---|---|
| Fast | 150 | 03:00 ± 4 | 166.8 ± 0.7 | 5.8 | ND |
| 170 | 03:52 ± 5 | 154.0 ± 1.0 | 8.7 | 4.17 | |
| 190 | 04:43 ± 2 | 130.2 ± 7.78 | 12.0 | 3.32 | |
| 203 | 05:15 ± 0 | 97.2 ± 13.44 | 14.7 | 2.26 | |
| 209 | 05:27 | 71.3 | 16.4 | 2.19 | |
| 215 | 05:37 | 56.3 | 18.6 | 1.84 | |
| Medium | 130 | 03:50 ± 6 | ND | 4.8 | ND |
| 150 | 05:07 ± 10 | 161.5 ± 2.1 | 7.0 | ND | |
| 170 | 06:45 ± 2 | 160.7 ± 1.9 | 9.4 | 4.06 | |
| 190 | 08:23 ± 19 | 139.5 ± 3.1 | 12.3 | 3.16 | |
| 203 | 09:36 ± 21 | 109.4 ± 6.6 | 15.3 | 2.03 | |
| 209 | 10:29 | 93.7 | 15.9 | 1.43 | |
| 215 | 11:05 | 78.7 | 17.6 | 1.26 | |
| Slow | 110 | 03:07 ± 17 | ND | 2.8 | ND |
| 130 | 04:54 ± 9 | ND | 5.0 | ND | |
| 150 | 06:45 ± 2 | 162.3 ± 1.4 | 7.4 | ND | |
| 170 | 09:06 ± 1 | 162.5 ± 0.2 | 9.5 | 4.16 | |
| 190 | 12:04 ± 4 | 140.7 ± 0.0 | 12.6 | 3.06 | |
| 203 | 14:51 ± 24 | 106.8 ± 2.1 | 15.3 | 1.92 | |
| 209 | 15:48 | 91.7 | 15.9 | 1.74 | |
| 215 | 16:36 | 78.0 | 16.8 | 1.32 | |
| 220 | 17:51 | 67.3 | 19.2 | 1.19 |
2.3. Antioxidant Assays
2.3.1. Folin-Ciocalteu (FC) Assay
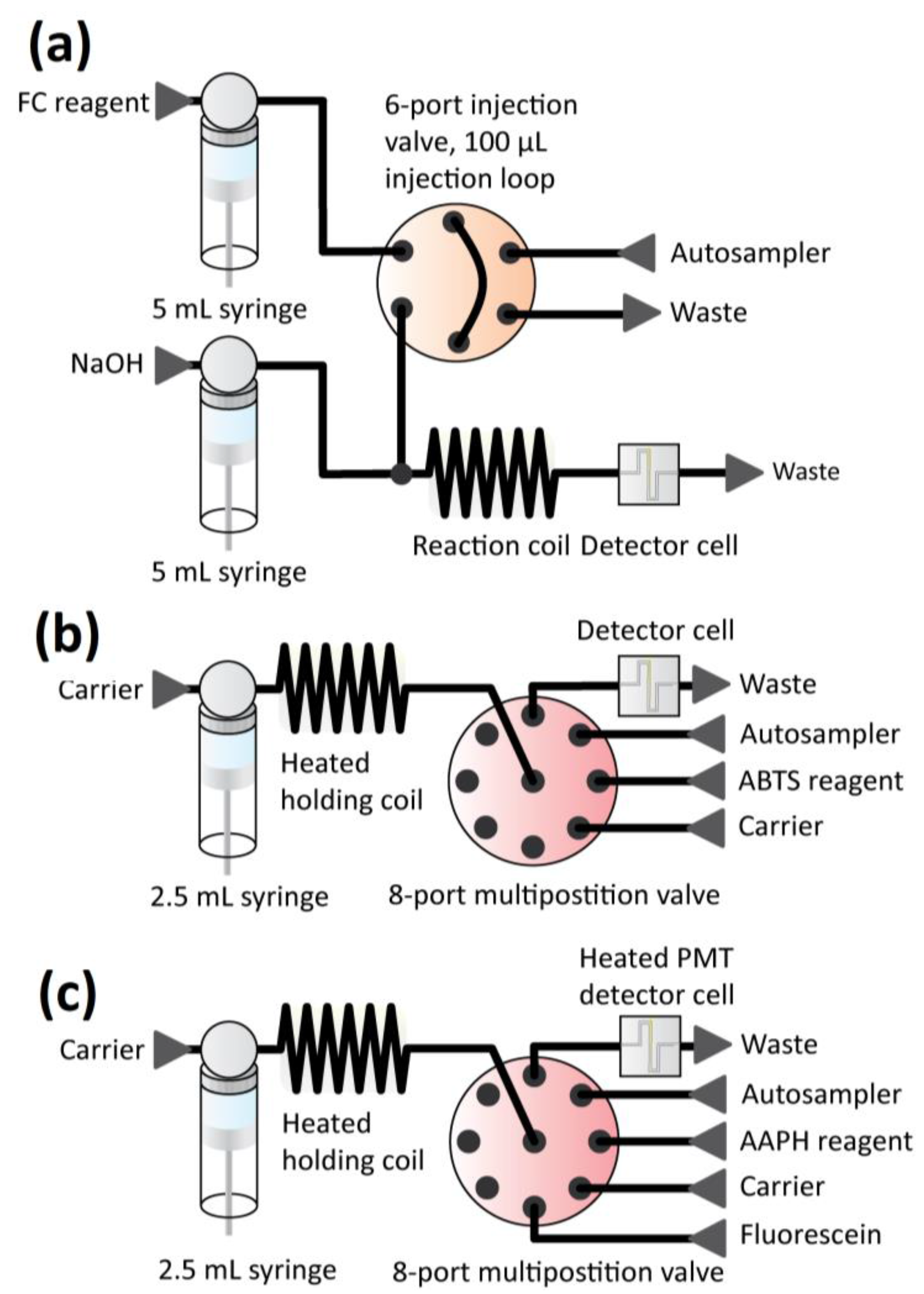
2.3.2. ABTS (2,2’-Azinobis-(3-Ethylbenzothiazoline-6-Sulphonate)) Assay
2.3.3. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.4. Validation of Assays
3. Results and Discussion
3.1. Methodology Validation
| Validation criterion | FC | ABTS | ORAC |
|---|---|---|---|
| Intraday RSD (%) | 0.9 at 30 mg/L GA | 0.4 at 3 mg/L GA | 4.7 at 10 mg/L GA |
| Interday RSD (%) | 2.1 at 30 mg/L GA | 4.4 at 3 mg/L GA | 8.2 at 10 mg/L GA |
| LoD (mg/L GA) | 1.6 | 0.12 | 0.43 |
| LoQ (mg/L GA) | 3.2 | 0.4 | 2.1 |
| Linearity range (mg/L GA) | 2–60 | 0.5–5 | 5–25 |
| R2 | 0.9996 | 0.9987 | 0.9993 |
| Recovery (%) | 93 (of 30 mg/L GA) | 89 (of 2 mg/L GA) | 78 (of 20 mg/L GA) |
3.2. Effects of Coffee Roasting Conditions on Antioxidant Contents

3.3. Significance of the Numbers Produced by the Different Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goodman, B.A. Coffee Consumption and Health; Nova Science Publishers: New York, NY, USA, 2012. [Google Scholar]
- Chu, Y.F. Coffee: Emerging Health Effects and Disease Prevention; Wiley-Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar]
- Richelle, M.; Tavazzi, I.; Offord, E. Comparison of the antioxidant activity of commonly consumed polyphenolic beverages (coffee, cocoa, and tea) prepared per cup serving. J. Agric. Food Chem. 2001, 49, 3438–3442. [Google Scholar]
- Nicoli, M.C.; Anese, M.; Manzocco, L.; Lerici, C.R. Antioxidant properties of coffee brews in relation to the roasting degree. Lebensm. Wiss. Technol. 1997, 30, 292–297. [Google Scholar]
- Anese, M.; Nicoli, M.C. Antioxidant properties of ready-to-drink coffee brews. J. Agric. Food Chem. 2003, 5, 942–946. [Google Scholar]
- Vicente, S.J.V.; Ishimoto, Y.; Torres, E.A.F.S. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats. J. Agric. Food Chem. 2014, 62, 116–122. [Google Scholar]
- Pulido, R.; Hernández-García, M.; Saura-Calixto, F. Contribution of beverages to the intake of lipophilic and hydrophilic antioxidants in the Spanish diet. Eur. J. Clin. Nutr. 2003, 57, 1275–1282. [Google Scholar]
- Svilaas, A.; Kaur Sakhi, A.; Frost Andersen, L.; Svilaas, T.; Ström, E.C.; Jacobs, D.R., Jr.; Ose, L.; Blomhoff, R. Intakes of antioxidants in coffee, wine, and vegetables are correlated with plasma carotenoids in humans. J. Nutr. 2004, 134, 562–567. [Google Scholar]
- Fukushima, Y.; Ohie, T.; Yonekawa, Y.; Yonemoto, K.; Aizawa, H.; Mori, Y.; Watanabe, M.; Takeuchi, M.; Hasegawa, M.; Taguchi, C.; Kondo, K. Coffee and green tea as a large source of antioxidant polyphenols in the Japanese population. J. Agric. Food Chem. 2009, 57, 1253–1259. [Google Scholar]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215–217. [Google Scholar]
- Perez-Jimenez, J.; Neveu, V.; Vos, F.; Scalbert, A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: An application of the phenol-explorer database. J. Agric. Food Chem. 2010, 58, 4959–4969. [Google Scholar]
- Chen, C.W.; Ho, C.T. Antioxidant properties of polyphenols extracted from green and black teas. J. Food Lipids 1995, 2, 35–46. [Google Scholar]
- Frei, B.; Higdon, J.V. Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. J. Nutr. 2003, 133, 3275–3284. [Google Scholar]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 70, 362–372. [Google Scholar]
- Madhava Naidu, M.; Sulochanamma, G.; Sampathu, S.R.; Srinivas, P. Studies on extraction and antioxidant potential of green coffee. Food Chem. 2008, 107, 377–384. [Google Scholar]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar]
- Moon, J.K.; Yoo, H.S.; Shibamoto, T. Role of roasting conditions in the level of chlorogenic acid content in coffee beans: Correlation with coffee acidity. J. Agric. Food Chem. 2009, 57, 5365–5369. [Google Scholar]
- Smrke, S.; Opitz, S.E.W.; Vovk, I.; Yeretzian, C. How does roasting affect the antioxidants of a coffee brew? Exploring the antioxidant capacity of coffee via on-line antioxidant assays coupled with size exclusion chromatography. Food Funct. 2013, 4, 1082–1092. [Google Scholar]
- Delgado-Andrade, C.; Morales, F.J. Unraveling the contribution of melanoidins to the antioxidant activity of coffee brews. J. Agric. Food Chem. 2005, 53, 1403–1407. [Google Scholar]
- Wang, H.Y.; Qian, H.; Yao, W.R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar]
- Prior, R.L.; Cao, G.H. In vivo total antioxidant capacity: Comparison of different analytical methods. Free Radic. Biol. Med. 1999, 27, 1173–1181. [Google Scholar]
- Prior, R.L.; Wu, X.L.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar]
- Magalhaes, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar]
- Daglia, M.; Papetti, A.; Gregotti, C.; Berte, F.; Gazzani, G. In vitro antioxidant and ex vivo protective activities of green and roasted coffee. J. Agric. Food Chem. 2000, 48, 1449–1454. [Google Scholar]
- Del Castillo, M.D.; Ames, J.M.; Gordon, M.H. Effect of roasting on the antioxidant activity of coffee brews. J. Agric. Food Chem. 2002, 50, 3698–3703. [Google Scholar]
- Nicoli, M.C.; Innocente, N.; Pittia, P.; Lerici, C.R. Staling of roasted coffee: Volatile release and oxidation reactions during storage. ASIC 15th Int. Sci. Colloq. Coffee 1993, 15, 557–566. [Google Scholar]
- Borrelli, R.C.; Visconti, A.; Mennella, C.; Anese, M.; Fogliano, V. Chemical characterization and antioxidant properties of coffee melanoidins. J. Agric. Food Chem. 2002, 50, 6527–6533. [Google Scholar]
- Cämmerer, B.; Kroh, L.W. Antioxidant activity of coffee brews. Eur. Food Res. Technol. 2006, 223, 469–474. [Google Scholar]
- Da Silveira Duarte, S.M.; de Abreu, C.M.P.; de Menezes, H.C.; dos Santos, M.H.; Gouvea, C.M.C.P. Effect of processing and roasting on the antioxidant activity of coffee brews. Food Sci. Technol. (Campinas) 2005, 25, 387–393. [Google Scholar]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar]
- Opitz, S.E.W.; Smrke, S.; Goodman, B.A.; Yeretzian, C. Methodology for the measurement of antioxidant capacity of coffee: A validated platform composed of three complementary antioxidant assays. In Processing and Impact on Antioxidants in Beverages; Preedy, V., Ed.; Academic Press: Oxford, UK, 2014; pp. 253–264. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar]
- Pellegrini, N.; Serafini, M.; Colombi, B.; del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar]
- Bisby, R.H.; Brooke, R.; Navaratnam, S. Effect of antioxidant oxidation potential in the oxygen radical absorption capacity (ORAC) assay. Food Chem. 2008, 108, 1002–1007. [Google Scholar]
- Iveković, D.; Milardović, S.; Roboz, M.; Grabarić, B.S. Evaluation of the antioxidant activity by flow injection analysis method with electrochemically generated ABTS radical cation. Analyst 2005, 130, 708–714. [Google Scholar]
- Watanabe, J.; Oki, T.; Takebayashi, J.; Yamasaki, K.; Takano-Ishikawa, Y.; Hino, A.; Yasui, A. Method validation by interlaboratory studies of improved hydrophilic oxygen radical absorbance capacity methods for the determination of antioxidant capacities of antioxidant solutions and food extracts. Anal. Sci. 2012, 28, 159–165. [Google Scholar]
- Huang, D.J.; Ou, B.X.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplateflourescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar]
- Prior, R.L.; Hoang, H.; Gu, L.W.; Wu, X.L.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.J.; Ou, B.X.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar]
- Vignoli, J.A.; Viegas, M.C.; Bassoli, D.G.; de Toledo Benassi, M. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res. Int. 2014, 61, 279–285. [Google Scholar]
- Hotta, H.; Sakamoto, H.; Nagano, S.; Osakai, T.; Tsujino, Y. Unusually large numbers of electrons for the oxidation of polyphenolic antioxidants. Biochim. Biophys. Acta 2001, 1526, 159–167. [Google Scholar]
- Namazian, M.; Zare, H.R. Electrochemistry of chlorogenic acid: Experimental and theoretical studies. Electrochim. Acta 2005, 50, 4350–4355. [Google Scholar]
- Scott, S.L.; Chen, W.J.; Bakac, A.; Espenson, J.H. Spectroscopic parameters, electrode-potentials, acid ionization-constants, and electron-exchange rates of the 2,2’-azinobis(3-ethylbenzothiazoline-6-sulfonate) radicals and ions. J. Phys. Chem. 1993, 97, 6710–6714. [Google Scholar]
- Nkhili, E.; Brat, P. Reexamination of the ORAC assay: Effect of metal ions. Anal. Bioanal. Chem. 2011, 400, 1451–1458. [Google Scholar]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2’-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar]
- Yeh, C.T.; Yen, G.C. Effects of phenolic acids on human phenolsulfotransferases in relation to their antioxidant activity. J. Agric. Food Chem. 2003, 51, 1474–1479. [Google Scholar]
- Pannala, A.S.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavonoid B-ring chemistry and antioxidant activity: Fast reaction kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar]
- Tian, X.; Schaich, K. Effects of molecular structure on kinetics and dynamics of the Trolox equivalent antioxidant capacity assay with ABTS+∙. J. Agric. Food Chem. 2013, 61, 5511–5519. [Google Scholar]
- Chun, O.K.; Kim, D.O. Consideration on equivalent chemicals in total phenolic assay of chlorogenic acid-rich plums. Food Res. Int. 2004, 37, 337–342. [Google Scholar]
- Hotta, H.; Nagano, S.; Ueda, M.; Tsujino, Y.; Koyama, J.; Osakai, T. Higher radical scavenging activities of polyphenolic antioxidants can be ascribed to chemical reactions following their oxidation. Biochim. Biophys. Acta 2002, 1572, 123–132. [Google Scholar]
- Nenadis, N.; Lazaridou, O.; Tsimidou, M.Z. Use of reference compounds in antioxidant activity assessment. J. Agric. Food Chem. 2007, 55, 5452–5460. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opitz, S.E.W.; Smrke, S.; Goodman, B.A.; Keller, M.; Schenker, S.; Yeretzian, C. Antioxidant Generation during Coffee Roasting: A Comparison and Interpretation from Three Complementary Assays. Foods 2014, 3, 586-604. https://doi.org/10.3390/foods3040586
Opitz SEW, Smrke S, Goodman BA, Keller M, Schenker S, Yeretzian C. Antioxidant Generation during Coffee Roasting: A Comparison and Interpretation from Three Complementary Assays. Foods. 2014; 3(4):586-604. https://doi.org/10.3390/foods3040586
Chicago/Turabian StyleOpitz, Sebastian E. W., Samo Smrke, Bernard A. Goodman, Marco Keller, Stefan Schenker, and Chahan Yeretzian. 2014. "Antioxidant Generation during Coffee Roasting: A Comparison and Interpretation from Three Complementary Assays" Foods 3, no. 4: 586-604. https://doi.org/10.3390/foods3040586
APA StyleOpitz, S. E. W., Smrke, S., Goodman, B. A., Keller, M., Schenker, S., & Yeretzian, C. (2014). Antioxidant Generation during Coffee Roasting: A Comparison and Interpretation from Three Complementary Assays. Foods, 3(4), 586-604. https://doi.org/10.3390/foods3040586




