Relationship Between Rennet Coagulation Properties of Milk, Cheese-Making Losses, and Cheese Yield in Manufacture of Parmigiano Reggiano PDO Cheese
Abstract
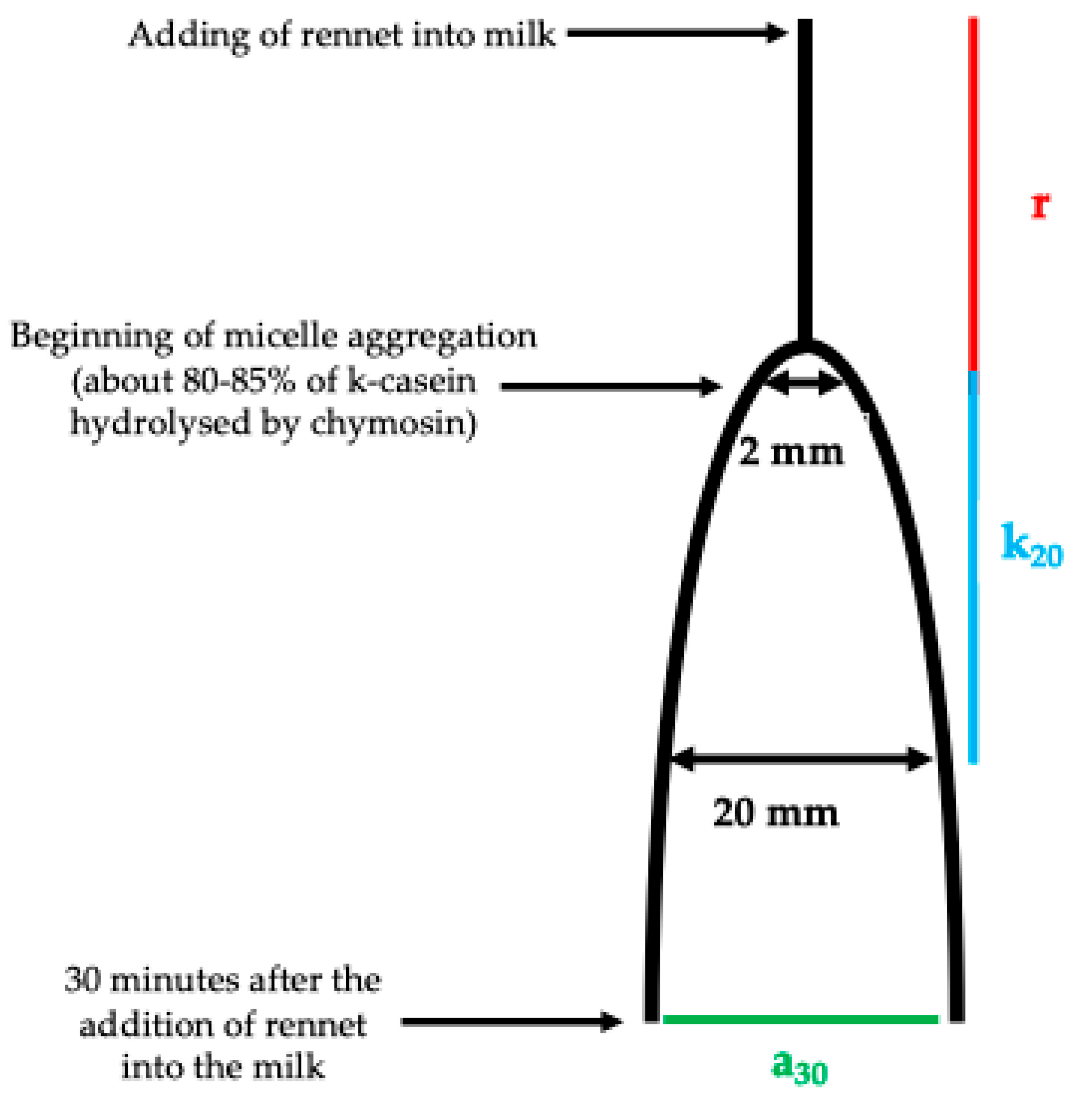
1. Introduction
2. Materials and Methods
2.1. Parmigiano Reggiano Cheese-Making
2.2. Experimental Design
- -
- Optimal (optimal RCP), including lactodynamographic types A, B, and AB (20 cheese-making trials);
- -
- Sub-optimal (sub-optimal RCP), including lactodynamographic types C, AE, EA, AD, and CC (20 cheese-making trials);
- -
- Poor (poor RCP), including lactodynamographic types D, E, and EF (20 cheese-making trials).
2.3. Sample Collection and Sampling Procedure
- -
- A sample of whole milk of the evening milking (W-milk), sampled directly from the creaming tank, before the start of the natural creaming process.
- -
- A sample of vat milk (V-milk), consisting of a mixture of the partially skimmed milk of the evening milking, obtained by natural creaming, and the whole milk of the morning milking. Sampling was performed directly in the vat milk at the beginning of the cheese-making process, before the addition of the whey starter.
- -
- A sample of residual cooked whey (C-whey), taken after the extraction of the cheese from the vat milk and after stirring the cooked whey for 5 min.
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results
- -
- Strength to cut was negatively correlated with curd firming time (p ≤ 0.001) and positively correlated with curd firmness (p ≤ 0.01);
- -
- Strength to compression showed a negative correlation with curd firming time and a positive correlation with curd firmness, both with p ≤ 0.001.
4. Discussion
4.1. Chemical Composition, Physico-Chemical Properties, and Rheological Parameters of Milk
4.2. Curd Rheological Properties, Cheese Yield, and Cheese-Making Losses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Britten, M.; Giroux, H.E. Rennet coagulation of heated milk: A review. Int. Dairy J. 2022, 124, 105–179. [Google Scholar] [CrossRef]
- Fox, P.F.; McSweeney, P.L.H. Cheese: An overview. In Cheese: Chemistry, Physics and Microbiology; General Aspects, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Elsevier Ltd.: San Diego, CA, USA; p. 5e21.
- Fox, P.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science, 2nd ed.; Springer: Boston, MA, USA, 2017; p. 217. [Google Scholar]
- Malacarne, M.; Franceschi, P.; Formaggioni, P.; Sandri, S.; Mariani, P.; Summer, A. Influence of micellar calcium and phosphorus on rennet coagulation properties of cows milk. J. Dairy Res. 2014, 81, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Bittante, G. Modeling rennet coagulation time and curd firmness of milk. J. Dairy Sci. 2011, 94, 5821–5832. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, P.D.; Andersen, K.K.; Hammershøj, M.; Poulsen, H.D.; Sørensen, J.; Bakman, M.; Qvist, K.B.; Larsen, L.B. Composition and effect of blending of noncoagulating, poorly coagulating, and well-coagulating bovine milk from individual Danish Holstein cows. J. Dairy Sci. 2011, 94, 4787–4799. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, P.; Malacarne, M.; Formaggioni, P.; Cipolat-Gotet, C.; Stocco, G.; Summer, A. Effect of season and cheese-factory on cheesemaking efficiency in Parmigiano Reggiano manufacture. Foods 2019, 8, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Beresford, T.P.; Fitzsimons, N.A.; Brennan, N.L.; Cogan, T.M. Recent advances in cheese microbiology. Int. Dairy J. 2011, 11, 59–274. [Google Scholar] [CrossRef]
- Franciosi, E.; Settanni, L.; Cologna, N.; Cavazza, A.; Poznanski, E. Microbial analysis of raw cows’ milk used for cheese-making: Influence of storage treatments on microbial composition and other technological traits. World J. Microbiol. Biotechnol. 2011, 27, 171–181. [Google Scholar] [CrossRef]
- Franceschi, P.; Malacarne, M.; Formaggioni, P.; Faccia, M.; Summer, A. Effects of milk storage temperature at the farm on the characteristics of Parmigiano Reggiano cheese: Chemical composition and proteolysis. Animals 2021, 11, 879. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.J.; Brown, R.J. Evaluation of Formagraph for comparing rennet solutions. J. Dairy Sci. 1982, 65, 1639–1642. [Google Scholar] [CrossRef]
- Bittante, G.; Penasa, M.; Cecchinato, A. Genetics and modeling of milk coagulation properties. J. Dairy Sci. 2012, 95, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Bonfatti, V.; Tuzzato, M.; Chiarot, G.; Carnier, P. Variation in milk coagulation properties does not affect cheese yield and composition of model cheese. Int. Dairy J. 2014, 39, 139–145. [Google Scholar] [CrossRef]
- Franceschi, P.; Formaggioni, P.; Barbanti, D.; Gonzalez Torres, Y.O.; Scotti, C.; Martuzzi, F. Association between herd size and the chemical composition and technological properties of milk intended for Parmigiano Reggiano PDO cheese. Foods 2025, 14, 494–510. [Google Scholar] [CrossRef]
- Ng-Kwai-Hang, K.F.; Politis, I.; Cue, R.I.; Marziali, A.S. Correlations between coagulation properties of milk and cheese yielding capacity and cheese composition. Can. Inst. Food Technol. J. 1989, 22, 291–294. [Google Scholar] [CrossRef]
- Pretto, D.; De Marchi, M.; Penasa, M.; Cassandro, M. Effect of milk composition and coagulation traits on Grana Padano cheese yield under field conditions. J. Dairy Res. 2013, 80, 1–5. [Google Scholar] [CrossRef]
- Wedholm, A.; Larsen, L.B.; Lindmark-Mansson, H.; Karlsson, A.H.; Andrén, A. Effect of protein composition on the cheese-making properties of milk from individual dairy cows. J. Dairy Sci. 2006, 89, 3296–3305. [Google Scholar] [CrossRef]
- Council Regulation (EU) No PDO-IT-02202 of 14 November 2016. Off. J European Union of 13 April 2018, C132/17–19. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52018XC0413(01) (accessed on 21 January 2026).
- Verdier-Metz, I.; Coulon, J.B.; Pradel, P. Relationship between milk fat and protein contents and cheese yield. Anim. Res. 2001, 50, 365–371. [Google Scholar] [CrossRef]
- Franceschi, P.; Malacarne, M.; Formaggioni, P.; Faccia, M.; Summer, A. Quantification of the effect of the cattle breed on milk cheese yield: Comparison between Italian Brown Swiss and Italian Friesian. Animals 2020, 10, 1331. [Google Scholar] [CrossRef] [PubMed]
- ISO9622:2013; Milk and Liquid Milk Products, Guidelines for the Application of Mid- Infrared Spectrometry. International Dairy Federation Standard: Brussels, Belgium, 2013.
- ISO13366:2006; Milk, Enumeration of Somatic Cells, Part 2: Guidance on the Operation of Fluoro-Opto-Electronic Counters. International Dairy Federation Standard: Brussels, Belgium, 2006.
- ISO16297:2013; Milk Bacterial Count: Protocol for the Evaluation of Alternative Methods. International Dairy Federation: Brussels, Belgium, 2013.
- Association of Official Analytical Chemists. Nitrogen (total) in milk, method no. 991.20. In Official Methods of Analysis of AOAC International, 18th ed.; Horowitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2005; pp. 10–12. [Google Scholar]
- Association of Official Analytical Chemists. Noncasein nitrogen content of milk, method no. 998.05. In Official Methods of Analysis of AOAC International, 18th ed.; Horowitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2005; pp. 50–51. [Google Scholar]
- Association of Official Analytical Chemists. Nonprotein nitrogen in whole milk, method no. 991.21. In Official Methods of Analysis of AOAC International, 18th ed.; Horowitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2005; pp. 12–13. [Google Scholar]
- Lynch, J.M.; Barbano, D.M.; Fleming, J.R. Indirect and direct determination of the casein content of milk by Kjeldahl nitrogen analysis: Collaborative study. J. AOAC Int. 1998, 81, 63–74. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Milk acidity, Titrimetric Methods, method no. 947.05. In Official Methods of Analysis of AOAC International, 15th ed.; Helrich, K., Ed.; AOAC International: Arlington, VA, USA, 1990; pp. 805–830. [Google Scholar]
- ISO5534:2004; Cheese and Processed Cheese, Determination of the Total Solids Content (Reference Method). International Dairy Federation Standard: Brussels, Belgium, 2004.
- IDF 27:1964; Determination of Ash Content of Processed Cheese Product. International Dairy Federation: Brussels, Belgium, 1964.
- Franceschi, P.; Martuzzi, F.; Formaggioni, P.; Malacarne, M.; Summer, A. Seasonal variations of the protein fractions and the mineral contents of the cheese whey in the Parmigiano Reggiano cheese manufacture. Agriculture 2023, 13, 165–176. [Google Scholar] [CrossRef]
- ISO9874:2006; Milk—Determination of Total Phosphorus Content—Method Using Molecular Absorption Spectrometry. International Dairy Federation Standard: Brussels, Belgium, 2006.
- ISO21422:2018; Milk, Milk Products, Infant Formula and Adult Nutritional, Determination of Chloride, Potentiometric Titration method. International Dairy Federation Standard: Brussels, Belgium, 2018.
- Careri, M.; Spagnoli, S.; Panari, G.; Zannoni, M.; Barbieri, G. Chemical parameters of the non-volatile fraction of ripened Parmigiano-Reggiano cheese. Int. Dairy J. 1996, 6, 147–155. [Google Scholar] [CrossRef]
- Ikonen, T.; Morri, S.; Tyrisevä, A.M.; Ruottinen, O.; Ojala, M. Genetic and phenotypic correlations between milk coagulation properties, milk production traits, somatic cell count, casein content and pH of milk. J. Dairy Sci. 2004, 87, 458–467. [Google Scholar] [CrossRef]
- Cassandro, M.; Comin, A.; Ojala, M.; Dal Zotto, R.; De Marchi, M.; Gallo, L.; Carnier, P.; Bittante, G. Genetic parameters of milk coagulation properties and their relationships with milk yield and quality traits in Italian Holstein cows. J. Dairy Sci. 2008, 91, 371–376. [Google Scholar] [CrossRef]
- Horne, D.S. Casein interactions: Casting light on the black boxes, the structure in dairy products. Int. Dairy J. 1998, 8, 171–177. [Google Scholar] [CrossRef]
- Gaucheron, F. The minerals of milk. Reprod. Nutr. Dev. 2005, 45, 473–483. [Google Scholar] [CrossRef]
- de la Fuente, M.A.; Olano, A.; Juarez, M. Distribution of calcium, magnesium, phosphorus, zinc, manganese, copper and iron between the soluble and colloidal phases of ewe’s and goat’s milk. Lait 1997, 77, 515–520. [Google Scholar] [CrossRef]
- Dalgleish, D.G.; Corredig, M. The structure of the casein micelle of milk and its changes during processing. Annu. Rev. Food Sci. Technol. 2012, 3, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, L.C. Studies on micellar calcium phosphate: Composition and apparent solubility product in milk over a wide pH range. J. Dairy Res. 1984, 51, 251–257. [Google Scholar] [CrossRef]
- Urech, E.; Puhan, Z.; Schällibaum, M. Changes in milk protein fraction as affected by subclinical mastitis. J. Dairy Sci. 1999, 82, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, P.; Faccia, M.; Malacarne, M.; Formaggioni, P.; Summer, A. Quantification of cheese yield reduction in manufacturing Parmigiano Reggiano from milk with non-compliant somatic cells count. Foods 2020, 9, 212–222. [Google Scholar] [CrossRef]
- Osorio, J.S.; Lohakare, J.; Bionaz, M. Biosynthesis of milk fat, protein, and lactose: Roles of transcriptional and posttranscriptional regulation. Physiol. Genom. 2016, 48, 231–256. [Google Scholar] [CrossRef]
- Costa, A.; Lopez-Villalobos, N.; Sneddon, N.W.; Shalloo, L.; Franzoi, M.; De Marchi, M.; Penasa, M. Invited review: Milk lactose—Current status and future challenges in dairy cattle. J. Dairy Sci. 2019, 102, 5883–5898. [Google Scholar] [CrossRef] [PubMed]
- Shennan, D.B.; Peaker, M. Transport of milk constituents by the mammary gland. Physiol. Rev. 2000, 80, 925–951. [Google Scholar] [CrossRef] [PubMed]
- Cant, J.P.; Trout, D.R.; Qiao, F.; Purdie, N.G. Milk synthetic response of the bovine mammary gland to an increase in the local concentration of arterial glucose. J. Dairy Sci. 2002, 85, 494–503. [Google Scholar] [CrossRef]
- Somers, J.; O’Brien, B.; Meany, W.; Kelly, A.L. Heterogeneity of proteolytic enzyme activities in milk samples of different somatic cell count. J. Dairy Res. 2003, 70, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.K.; Suman, K.; Charan, S.; Sehgal, J.P. Milk differential cell counts and compositional changes in cows during different physiological stages. Milchwissenschaft 2008, 63, 239–242. [Google Scholar]
- Emmons, D.B.; Dubeé, C.; Modler, H.W. Transfer of protein from milk to cheese. J. Dairy Sci. 2003, 86, 469–485. [Google Scholar] [CrossRef]
- Franceschi, P.; Formaggioni, P.; Brasca, M.; Natrella, G.; Faccia, M.; Malacarne, M.; Summer, A. Fatty acids composition and lipolysis of Parmigiano Reggiano PDO cheese: Effect of the milk cooling temperature at the farm. Anim. Biosci. 2023, 36, 132–143. [Google Scholar] [CrossRef]
- Barbano, D.M.; Rasmussen, R.R.; Lynch, J.M. Influence of milk somatic cell count and milk age on cheese yield. J. Dairy Sci. 1991, 74, 369–388. [Google Scholar] [CrossRef]
- Politis, I.; Ng-Kwai-Hang, K.F. Association between somatic cell count of milk and cheese-yielding capacity. J. Dairy Sci. 1988, 71, 1720–1727. [Google Scholar] [CrossRef]
- Klei, L.; Yun, J.; Sapru, A.; Lynch, J.; Barbano, D.M.; Sears, P.; Galton, D. Effects of milk somatic cell count on Cottage cheese yield and quality. J. Dairy Sci. 1998, 81, 1205–1213. [Google Scholar] [CrossRef]
- Kethireddipalli, P.; Hill, A.R. Rennet coagulation and cheesemaking properties of thermally processed milk: Overview and recent developments. J. Agric. Food Chem. 2015, 63, 9389–9403. [Google Scholar] [CrossRef] [PubMed]
- Green, M.L.; Grandison, A.S. Secondary (non-enzymatic) phase of rennet coagulation and post-coagulation phenomena. In Cheese: Chemistry, Physics and Microbiology; Fox, P.F., Ed.; Chapman & Hall: London, UK, 1993; Volume 1, pp. 101–140. [Google Scholar]
- De Marchi, M.; Dal Zotto, R.; Cassandro, M.; Bittante, G. Milk coagulation ability of five dairy cattle breeds. J. Dairy Sci. 2007, 90, 3986–3992. [Google Scholar] [CrossRef] [PubMed]
| Operation | Number | Description |
|---|---|---|
| Duration of experimental design | 2 years | Two consecutive years |
| Cheese factories involved | 10 cheese factories | Located in the Parma and Reggio Emilia provinces |
| Trials performed | 20 experimental trials | One trial per cheese factory per year |
| Cheese-making processes | 60 cheese-making processes | Three cheese-making processes per cheese factory per trial: optimal, sub-optimal, and poor rennet coagulation properties |
| Clotting Time (Minutes) | Curd Firmness (Millimetres) | Lactodynamograms Type | Rennet Coagulation Classes |
|---|---|---|---|
| Less than 5 | Between 0 to 100 | DD | Poor |
| Between 5 and 8 | Less than 30 | C | Suboptimal |
| Between 5 and 8 | Between 30 to 53 | AB | Optimal |
| Between 5 and 8 | Between 54 to 100 | CC | Suboptimal |
| Between 8 and 11 | Less than 30 | C | Suboptimal |
| Between 8 and 11 | Between 30 to 40 | AB | Optimal |
| Between 8 and 11 | Between 40 to 100 | AD | Suboptimal |
| Between 11 and 17.30 | Less than 19 | AE | Suboptimal |
| Between 11 and 17.30 | Between 19 to 40 | A | Optimal |
| Between 11 and 17.30 | Between 40 to 100 | B | Optimal |
| Between 17.30 and 20 | Less than 19 | E | Poor |
| Between 17.30 and 20 | Between 19 to 40 | EA | Suboptimal |
| Between 17.30 and 20 | Between 40 to 100 | B | Optimal |
| Between 20 and 22 | Less than 19 | E | Poor |
| Between 20 and 22 | Between 19 to 100 | EA | Suboptimal |
| Between 22 and 26 | Less than 100 | E | Poor |
| Between 26 and 28 | Less than 4 | EF | Poor |
| Between 26 and 28 | Between 4 to 100 | E | Poor |
| Between 28 and 30 | Less than 100 | EF | Poor |
| Optimal n 1 = 20 | Sub-Optimal n 1 = 20 | Poor n 1 = 20 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Measure Units | Mean | Mean | Mean | SE 2 | p 3 | |||
| Fat | g/100 g | 3.64 | b | 3.68 | b | 3.58 | a | 0.03 | * |
| Crude protein | g/100 g | 3.25 | 3.24 | 3.24 | 0.02 | NS | |||
| Casein | g/100 g | 2.51 | 2.50 | 2.50 | 0.02 | NS | |||
| Somatic cells | 103 cells/mL | 249 | 250 | 243 | 32 | NS | |||
| Total bacterial count | 103 CFU/mL | 49 | 54 | 36 | 16 | NS | |||
| Optimal n 1 = 20 | Sub-Optimal n 1 = 20 | Poor n 1 = 20 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Measure Units | Mean | Mean | Mean | SE 2 | p 3 | |||
| Lactose | g/100 g | 5.00 | b | 5.04 | b | 4.91 | a | 0.03 | *** |
| Fat | g/100 g | 2.75 | 2.72 | 2.73 | 0.03 | NS | |||
| Crude protein | g/100 g | 3.28 | 3.27 | 3.26 | 0.04 | NS | |||
| Whey protein | g/100 g | 0.75 | 0.75 | 0.74 | 0.01 | NS | |||
| Casein | g/100 g | 2.53 | 2.52 | 2.52 | 0.02 | NS | |||
| Casein number | % | 77.13 | 77.06 | 77.30 | 0.16 | NS | |||
| NPN × 6.38 | g/100 g | 0.17 | 0.17 | 0.16 | 0.01 | NS | |||
| Fat-to-casein ratio | Value | 1.09 | 1.08 | 1.08 | 0.02 | NS | |||
| Calcium | mg/100 g | 122.51 | c | 115.73 | b | 110.10 | a | 0.94 | ** |
| Phosphorus | mg/100 g | 95.02 | c | 91.14 | b | 88.78 | a | 0.72 | *** |
| Magnesium | mg/100 g | 11.97 | b | 10.86 | b | 10.03 | a | 0.11 | * |
| Citric acid | mg/100 g | 181.10 | c | 172.13 | b | 166.47 | a | 1.71 | * |
| Chloride | mg/100 g | 90.18 | a | 91.06 | a | 97.94 | b | 1.18 | * |
| Somatic cell count | 103 cells/mL | 172 | a | 157 | a | 220 | b | 11 | * |
| Optimal n 1 = 20 | Sub-Optimal n 1 = 20 | Poor n 1 = 20 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Measure Units | Mean | Mean | Mean | SE 2 | p 3 | |||
| Titratable acidity | °SH/50 mL | 3.44 | c | 3.30 | b | 3.08 | a | 0.02 | *** |
| pH | Value | 6.68 | a | 6.70 | b | 6.72 | b | 0.01 | * |
| Clotting time | minutes | 15.50 | a | 18.53 | b | 21.18 | c | 0.27 | *** |
| Curd firming time | minutes | 6.24 | a | 9.02 | b | 10.92 | c | 0.87 | * |
| Curd firmness | millimetres | 35.73 | c | 28.82 | b | 17.02 | a | 1.22 | *** |
| Strength to cut | grams | 68.97 | b | 64.43 | b | 44.38 | a | 2.86 | ** |
| Strength to compression | grams | 31.48 | b | 30.49 | b | 25.70 | a | 1.12 | ** |
| Optimal n 1 = 20 | Sub-Optimal n 1 = 20 | Poor n 1 = 20 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Measure Units | Mean | Mean | Mean | SE 2 | p 3 | |||
| Actual cheese yield | Kg/100 kg | 8.79 | b | 8.56 | ab | 8.08 | a | 0.10 | ** |
| Protein losses | % | 26.90 | 26.77 | 26.52 | 0.16 | NS | |||
| Fat losses | % | 14.23 | a | 15.48 | ab | 16.72 | b | 0.58 | * |
| Calcium losses | % | 35.12 | 35.99 | 35.48 | 0.48 | NS | |||
| Phosphorus losses | % | 48.40 | 48.95 | 49.44 | 0.38 | NS | |||
| Magnesium losses | % | 74.88 | 75.60 | 76.13 | 1.28 | NS | |||
| Calcium | Phosphorus | Citric Acid | ||||
|---|---|---|---|---|---|---|
| r 1 | p 2 | r 1 | p 2 | r 1 | p 2 | |
| Clotting time | −0.378 | ** | −0.376 | ** | −0.479 | ** |
| Curd firming time | −0.481 | ** | −0.451 | ** | −0.237 | NS |
| Curd firmness | 0.542 | *** | 0.672 | * | 0.341 | * |
| Strength to cut | 0.512 | *** | 0.609 | *** | 0.080 | NS |
| Strength to compression | 0.511 | *** | 0.624 | *** | 0.302 | NS |
| Strength to Cut | Strength to Compression | |||
|---|---|---|---|---|
| r 1 | p 2 | r 1 | p 2 | |
| Clotting time | −0.218 | NS | −0.371 | NS |
| Curd firming time | −0.527 | *** | −0.672 | *** |
| Curd firmness | 0.450 | ** | 0.656 | *** |
| Strength to cut | - | - | 0.549 | *** |
| Strength to compression | 0.549 | *** | - | - |
| Actual Cheese Yield | Protein Losses | Fat Losses | ||||
|---|---|---|---|---|---|---|
| r 1 | p 2 | r 1 | p 2 | r 1 | p 2 | |
| Clotting time | −0.089 | NS | 0.101 | NS | −0.063 | NS |
| Curd firming time | −0.505 | ** | −0.099 | NS | 0.578 | *** |
| Curd firmness | 0.456 | ** | −0.054 | NS | −0.486 | * |
| Strength to cut | 0.523 | *** | −0.068 | NS | −0.129 | NS |
| Strength to compression | 0.637 | *** | 0.037 | NS | −0.440 | * |
| Actual cheese yield | - | - | 0.114 | NS | −0.355 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Franceschi, P.; Barbanti, D.; Formaggioni, P.; Scotti, C.; Giambiasi, P.; Martuzzi, F. Relationship Between Rennet Coagulation Properties of Milk, Cheese-Making Losses, and Cheese Yield in Manufacture of Parmigiano Reggiano PDO Cheese. Foods 2026, 15, 428. https://doi.org/10.3390/foods15030428
Franceschi P, Barbanti D, Formaggioni P, Scotti C, Giambiasi P, Martuzzi F. Relationship Between Rennet Coagulation Properties of Milk, Cheese-Making Losses, and Cheese Yield in Manufacture of Parmigiano Reggiano PDO Cheese. Foods. 2026; 15(3):428. https://doi.org/10.3390/foods15030428
Chicago/Turabian StyleFranceschi, Piero, Davide Barbanti, Paolo Formaggioni, Cristina Scotti, Paola Giambiasi, and Francesca Martuzzi. 2026. "Relationship Between Rennet Coagulation Properties of Milk, Cheese-Making Losses, and Cheese Yield in Manufacture of Parmigiano Reggiano PDO Cheese" Foods 15, no. 3: 428. https://doi.org/10.3390/foods15030428
APA StyleFranceschi, P., Barbanti, D., Formaggioni, P., Scotti, C., Giambiasi, P., & Martuzzi, F. (2026). Relationship Between Rennet Coagulation Properties of Milk, Cheese-Making Losses, and Cheese Yield in Manufacture of Parmigiano Reggiano PDO Cheese. Foods, 15(3), 428. https://doi.org/10.3390/foods15030428










