Optimization of Ultrasound Pretreatment for Enhanced Drying Efficiency and Piperine Retention in Black Pepper (Piper nigrum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
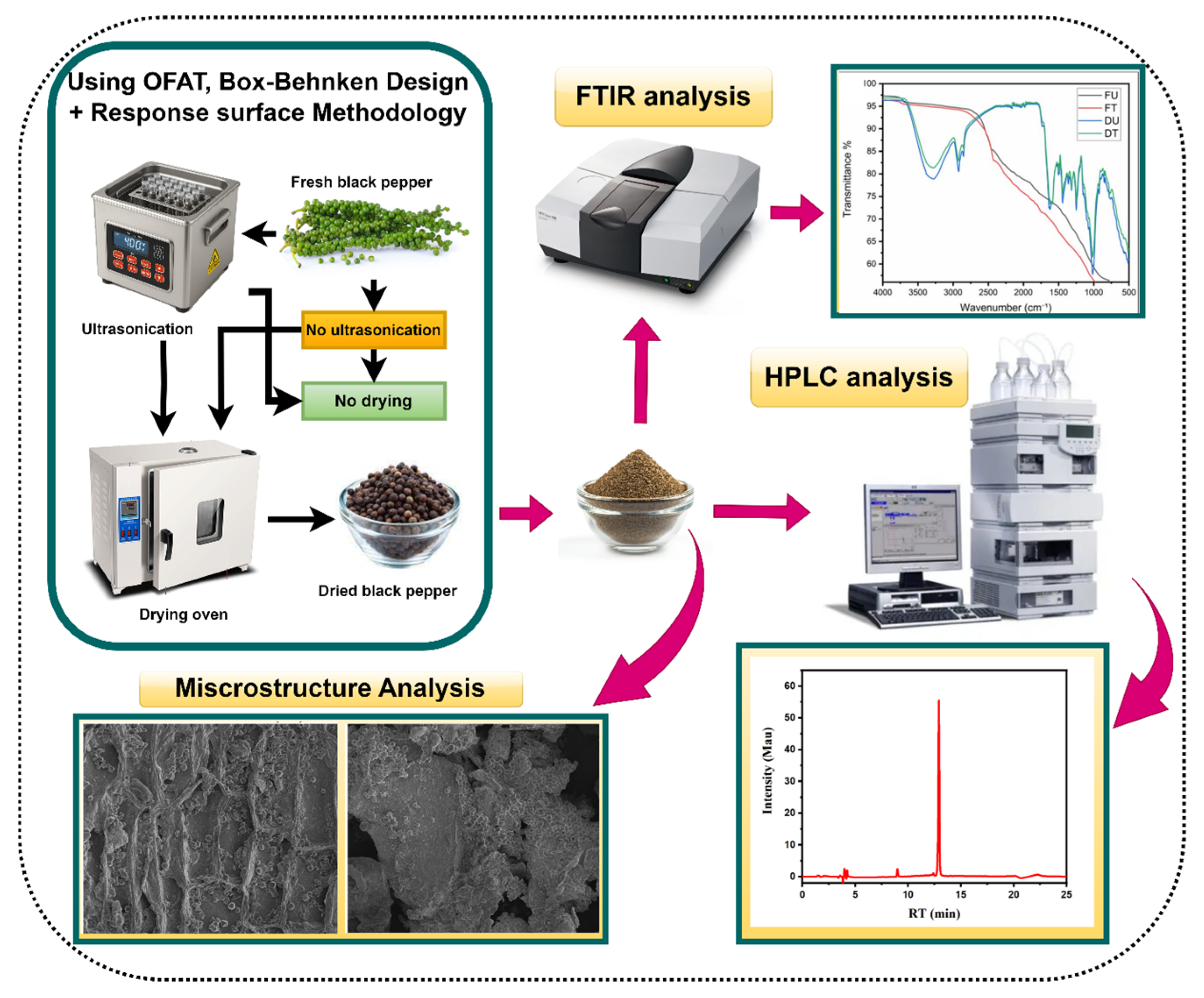
2.2. Experimental Design and Optimization Strategy
2.2.1. Convective Drying Procedure
2.2.2. Energy Consumption Analysis and Ultrasound Intensity Quantification
2.2.3. Mechanistic Modeling of Mass Transfer
2.3. Analytical Methodologies
2.3.1. Moisture Content Determination
2.3.2. Determination of Piperine Content via UV-Spectrophotometry
2.3.3. Determination of Piperine Content via High-Performance Liquid Chromatography
2.4. Microstructural and Chemical Characterization
2.4.1. Scanning Electron Microscopy (SEM) Analysis
2.4.2. Fourier Transform Infrared (FTIR) Spectroscopy
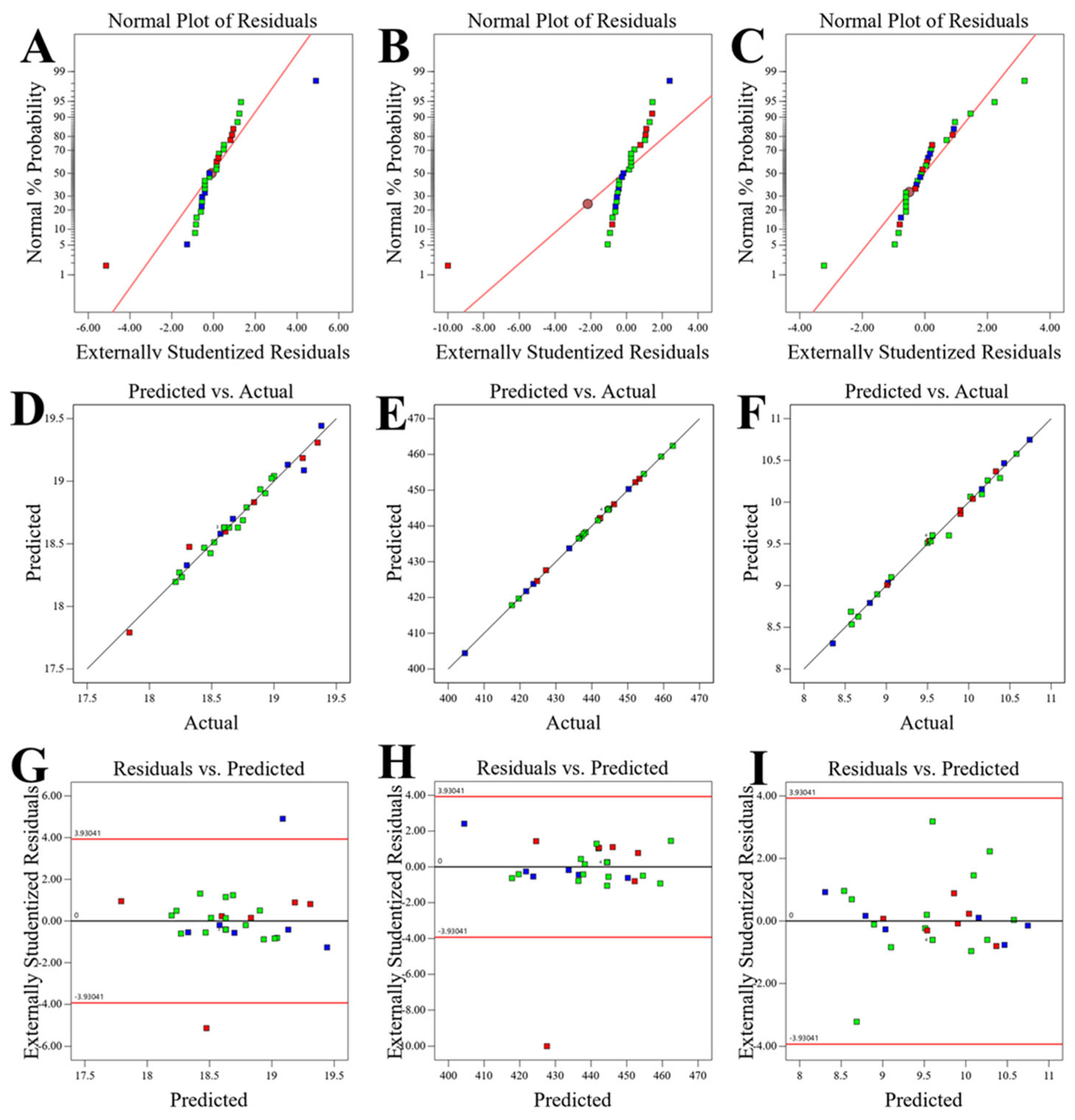
2.4.3. Statistical Analysis and Model Validation
3. Results
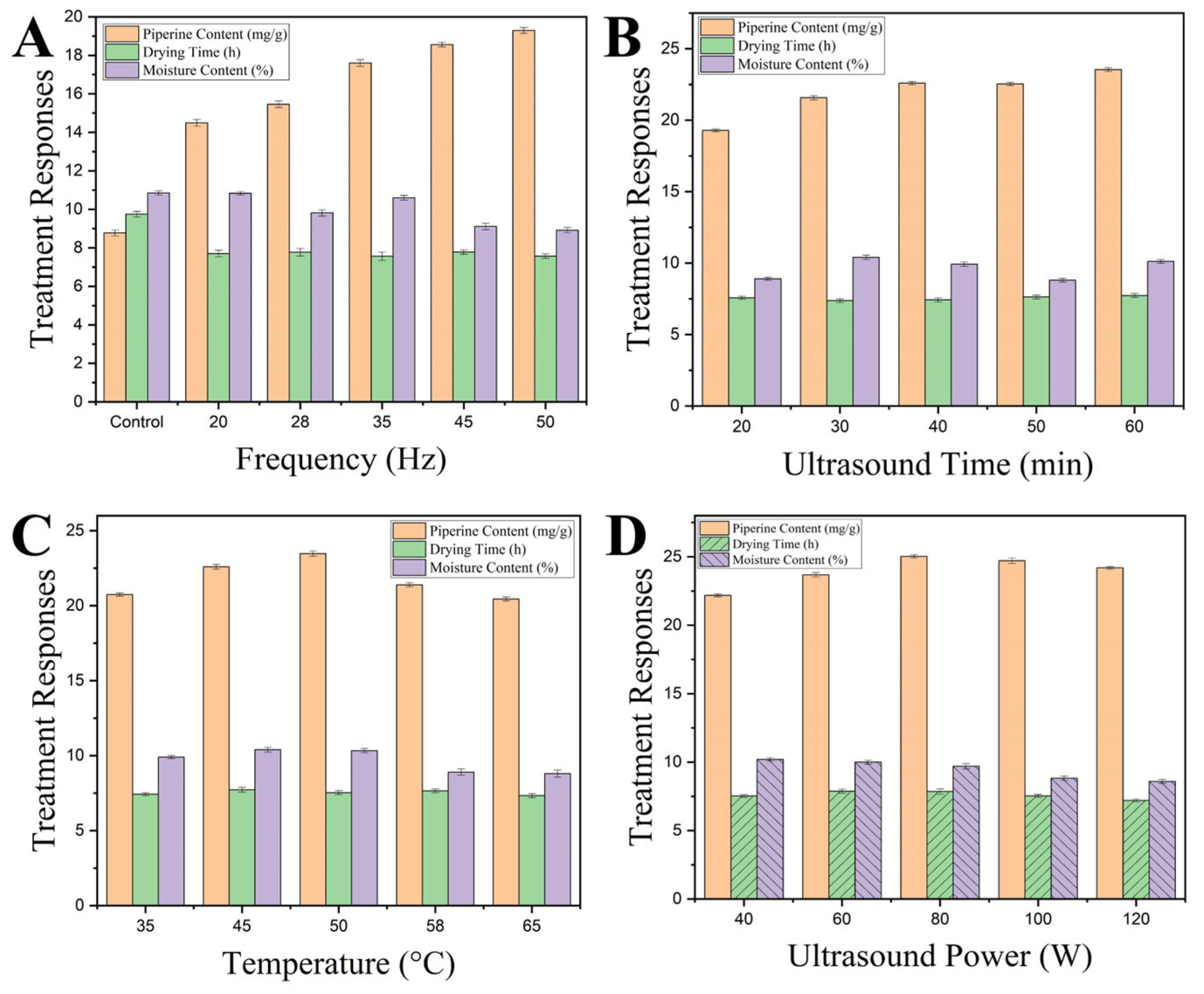
3.1. One-Factor-At-a-Time (OFAT) Parameter Screening
3.2. Response Surface Methodology and Box–Behnken Design Optimization
3.2.1. Box–Behnken Design Implementation and Model Development
3.2.2. Multi-Objective Optimization and Desirability Analysis
3.3. Confirmation Study and Dual-Method Analytical Validation
3.4. Mechanistic Insights and Analytical Validation of Ultrasound Pretreatment for Black Pepper Processing
3.4.1. Mechanistic Synthesis of Ultrasound Effects on Mass Transfer Kinetics and Piperine Stability
3.4.2. Analytical Sensitivity and Method Validation
3.5. Mechanisms of Ultrasound-Assisted Drying on Piperine Stability, Structural Integrity, and Mass Transfer Enhancement
3.5.1. Compound Stability
3.5.2. Ultrasound-Induced Microstructural Modifications
3.5.3. Mass Transfer Enhancement and Piperine Yield Increase
3.5.4. Energy Consumption and Process Scalability Implications
3.5.5. Kinetic Parameters and Effective Moisture Diffusivity
3.6. Limitations and Future Directions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RSM | Response Surface Methodology |
| OFAT | One-Factor-At-a-Time |
| UV | Ultraviolet (spectrophotometry) |
| HPLC | High-Performance Liquid Chromatography |
| FTIR | Fourier Transform Infrared (spectroscopy) |
| SEM | Scanning Electron Microscopy |
| BBD | Box–Behnken Design |
| ANOVA | Analysis of Variance |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| RSD | Relative Standard Deviation |
| DAD | Diode Array Detector |
| ICH | International Council for Harmonisation (guidelines) |
| QbD | Quality by Design |
| RP-HPLC | Reverse Phase High-Performance Liquid Chromatography |
| DI | Desirability Index |
| C.V. | Coefficient of Variation |
| STD | Standard Deviation |
| SD | Standard Deviation |
| kHz | kilohertz |
| W/cm3 | Watts per cubic centimeter |
| μg/mL | micrograms per milliliter |
| min | minutes |
| GB/T | Guobiao/Tuijian (Chinese National Standard/Recommended) |
| KBr | Potassium Bromide |
References
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine—The Bioactive Compound of Black Pepper: From Isolation to Medicinal Formulations. Compr. Rev. Food Sci. Food Saf. 2017, 16, 124–140. [Google Scholar] [CrossRef]
- Johnson, N.A.N.; Adade, S.Y.S.S.; Ekumah, J.N.; Kwadzokpui, B.A.; Xu, J.; Xu, Y.; Chen, Q. A Comprehensive Review of Analytical Techniques for Spice Quality and Safety Assessment in the Modern Food Industry. Crit. Rev. Food Sci. Nutr. 2025, 65, 7225–7250. [Google Scholar] [CrossRef]
- Namjoyan, F.; Hejazi, H.; Ramezani, Z. Evaluation of Drying Process on the Composition of Black Pepper Ethanolic Extract by High Performance Liquid Chromatography With Diode Array Detector. Pharm. Policy Law 2015, 17, 81–89. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.; Meullemiestre, A.; Abert-vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; Wiley-Interscience: Hoboken, NJ, USA, 2015; ISBN 9780470174463. [Google Scholar]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Lučić, M.; Potkonjak, N.; Sredović Ignjatović, I.; Lević, S.; Dajić-Stevanović, Z.; Kolašinac, S.; Belović, M.; Torbica, A.; Zlatanović, I.; Pavlović, V.; et al. Influence of Ultrasonic and Chemical Pretreatments on Quality Attributes of Dried Pepper (Capsicum annuum). Foods 2023, 12, 2468. [Google Scholar] [CrossRef]
- Yilmaz, D.; Tekin-Cakmak, Z.H.; Karasu, S. Impact of Ultrasound Pretreatment and Temperature on Drying Kinetics and Quality Characteristics of Blood Orange Slices: Comparison with Different Drying Methods. Processes 2025, 13, 1596. [Google Scholar] [CrossRef]
- Lucio-Juárez, J.S.; Moscosa-Santillán, M.; González-García, R.; Grajales-Lagunes, A.; Ruiz-Cabrera, M.A. Ultrasonic Assisted Pre-Treatment Method for Enhancing Mass Transfer during the Air-Drying of Habanero Chili Pepper (Capsicum chinense). Int. J. Food Prop. 2013, 16, 867–881. [Google Scholar] [CrossRef]
- Johnson, N.A.N.; Ekumah, J.-N.; Adade, S.Y.-S.S.; Li, Y.; Betchem, G.; Issaka, E.; Ma, Y. Phytochemical and Structural Changes of Chickpea Beverage Prepared Using Ultrasound-Assisted Fermentation with Optimized Ultrasound Parameters Modelled by Response Surface Methodology. Beverages 2023, 9, 62. [Google Scholar] [CrossRef]
- Schössler, K.; Jäger, H.; Knorr, D. Effect of Continuous and Intermittent Ultrasound on Drying Time and Effective Diffusivity during Convective Drying of Apple and Red Bell Pepper. J. Food Eng. 2012, 108, 103–110. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1975. [Google Scholar]
- Azman, P.N.M.A.; Shamsudin, R.; Man, H.C.; Ya’acob, M.E. Some Physical Properties and Mass Modelling of at Di Ff Erent Maturity Levels. Processes 2020, 8, 1314. [Google Scholar] [CrossRef]
- Azman, P.N.M.A.; Shamsudin, R.; Che Man, H.; Ya’acob, M.E. Mass Modelling of Pepper Berries (Piper nigrum L.) with Some Physical. Food Res. 2021, 5, 80–84. [Google Scholar] [CrossRef]
- Shreelavaniya, R.; Kamaraj, S. Effect of Moisture Content on Physical Properties of Black Pepper. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 4873–4879. [Google Scholar] [CrossRef]
- Nemo, R.; Bacha, K. Microbial, Physicochemical and Proximate Analysis of Selected Ethiopian Traditional Fermented Beverages. LWT—Food Sci. Technol. 2020, 131, 109713. [Google Scholar] [CrossRef]
- Patil, A.K.; Jalalpure, S.S.; Chimagave, S.S.; Kurangi, B.K. UV-Spectrophotometric Method Development and Validation for Piperine Estimation in Black Pepper, Ayurvedic Formulation and Novel Nano Formulation: A Perfect Quality Assessment Tool. Indian J. Pharm. Educ. Res. 2024, 58, 305–315. [Google Scholar] [CrossRef]
- GB/T 17528-2009; Determination of Piperine Content—Method Using HPLC. Standardization Administration of China. General Administration of Quality Supervision, Inspection and Quarantine of China: Beijing, China, 2009.
- Parab Gaonkar, V.; Mannur, V.K.; Hullatti, K. Quality Assessment and Analytical Quality by Design-Based RP-HPLC Method Development for Quantification of Piperine in Piper nigrum L. Future J. Pharm. Sci. 2022, 8, 1–9. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Rybak, K.; Pobiega, K.; Ossowski, S. Effect of Ultrasonic Pre-Treatment on the Textural, Structural, and Chemical Properties of Fermented Red Bell Peppers. Appl. Sci. 2025, 15, 2988. [Google Scholar] [CrossRef]
- Deng, L.; Huang, G. Ultrasound-Assisted Extraction, Optimization, Characteristics and Antioxidant Activity of Piper nigrum L. Polysaccharides. Ultrason. Sonochem. 2025, 116, 107309. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A Clean, Green Extraction Technology. TrAC—Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Radziejewska-Kubzdela, E.; Szadzińska, J.; Biegańska-Marecik, R.; Spiżewski, T.; Mierzwa, D. Effect of Ultrasound on Mass Transfer during Vacuum Impregnation and Selected Quality Parameters of Products: A Case Study of Carrots. Ultrason. Sonochem. 2023, 99, 106592. [Google Scholar] [CrossRef]
- Briars, R.; Paniwnyk, L. Examining the Extraction of Artemisinin from Artemisia Annua Using Ultrasound. AIP Conf. Proc. 2012, 1433, 581–585. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in Application of Ultrasound in Food Processing: A Review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef] [PubMed]
- Yolmeh, M.; Jafari, S.M. Applications of Response Surface Methodology in the Food Industry Processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- Alp, D.; Bulantekin, Ö. The Microbiological Quality of Various Foods Dried by Applying Different Drying Methods: A Review. Eur. Food Res. Technol. 2021, 247, 1333–1343. [Google Scholar] [CrossRef]
- Ontario Agency for Health Protection and Promotion (Public Health Ontario); Parto, N. Case Study: Pathogens and Spices; Canadian Electronic Library: Ottawa, ON, Canada, 2016; pp. 1–40. [Google Scholar]
- Keller, S.E.; Vandoren, J.M.; Grasso, E.M.; Halik, L.A. Growth and Survival of Salmonella in Ground Black Pepper (Piper nigrum). Food Microbiol. 2013, 34, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, U.; Slim, D.; Abbagana, M.; Yero, S.A. Alkaleri Metakaolin Powder Performance Evaluation by Response Surface Method. Int. J. Adv. Eng. Manag. 2024, 6, 34–45. [Google Scholar] [CrossRef]
- Gupta, I.; Adina, S.N.; Aqilb, M.; Mujeeba, M.; Ahad, A. Computer-aided box-behnken outlook towards optimization of extraction of piperine from Piper longum L. fruits. World J. Pharm. Res. 2022, 11, 1439–1455. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 106, ISBN 9781119113478. [Google Scholar]
- Johnson, N.A.N.; Ekumah, J.; Ma, Y.; Dzidzorgbe, N.; Akpabli-tsigbe, K.; Solomon, S.Y.; Manching, X.; Quaisie, J.; Kwaw, E.; Wang, C. Optimization of Fermentation Parameters for the Production of a Novel Selenium Enriched Mulberry (Morus nigra) Wine. LWT 2023, 178, 114608–114620. [Google Scholar] [CrossRef]
- Whitcomb, P.J.; Anderson, M.J. RSM Simplified: Optimizing Processes Using Response Surface Methods for Design of Experiments, 1st ed.; Taylor and Francis: New York, NY, USA, 2004. [Google Scholar]
- Giannakourou, M.C.; Lazou, A.E.; Dermesonlouoglou, E.K. Optimization of Osmotic Dehydration of Tomatoes in Solutions of Non-Conventional Sweeteners by Response Surface Methodology and Desirability Approach. Foods 2020, 9, 1393. [Google Scholar] [CrossRef]
- Namjoo, M.; Dibagar, N.; Golbakhshi, H.; Figiel, A.; Masztalerz, K. RSM-Based Optimization Analysis for Cold Plasma and Ultrasound-Assisted Drying of Caraway Seed. Foods 2024, 13, 3084. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Lee, J.G.; Chae, Y.; Shin, Y.; Kim, Y.J. Chemical Composition and Antioxidant Capacity of Black Pepper Pericarp. Appl. Biol. Chem. 2020, 63, 1–9. [Google Scholar] [CrossRef]
- Nowacka, M.; Wiktor, A.; Sledz, M.; Jurek, N.; Witrowa-Rajchert, D. Drying of Ultrasound Pretreated Apple and Its Selected Physical Properties. J. Food Eng. 2012, 113, 427–433. [Google Scholar] [CrossRef]
- Fu, X.; Belwal, T.; Cravotto, G.; Luo, Z. Ultrasonics—Sonochemistry Sono-Physical and Sono-Chemical Effects of Ultrasound: Primary Applications in Extraction and Freezing Operations and Influence on Food Components. Ultrason. Sonochem. 2020, 60, 104726. [Google Scholar] [CrossRef]
- ElGamal, R.; Song, C.; Rayan, A.M.; ElMasry, G. Thermal Degradation of Bioactive Compounds during Drying Process of Horticultural and Agronomic Products: A Comprehensive Overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Yüksel, A.; Kutlu, N.; Aslam, R.; Sahni, P.; Yoo, S. Individual and Interactive Effect of Ultrasound Pre-Treatment on Drying Kinetics and Biochemical Qualities of Food: A Critical Review. Ultrason. Sonochem. 2023, 92, 106261. [Google Scholar] [CrossRef]
- Kurangi, B.; Jalalpure, S. A Validated Stability-Indicating Rp-Hplc Method for Piperine Estimation in Black Pepper, Marketed Formulation and Nanoparticles. Indian J. Pharm. Educ. Res. 2020, 54, s677–s686. [Google Scholar] [CrossRef]
- Shrestha, S.; Chaudhary, N.; Sah, R.; Malakar, N. Analysis of Piperine in Black Pepper by High Performance Liquid Chromatography. J. Nepal Chem. Soc. 2020, 41, 80–86. [Google Scholar] [CrossRef]
- Gouda, M.; El-Din Bekhit, A.; Tang, Y.; Huang, Y.; Huang, L.; He, Y.; Li, X. Recent Innovations of Ultrasound Green Technology in Herbal Phytochemistry: A Review. Ultrason. Sonochem. 2021, 73, 105538. [Google Scholar] [CrossRef] [PubMed]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Sequential Microwave-Ultrasound-Assisted Extraction for Isolation of Piperine from Black Pepper (Piper nigrum L.). Food Bioprocess Technol. 2017, 10, 2199–2207. [Google Scholar] [CrossRef]
- Soares, J.F.; Prá, V.D.; Barrales, F.M.; Dos Santos, P.; Kuhn, R.C.; Rezende, C.A.; Martínez, J.; Mazutti, M.A. Extraction of Rice Bran Oil Using Supercritical CO2 Combined with Ultrasound. Braz. J. Chem. Eng. 2018, 35, 785–794. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, C.; Chen, Z.; Wang, H.; Wang, Y.; Bai, H. A Review: Study on the Enhancement Mechanism of Heat and Moisture Transfer in Deformable Porous Media. Processes 2023, 11, 2699. [Google Scholar] [CrossRef]
- Yao, Y. Enhancement of Mass Transfer by Ultrasound: Application to Adsorbent Regeneration and Food Drying/Dehydration. Ultrason. Sonochem. 2016, 31, 512–531. [Google Scholar] [CrossRef] [PubMed]


| Source | Piperine Content | Drying Time | Moisture Content | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean Square | F-Value | p-Value | Mean Square | F-Value | p-Value | Mean Square | F-Value | p-Value | |
| Model | 0.2655 | 43.00 | <0.0001 | 350.07 | 12,034.67 | <0.0001 | 0.8800 | 168.74 | <0.0001 |
| Linear | |||||||||
| A-Frequency | 0.3816 | 61.82 | <0.0001 | 339.52 | 11,672.21 | <0.0001 | 0.7450 | 142.86 | <0.0001 |
| B-Ultrasound Time | 0.0184 | 2.98 | 0.1062 | 414.31 | 14,243.11 | <0.0001 | 2.48 | 476.39 | <0.0001 |
| C-Temperature | 0.5590 | 90.55 | <0.0001 | 142.21 | 4888.93 | <0.0001 | 3.85 | 738.91 | <0.0001 |
| D-Ultrasound Power | 0.0972 | 15.75 | 0.0014 | 473.14 | 16,265.60 | < 0.0001 | 0.1220 | 23.40 | 0.0003 |
| Quadratic | |||||||||
| A2 | 0.1484 | 24.04 | 0.0002 | 51.42 | 1767.58 | <0.0001 | 1.01 | 194.48 | <0.0001 |
| B2 | 0.2342 | 37.93 | <0.0001 | 356.20 | 12,245.58 | <0.0001 | 0.1333 | 25.55 | 0.0002 |
| C2 | 0.0146 | 2.37 | 0.1459 | 159.55 | 5485.10 | <0.0001 | 0.0987 | 18.92 | 0.0007 |
| D2 | 0.1687 | 27.32 | 0.0001 | 420.38 | 14,452.10 | <0.0001 | 0.1048 | 20.09 | 0.0005 |
| Interactions | |||||||||
| AB | 0.0012 | 0.1984 | 0.6628 | 46.31 | 1591.99 | <0.0001 | 0.0072 | 1.39 | 0.2588 |
| AC | 0.1560 | 25.27 | 0.0002 | 194.88 | 6699.70 | <0.0001 | 0.0529 | 10.14 | 0.0066 |
| AD | 1.35 | 217.97 | <0.0001 | 1242.56 | 42,717.22 | <0.0001 | 1.82 | 349.48 | <0.0001 |
| BC | 0.0342 | 5.54 | 0.0337 | 890.43 | 30,611.34 | <0.0001 | 0.3844 | 73.71 | <0.0001 |
| BD | 0.4422 | 71.64 | <0.0001 | 94.28 | 3241.33 | <0.0001 | 1.09 | 209.41 | <0.0001 |
| CD | 0.0056 | 0.9112 | 0.3560 | 16.52 | 568.08 | <0.0001 | 0.0900 | 17.26 | 0.0010 |
| Diagnosis Statistics | |||||||||
| Lack of Fit | 0.0077 | 3.36 | 0.1272 | 0.0375 | 4.69 | 0.0749 | 0.0041 | 0.5126 | 0.8216 |
| R2 | 0.9773 | 0.9999 | 0.9941 | ||||||
| R2 Adjusted | 0.9545 | 0.9998 | 0.9882 | ||||||
| R2 Predicted | 0.8793 | 0.9995 | 0.9769 | ||||||
| Adequate precision | 29.2220 | 472.6465 | 46.9889 | ||||||
| Mean | 18.69 | 439.00 | 9.60 | ||||||
| C.V. % | 0.4203 | 0.0389 | 0.7523 | ||||||
| STD | 0.0786 | 0.1706 | 0.0722 | ||||||
| Second-order polynomial models for piperine ultrasound parameters | |||||||||
| Response | Second-order polynomial model | ||||||||
| Piperine Content | 18.63 − 0.178333 A − 0.0391667 B − 0.215833 C − 0.09 D + 0.0175 AB + 0.1975 AC − 0.58 AD − 0.0925 BC + 0.3325 BD + 0.0375 CD − 0.15125 A2 + 0.19 B2 − 0.0475 C2 + 0.16125 D2 | ||||||||
| Drying Time | 444.51 − 5.31917 A − 5.87583 B + 3.4425 C + 6.27917 D − 3.4025 AB − 6.98 AC + 17.625 AD + 14.92 BC − 4.855 BD + 2.0325 CD − 2.81542 A2 − 7.41042 B2 + 4.95958 C2 − 8.05042 D2 | ||||||||
| Moisture Content | 9.6 + 0.249167 A − 0.455 B − 0.566667 C + 0.100833 D − 0.0425 AB − 0.115 AC − 0.675 AD − 0.31 BC + 0.5225 BD + 0.15 CD − 0.395417 A2 + 0.143333 B2 + 0.123333 C2 + 0.127083 D2 | ||||||||
| Parameter | Predicted Mean | UV-Observed Mean ± SD | HPLC-Observed Mean ± SD | Method Correlation |
|---|---|---|---|---|
| Piperine Content (mg/g) | 18.63 | 18.64 ± 0.11 | 39.51 ± 0.12 | 1:2.12 * |
| Drying Time (min) | 444.51 | 444.41 ± 0.12 | 444.41 ± 0.12 | N/A |
| Moisture Content (%) | 9.6 | 9.53 ± 0.13 | 9.53 ± 0.13 | N/A |
| Method | Range (µg/mL) | Linear Equation | R2 | LOD (µg/mL) | LOQ (µg/mL) | %RSD |
|---|---|---|---|---|---|---|
| HPLC | 0.4–10.0 | y = 83.6213x − 5.3384 | 0.99949 | 0.339 | 1.026 | 1.14 |
| UV | 2.0–10.0 | y = 0.0540x + 0.0180 | 0.99979 | 0.473 | 1.433 | 1.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Johnson, N.A.N.; Adade, S.Y.-S.S.; Ekumah, J.-N.; Kwadzokpui, B.A.; Boasiako, T.A.; Xu, Y. Optimization of Ultrasound Pretreatment for Enhanced Drying Efficiency and Piperine Retention in Black Pepper (Piper nigrum L.). Foods 2026, 15, 86. https://doi.org/10.3390/foods15010086
Johnson NAN, Adade SY-SS, Ekumah J-N, Kwadzokpui BA, Boasiako TA, Xu Y. Optimization of Ultrasound Pretreatment for Enhanced Drying Efficiency and Piperine Retention in Black Pepper (Piper nigrum L.). Foods. 2026; 15(1):86. https://doi.org/10.3390/foods15010086
Chicago/Turabian StyleJohnson, Nana Adwoa Nkuma, Selorm Yao-Say Solomon Adade, John-Nelson Ekumah, Bridget Ama Kwadzokpui, Turkson Antwi Boasiako, and Yi Xu. 2026. "Optimization of Ultrasound Pretreatment for Enhanced Drying Efficiency and Piperine Retention in Black Pepper (Piper nigrum L.)" Foods 15, no. 1: 86. https://doi.org/10.3390/foods15010086
APA StyleJohnson, N. A. N., Adade, S. Y.-S. S., Ekumah, J.-N., Kwadzokpui, B. A., Boasiako, T. A., & Xu, Y. (2026). Optimization of Ultrasound Pretreatment for Enhanced Drying Efficiency and Piperine Retention in Black Pepper (Piper nigrum L.). Foods, 15(1), 86. https://doi.org/10.3390/foods15010086






