Enzyme-Assisted Tenderization and Vitamin E-Loaded Liposome Coating for Garlic Scape Quality Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Optimization of Enzyme Concentration and Treatment Time
2.2.1. Sample Preparation for Enzyme Optimization
2.2.2. Color Measurement
2.2.3. Texture Profile Analysis Method
2.2.4. Optical Microscopic Analysis
2.2.5. Enzyme Activity Assay
2.3. Optimization Strategy of Vitamin E Content
2.4. Characterization of Liposome-Loaded Garlic Scapes
2.4.1. Preparation of Liposomes
2.4.2. Liposome Treatment of Garlic Scapes
2.4.3. Color Profile Analysis
2.4.4. Texture Profile Analysis
2.4.5. Optical Microscopic Images
2.4.6. Enzyme Activity
2.4.7. Vitamin Content Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Color Characteristics of Enzyme-Treated Garlic Scapes
3.2. Texture Profile of Enzyme-Treated Samples
3.3. Microstructural Alterations Induced by Enzymes
3.4. Residual Enzyme Activities of Treated Solution
3.5. Optimization of Vitamin E Content
3.6. Color and Microscopic Observation
3.7. Texture
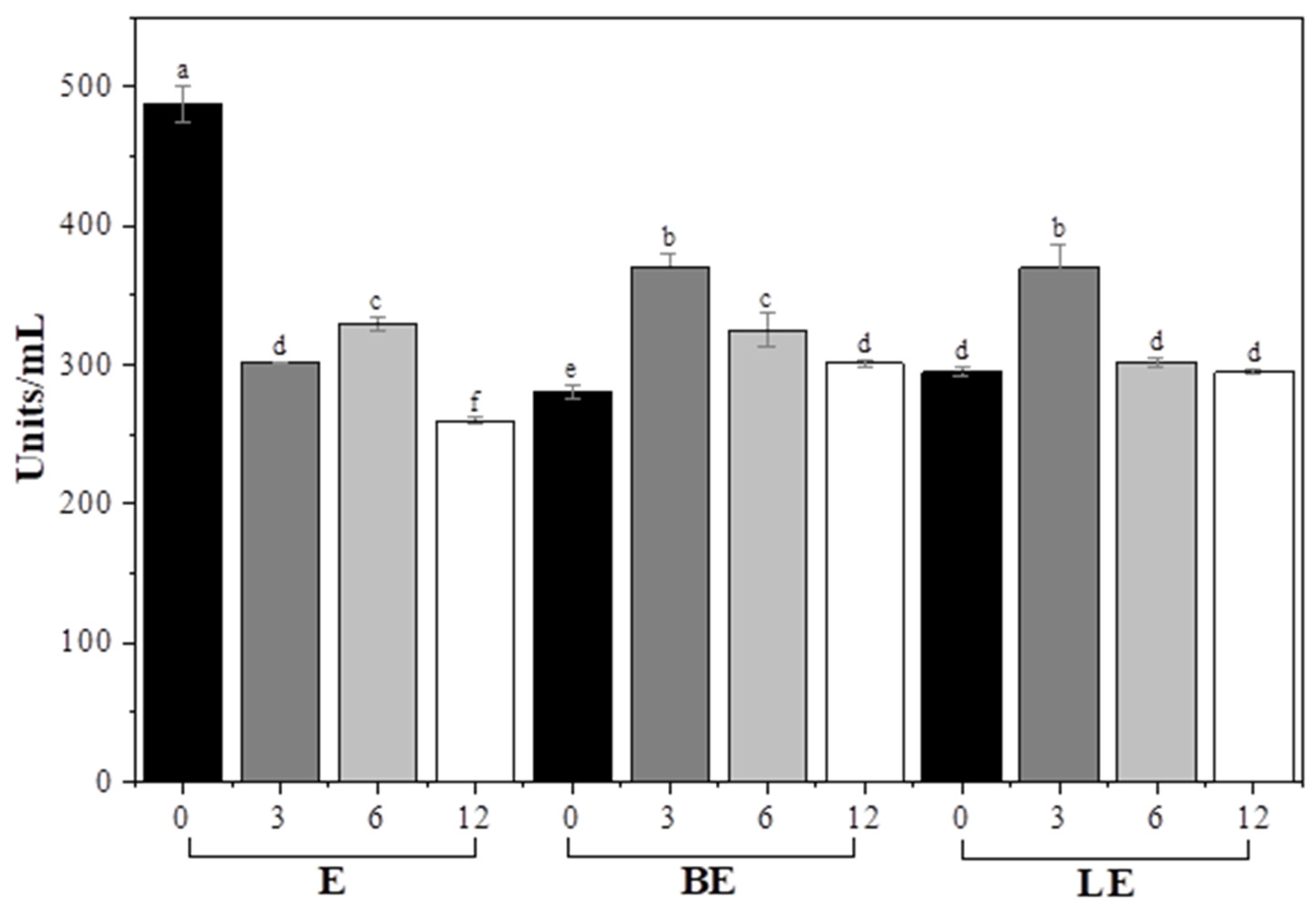
3.8. Enzyme Activity and Stability in Liposomal Formulations
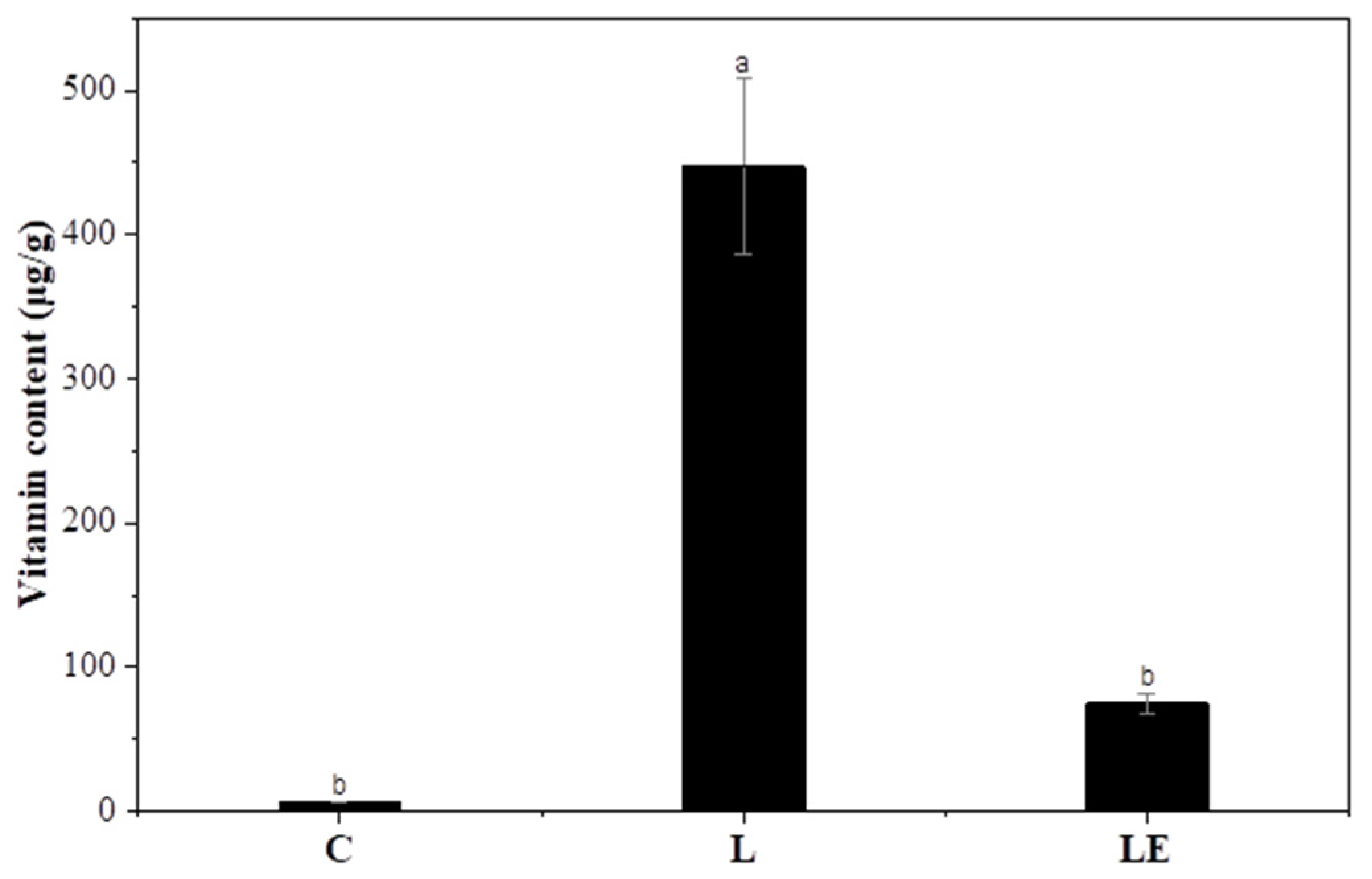
3.9. Vitamin E Enrichment of Garlic Scapes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| B | Blank liposome |
| BE | Enzyme combined with blank liposome |
| C | Untreated control sample |
| E | Enzyme solution |
| ΔE | Total color difference |
| L | Vitamin E-loaded liposome |
| LE | Enzyme combined with vitamin E-loaded liposome |
| PdI | Polydispersity index |
| PT | Plantase PT |
| UF | Plantase UF |
| V | Vitamin E content in liposomes |
References
- Hernigou, P.; Bumbasirevic, M.; Pecina, M.; Scarlat, M.M. Eight billion people, sixteen billion hip joints today: Are future orthopedists prepared to treat a world of ultra-old patients and centenarians in 2050? Int. Orthop. 2024, 48, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, Y.; Liu, Y.; Ma, A.; Zhong, F.; Gao, T.; Cai, J.; Chen, Y.; Wang, Y.; Zhou, W. Relationships between oral function, dietary intake and nutritional status in older adults aged 75 years and above: A cross-sectional study. BMC Public Health 2024, 24, 1465. [Google Scholar] [CrossRef]
- McGrogan, C.; Matcham, F.; Dawes, H.; Ang, C.S.; Cartner, H.; Branley-Bell, D. Exploring relationships between chewing difficulty, eating experience, pain, and well-being: A cross-sectional self-report study. Clin. Nutr. Open Sci. 2024, 57, 177–202. [Google Scholar] [CrossRef]
- Lin, M.; Chang, S.; Jea, Y.; Chen, W.; Lee, T. Effects of dietary garlic scape meal on the growth and meat characteristics of geese. Br. Poult. Sci. 2015, 56, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Itrat, N.; Nisa, M.U.; Al-Asmari, F.; Ramadan, M.F.; Zongo, E. A double-blind, randomized control trial to investigate the therapeutic potential of garlic scapes for high apoprotein E levels in a high-Fat diet-induced hypercholesteremic rat model. Food Sci. Nutr. 2024, 12, 7607–7619. [Google Scholar] [CrossRef]
- Sasi, M.; Kumar, S.; Kumar, M.; Thapa, S.; Prajapati, U.; Tak, Y.; Changan, S.; Saurabh, V.; Kumari, S.; Kumar, A. Garlic (Allium sativum L.) bioactives and its role in alleviating oral pathologies. Antioxidants 2021, 10, 1847. [Google Scholar] [CrossRef]
- Eryılmaz, O.; Ovalı, S. Investigation and Analysis of new fiber from Allium fistulosum L. (Scallion) plant’s tassel and its suitability for fiber-reinforced composites. Uludağ Üniversitesi Mühendislik Fakültesi Derg. 2024, 29, 51–66. [Google Scholar]
- Fenwick, G.R.; Hanley, A.B.; Whitaker, J.R. The genus allium—Part 3. Crit. Rev. Food Sci. Nutr. 1985, 23, 1–73. [Google Scholar] [CrossRef]
- Yan, J.-K.; Zhu, J.; Liu, Y.; Chen, X.; Wang, W.; Zhang, H.; Li, L. Recent advances in research on Allium plants: Functional ingredients, physiological activities, and applications in agricultural and food sciences. Crit. Rev. Food Sci. Nutr. 2023, 63, 8107–8135. [Google Scholar] [CrossRef]
- Liu, T.; Zheng, J.; Du, J.; He, G. Food processing and nutrition strategies for improving the health of elderly people with dysphagia: A review of recent developments. Foods 2024, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- de Souza, T.S.; Kawaguti, H.Y. Cellulases, hemicellulases, and pectinases: Applications in the food and beverage industry. Food Bioprocess Technol. 2021, 14, 1446–1477. [Google Scholar] [CrossRef]
- Cheng, L.; Hu, X.; Hong, Y.; Li, Z.; Li, C.; Gu, Z. Reduction of inhibitory effects on cellulose degradation with pectinase treatment in potato residue. Ind. Crops Prod. 2023, 192, 116010. [Google Scholar] [CrossRef]
- Sakamoto, K.; Shibata, K.; Ishihara, M. Decreased hardness of dietary fiber-rich foods by the enzyme-infusion method. Biosci. Biotechnol. Biochem. 2006, 70, 1564–1570. [Google Scholar] [CrossRef]
- Luo, M.; Akoetey, W.; Martí, N.; Saura, D.; Hosseinian, F. Impact of Ultrasound-Treated Emulsion Gels on the Structure of Purees for Oropharyngeal Dysphagia. Molecules 2025, 30, 3933. [Google Scholar] [CrossRef]
- Żmuda, P.; Khaidakov, B.; Krasowska, M.; Czapska, K.; Dobkowski, M.; Guzowski, J.; Kowalczyk, P.; Lemke, K.; Folwarski, M.; Foryś, A. Bioavailability of liposomal vitamin C in powder form: A randomized, double-blind, cross-over trial. Appl. Sci. 2024, 14, 7718. [Google Scholar] [CrossRef]
- Fan, C.; Feng, T.; Wang, X.; Xia, S.; Swing, C.J. Liposomes for encapsulation of liposoluble vitamins (A, D, E and K): Comparation of loading ability, storage stability and bilayer dynamics. Food Res. Int. 2023, 163, 112264. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tang, W.; Pu, C.; Li, R.; Sun, Q.; Wang, H. Improved stability of liposome-stabilized emulsions as a co-encapsulation delivery system for vitamin B 2, vitamin E and β-carotene. Food Funct. 2022, 13, 2966–2984. [Google Scholar] [CrossRef]
- Marsanasco, M.; Márquez, A.L.; Wagner, J.R.; del V. Alonso, S.; Chiaramoni, N.S. Liposomes as vehicles for vitamins E and C: An alternative to fortify orange juice and offer vitamin C protection after heat treatment. Food Res. Int. 2011, 44, 3039–3046. [Google Scholar] [CrossRef]
- Huang, Z.; Brennan, C.S.; Zhao, H.; Liu, J.; Guan, W.; Mohan, M.S.; Stipkovits, L.; Zheng, H.; Kulasiri, D. Fabrication and assessment of milk phospholipid-complexed antioxidant phytosomes with vitamin C and E: A comparison with liposomes. Food Chem. 2020, 324, 126837. [Google Scholar] [CrossRef]
- Ortega, R.M.; Requejo, A.M.; Lopez-Sobaler, A.M.; Navia, B.; Perea, J.M.; Andrés, P.; Robles, F. Cognitive function in elderly people is influenced by vitamin E status. J. Nutr. 2002, 132, 2065–2068. [Google Scholar] [CrossRef]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Effects of vitamin E on cognitive performance during ageing and in Alzheimer’s disease. Nutrients 2014, 6, 5453–5472. [Google Scholar] [CrossRef]
- Kim, H.-T.; Lee, J.; Jo, Y.-J.; Choi, M.-J. Application of liposome encapsulating lactobacillus curvatus extract in cosmetic emulsion lotion. Materials 2021, 14, 7571. [Google Scholar] [CrossRef]
- Taouzinet, L.; Fatmi, S.; Khellouf, A.; Lahiani-Skiba, M.; Skiba, M.; Iguer-Ouada, M. Drug release, stability and efficiency of vitamin E loaded in liposomes for bovine sperm protection in cryopreservation medium. CryoLetters 2022, 43, 50–57. [Google Scholar] [CrossRef]
- Uluisik, S.; Seymour, G.B. Pectate lyases: Their role in plants and importance in fruit ripening. Food Chem. 2020, 309, 125559. [Google Scholar] [CrossRef] [PubMed]
- Mirani, A.; Goli, M. Production of the eggplant-fiber incorporated cupcake and evaluating its chemical, textural and colorimetric properties over a ten-day storage time. J. Food Process. Preserv. 2021, 45, e15311. [Google Scholar] [CrossRef]
- Hategekimana, J.; Masamba, K.G.; Ma, J.; Zhong, F. Encapsulation of vitamin E: Effect of physicochemical properties of wall material on retention and stability. Carbohydr. Polym. 2015, 124, 172–179. [Google Scholar] [CrossRef]
- Kuhlman, B.; Hansen, J.; Jørgensen, B.; du Toit, W.; Moore, J.P. The effect of enzyme treatment on polyphenol and cell wall polysaccharide extraction from the grape berry and subsequent sensory attributes in Cabernet Sauvignon wines. Food Chem. 2022, 385, 132645. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, A.K.; Nijsse, J.; Bialek, L.; Bouwens, L.; Hendrickx, M.E.; Van Loey, A.M. Effect of enzyme homogenization on the physical properties of carrot cell wall suspensions. Food Bioprocess Technol. 2015, 8, 1377–1385. [Google Scholar] [CrossRef]
- Kim, K.-I.; Lee, S.; Lee, J.; Lee, J.; Min, S.-G.; Choi, M.-J. Effect of liposome-coated hemicellulase on the tenderization of burdock. Korean J. Food Sci. Technol. 2015, 47, 698–703. [Google Scholar] [CrossRef]
- Abril, B.; Bou, R.; García-Pérez, J.V.; Benedito, J. Role of enzymatic reactions in meat processing and use of emerging technologies for process intensification. Foods 2023, 12, 1940. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Huang, B.-C.; Lin, Y.-H.; Wang, C.-Y. Enzymatic impregnation by high hydrostatic pressure for preparing shape-retaining softened broccoli and carrot. Food Biosci. 2024, 59, 103848. [Google Scholar] [CrossRef]
- De Laet, E.; Bernaerts, T.; Morren, L.; Vanmarcke, H.; Van Loey, A.M. The Use of Different Cell Wall Degrading Enzymes for Pectin Extraction from Carrot Pomace, in Comparison to and in Combination with an Acid Extraction. Foods 2025, 14, 435. [Google Scholar] [CrossRef]
- Zdunek, A.; Kozioł, A.; Pieczywek, P.; Cybulska, J. Evaluation of the nanostructure of pectin, hemicellulose and cellulose in the cell walls of pears of different texture and firmness. Food Bioprocess Technol. 2014, 7, 3525–3535. [Google Scholar] [CrossRef]
- Tucker, G.; Yin, X.; Zhang, A.; Wang, M.; Zhu, Q.; Liu, X.; Grierson, D. Ethylene and fruit softening. Food Qual. Saf. 2017, 1, 253–267. [Google Scholar] [CrossRef]
- Hernández, S.; Gallego, M.; Verdú, S.; Barat, J.M.; Talens, P.; Grau, R. Physicochemical characterization of texture-modified pumpkin by vacuum enzyme impregnation: Textural, chemical, and image analysis. Food Bioprocess Technol. 2023, 16, 122–134. [Google Scholar] [CrossRef]
- Li, Q.; Coffman, A.M.; Ju, L.-K. Development of reproducible assays for polygalacturonase and pectinase. Enzym. Microb. Technol. 2015, 72, 42–48. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, L.; Xu, Y.; Lu, H.; Wu, Q.; Li, W.; Zhang, C.; Li, X. Three molecular modification strategies to improve the thermostability of xylanase XynA from Streptomyces rameus L2001. Foods 2023, 12, 879. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, C.; Dong, W.; Lu, H.; Yang, Y.; Li, W.; Xu, Y.; Li, X. Enhanced thermostability of xylanase XynA via computationally designed assembly of multiple N-terminal disulfide bridges. Process Biochem. 2024, 138, 67–78. [Google Scholar] [CrossRef]
- Nezhad, N.G.; Abd Rahman, R.N.Z.R.; Normi, Y.M.; Oslan, S.N.; Shariff, F.M.; Leow, T.C. Recent advances in simultaneous thermostability-activity improvement of industrial enzymes through structure modification. Int. J. Biol. Macromol. 2023, 232, 123440. [Google Scholar] [CrossRef]
- Lee, J.; Xiong, Y.L.; Choi, M.-J. Effect of micro-and nano-sized emulsion fillers combined with trans-cinnamaldehyde on the physicochemical and rheological characteristics of myofibrillar protein gels. Food Hydrocoll. 2023, 143, 108865. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zhang, L.; Xue, Y.; Zhang, K. Mechanistic Insights into Vegetable Color Stability: Discoloration Pathways and Emerging Protective Strategies. Foods 2025, 14, 2222. [Google Scholar] [CrossRef]
- Ru, X.; You, W.; Zhang, J.; Xu, F.; Wu, Z.; Jin, P.; Zheng, Y.; Cao, S. γ-aminobutyric acid treatment inhibits browning and promotes storage quality by regulating reactive oxygen species and membrane lipid metabolism in fresh-cut stem lettuce. Food Chem. 2024, 459, 140420. [Google Scholar] [CrossRef]
- Kim, S. Antioxidant compounds for the inhibition of enzymatic browning by polyphenol oxidases in the fruiting body extract of the edible mushroom Hericium erinaceus. Foods 2020, 9, 951. [Google Scholar] [CrossRef]
- Han, J.-A. The effect of different pretreatments followed by enzyme reaction on preparing shape-retaining softened burdock. Food Chem. 2021, 353, 129440. [Google Scholar] [CrossRef]
- Buergy, A.; Rolland-Sabaté, A.; Leca, A.; Renard, C.M. Pectin modifications in raw fruits alter texture of plant cell dispersions. Food Hydrocoll. 2020, 107, 105962. [Google Scholar] [CrossRef]
- Chaize, B.; Colletier, J.-P.; Winterhalter, M.; Fournier, D. Encapsulation of enzymes in liposomes: High encapsulation efficiency and control of substrate permeability. Artif. Cells Blood Substit. Biotechnol. 2004, 32, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Chibowski, E.; Szcześ, A. Zeta potential and surface charge of DPPC and DOPC liposomes in the presence of PLC enzyme. Adsorption 2016, 22, 755–765. [Google Scholar] [CrossRef]
- Marsanasco, M.; Calabró, V.; Piotrkowski, B.; Chiaramoni, N.S.; del V. Alonso, S. Fortification of chocolate milk with omega-3, omega-6, and vitamins E and C by using liposomes. Eur. J. Lipid Sci. Technol. 2016, 118, 1271–1281. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, J.; Jin, R.; Cheng, R.; Wang, X.; Yuan, C.; Gan, C. Physicochemical properties, antioxidant activities and in vitro sustained release behaviour of co-encapsulated liposomes as vehicle for vitamin E and β-carotene. J. Sci. Food Agric. 2022, 102, 5759–5767. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yi, X.; Gao, X.; Long, Z.; Guo, J.; Xia, G.; Shen, X. Stabilization of β-Carotene Liposomes with Chitosan–Lactoferrin Coating System: Vesicle Properties and Anti-Inflammatory In Vitro Studies. Foods 2025, 14, 968. [Google Scholar] [CrossRef]
- Hamedinasab, H.; Rezayan, A.H.; Mellat, M.; Mashreghi, M.; Jaafari, M.R. Development of chitosan-coated liposome for pulmonary delivery of N-acetylcysteine. Int. J. Biol. Macromol. 2020, 156, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Kim, H.K.; Choo, J.; Seong, G.H.; Hien, T.B.D.; Lee, E. Effects of operating parameters on the efficiency of liposomal encapsulation of enzymes. Colloids Surf. B Biointerfaces 2012, 94, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Pisani, S.; Di Martino, D.; Cerri, S.; Genta, I.; Dorati, R.; Bertino, G.; Benazzo, M.; Conti, B. Investigation and comparison of active and passive encapsulation methods for loading proteins into liposomes. Int. J. Mol. Sci. 2023, 24, 13542. [Google Scholar] [CrossRef] [PubMed]


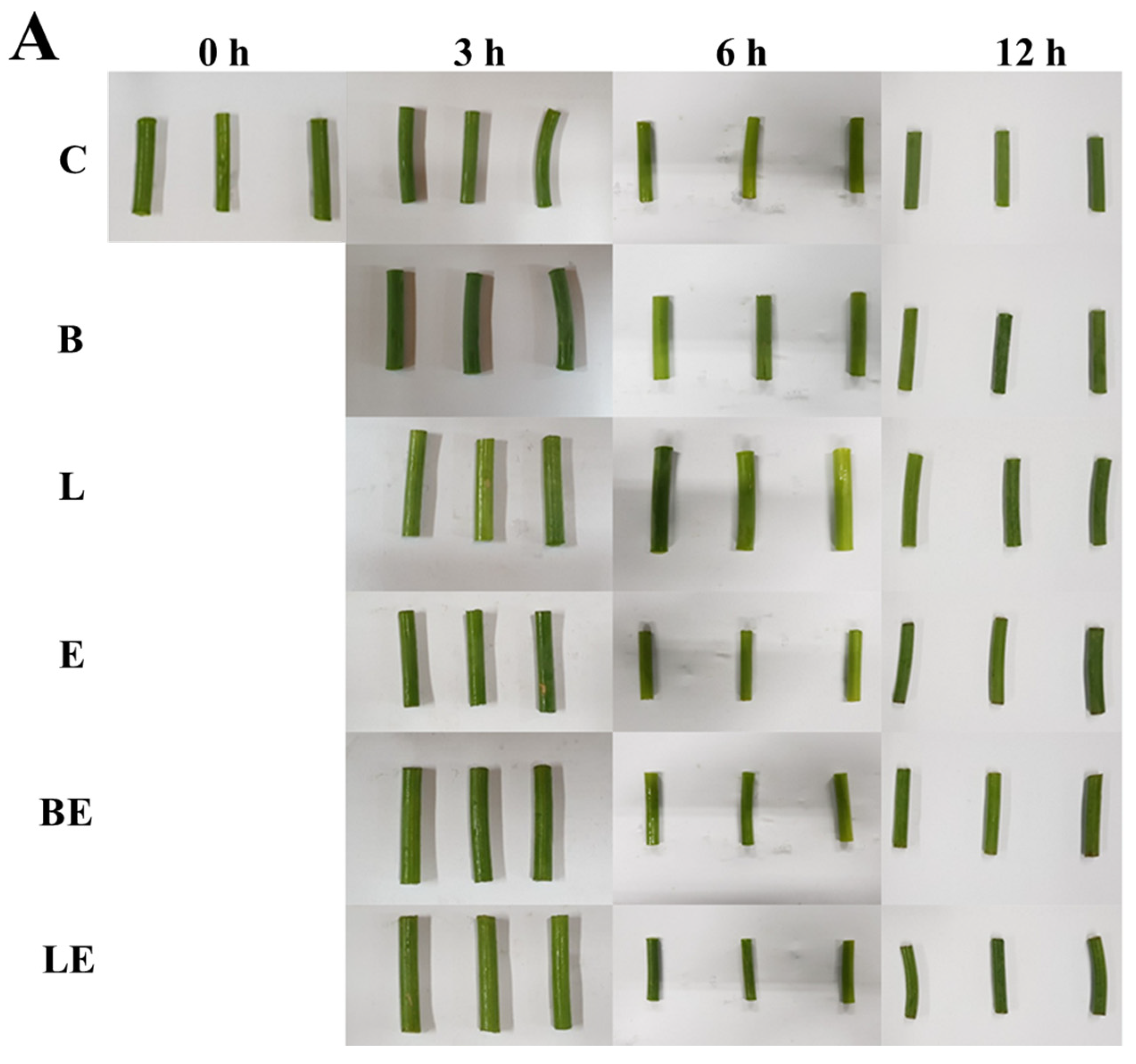
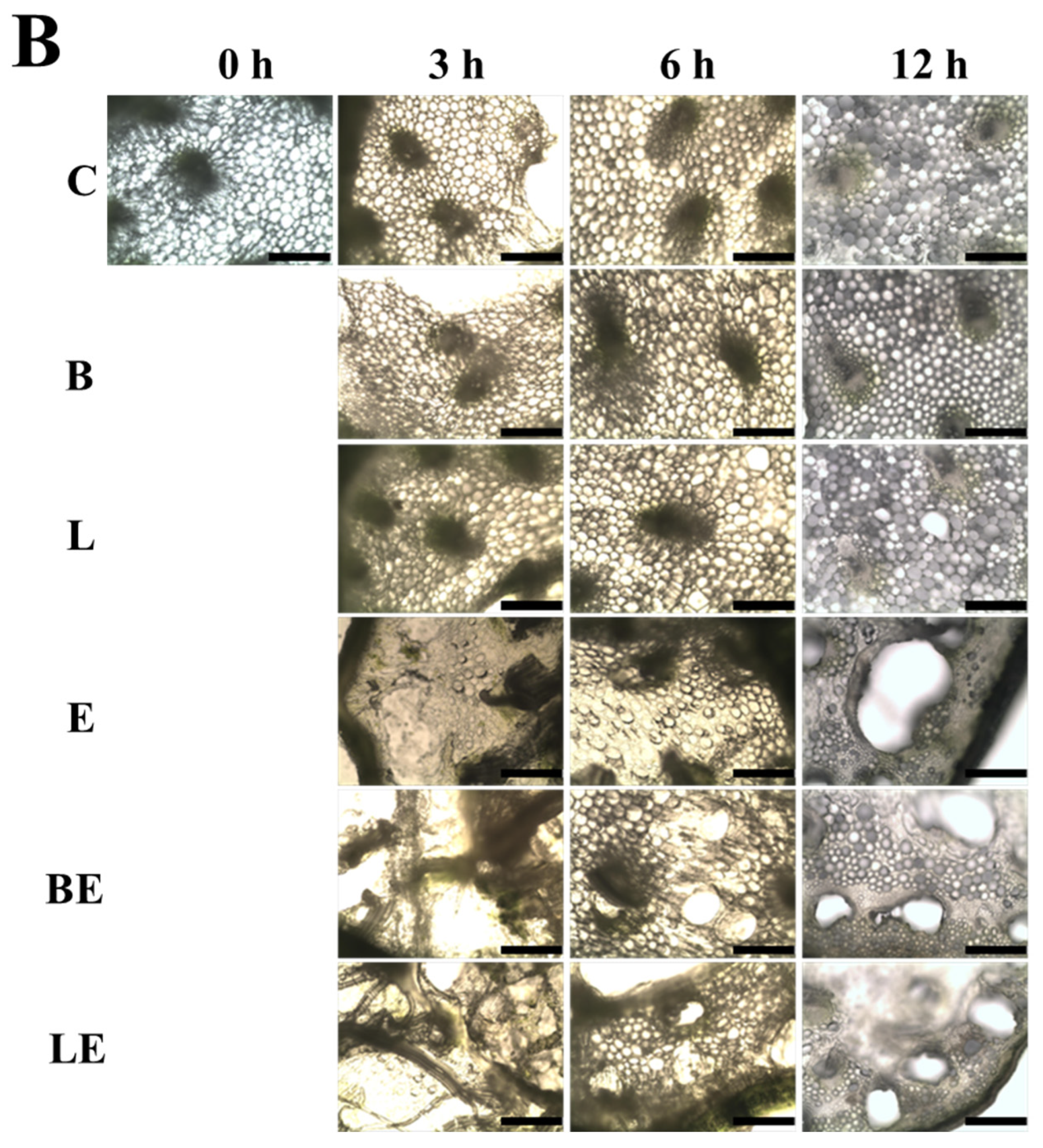
| Treatment | Concentration (%) | L* | a* | b* | ΔE |
|---|---|---|---|---|---|
| UF | 0 | 35.08 ± 2.09 d | −12.05 ± 0.84 b | 15.50 ± 1.40 bc | - |
| 1 | 36.44 ± 2.15 bcd | −10.68 ± 0.46 a | 15.10 ± 1.14 c | 3.75 ± 2.13 b | |
| 2 | 35.42 ± 0.57 cd | −12.16 ± 0.49 b | 15.43 ± 0.71 bc | 0.20 ± 0.11 b | |
| 3 | 40.95 ± 2.56 a | −12.32 ± 1.03 b | 18.66 ± 1.56 a | 27.26 ± 18.91 a | |
| PT | 0 | 35.08 ± 2.09 d | −12.05 ± 0.84 b | 15.50 ± 1.40 bc | - |
| 1 | 37.60 ± 0.75 bc | −11.17 ± 0.50 a | 15.39 ± 0.62 bc | 3.58 ± 1.17 b | |
| 2 | 35.41 ± 0.52 cd | −12.09 ± 0.17 b | 15.31 ± 0.23 bc | 0.12 ± 0.08 b | |
| 3 | 37.79 ± 1.43 b | −12.11 ± 0.52 b | 16.67 ± 0.68 b | 5.74 ± 4.60 b | |
| Treatment | Treatment duration (h) | ||||
| Control | 0 | 35.08 ± 2.09 a | −12.05 ± 0.84 a | 15.50 ± 1.40 a | - |
| 12 | 37.43 ± 2.21 a | −12.01 ± 0.68 a | 15.93 ± 1.37 a | 3.86 ± 4.97 a | |
| 24 | 35.08 ± 2.09 a | −12.05 ± 0.84 a | 15.50 ± 1.40 a | 2.05 ± 2.07 a | |
| 48 | 35.20 ± 0.96 a | −11.80 ± 0.70 a | 15.22 ± 0.74 a | 0.71 ± 0.62 a | |
| UF | 0 | 35.08 ± 2.09 c | −12.05 ± 0.84 a | 15.50 ± 1.40 c | - |
| 12 | 38.89 ± 1.78 a | −12.97 ± 0.23 bc | 18.07 ± 0.81 a | 12.91 ± 9.27 a | |
| 24 | 35.52 ± 1.38 bc | −12.34 ± 0.87 ab | 16.55 ± 1.22 bc | 2.28 ± 0.73 b | |
| 48 | 37.43 ± 0.60 ab | −13.47 ± 0.28 c | 17.62 ± 0.60 ab | 4.94 ± 0.99 ab | |
| PT | 0 | 35.08 ± 2.09 ab | −12.05 ± 0.84 ab | 15.50 ± 1.40 b | - |
| 12 | 34.62 ± 2.17 b | −11.71 ± 1.11 a | 15.78 ± 1.65 ab | 3.30 ± 2.79 a | |
| 24 | 37.41 ± 1.94 a | −13.15 ± 0.76 b | 17.62 ± 1.42 a | 8.73 ± 8.41 a | |
| 48 | 35.37 ± 1.96 ab | −12.29 ± 0.67 ab | 16.51 ± 1.63 ab | 4.05 ± 3.33 a |
| Treatment | Concentration (%) | Hardness (g) | Adhesiveness (mJ) |
| UF | 0 | 6046 ± 1088 ab | 0.54 ± 0.19 d |
| 1 | 7236 ± 213 a | 0.38 ± 0.07 d | |
| 2 | 6861 ± 1323 a | 5.58 ± 1.94 b | |
| 3 | 6060 ± 666 ab | 0.48 ± 0.07 d | |
| PT | 0 | 6046 ± 1088 ab | 0.54 ± 0.19 d |
| 1 | 5132 ± 1525 bc | 4.58 ± 5.19 c | |
| 2 | 4101 ± 860 cd | 4.39 ± 2.21 bc | |
| 3 | 2815 ± 1062 d | 8.20 ± 2.01 a | |
| Treatment | Treatment duration (h) | Hardness (g) | Adhesiveness (mJ) |
| Control | 0 | 6046 ± 1088 b | 0.54 ± 0.19 c |
| 12 | 7518 ± 354 ab | 0.30 ± 0.09 c | |
| 24 | 7025 ± 1290 ab | 4.12 ± 0.39 bc | |
| 48 | 8781 ± 1241 a | 22.32 ± 2.82 a | |
| UF | 0 | 6046 ± 1088 b | 0.54 ± 0.19 c |
| 12 | 7053 ± 1842 ab | 0.20 ± 0.00 c | |
| 24 | 6861 ± 1323 ab | 5.58 ± 1.94 bc | |
| 48 | 7260 ± 1436 ab | 2.19 ± 0.30 bc | |
| PT | 0 | 6360 ± 1187 b | 0.54 ± 0.19 c |
| 12 | 5396 ± 438 b | 0.30 ± 0.11 c | |
| 24 | 5527 ± 495 b | 8.48 ± 5.58 b | |
| 48 | 2999 ± 1623 c | 28.34 ± 11.19 a |
| Treatment | Particle Size (nm) | PdI | [-] ζ-Potential (mV) |
|---|---|---|---|
| V0 | 151 ± 2 e | 0.23 ± 0.01 c | 36.00 ± 1.73 a |
| V0.025 | 1196 ± 71 b | 0.46 ± 0.09 b | 12.10 ± 0.30 cd |
| V0.05 | 1306 ± 104 a | 0.32 ± 0.05 c | 11.43 ± 0.59 d |
| V0.1 | 291 ± 12 d | 0.66 ± 0.04 a | 28.13 ± 1.23 b |
| V0.2 | 404 ± 9 c | 0.65 ± 0.02 a | 13.73 ± 0.12 c |
| E | 575 ± 41 b | 0.79 ± 0.04 a | 13.57 ± 0.34 b |
| L | 291 ± 12 c | 0.66 ± 0.04 a | 28.13 ± 1.23 a |
| LE | 1214 ± 95 a | 0.81 ± 0.16 a | 12.97 ± 0.39 b |
| Treatment | Treatment Duration (h) | L* | a* | b* | ΔE |
|---|---|---|---|---|---|
| C | 3 | 35.33 ± 1.95 aBC | −9.98 ± 1.04 aA | 12.76 ± 1.38 aC | |
| 6 | 36.04 ± 1.21 aB | −10.90 ± 0.28 aA | 13.77 ± 0.46 aD | ||
| 12 | 36.58 ± 3.12 aA | −10.74 ± 0.78 aAB | 13.90 ± 1.41 aAB | ||
| E | 3 | 37.02 ± 1.47 aAB | −11.30 ± 0.72 aB | 14.89 ± 1.11 bB | 114.77 ± 20.54 aB |
| 6 | 36.70 ± 0.78 aB | −12.88 ± 1.20 bC | 17.21 ± 1.36 bB | 9.75 ± 5.66 bB | |
| 12 | 32.33 ± 2.14 bB | −10.74 ± 1.14 aAB | 13.91 ± 1.74 aAB | 12.85 ± 9.21 bA | |
| B | 3 | 34.33 ± 2.24 abC | −10.42 ± 0.81 aA | 12.94 ± 0.90 bC | 87.17 ± 8.54 aC |
| 6 | 36.13 ± 1.19 aB | −12.75 ± 0.43 bC | 16.64 ± 0.48 aB | 6.62 ± 2.94 bB | |
| 12 | 11.49 ± 1.33 bB | −10.16 ± 0.69 aA | 12.62 ± 1.24 bB | 10.99 ± 6.96 bA | |
| BE | 3 | 34.62 ± 2.22 bC | −10.40 ± 0.98 aA | 14.31 ± 1.18 bB | 105.89 ± 15.47 aB |
| 6 | 41.77 ± 2.94 aA | −14.18 ± 0.78 cD | 20.35 ± 1.40 aA | 48.26 ± 29.36 bA | |
| 12 | 33.90 ± 1.36 bB | −11.44 ± 0.70 bB | 15.34 ± 0.94 bA | 6.22 ± 3.03 cA | |
| L | 3 | 37.90 ± 1.78 aA | −12.10 ± 0.34 cB | 16.80 ± 0.92 aA | 148.36 ± 20.43 aA |
| 6 | 35.77 ± 0.32 bB | −11.74 ± 0.13 bB | 15.56 ± 0.22 bC | 2.07 ± 0.47 bB | |
| 12 | 32.15 ± 0.60 cB | −10.08 ± 0.31 aA | 12.46 ± 0.31 cB | 11.29 ± 3.01 bA | |
| LE | 3 | 37.11 ± 0.51 aAB | −11.89 ± 0.22 bB | 16.10 ± 0.35 aA | 133.22 ± 6.41 aA |
| 6 | 32.54 ± 0.09 bC | −10.31 ± 0.41 aA | 12.28 ± 0.44 bE | 7.53 ± 0.89 bB | |
| 12 | 33.61 ± 2.59 bB | −11.68 ± 1.30 bB | 15.06 ± 2.24 aA | 11.11 ± 7.36 bA |
| Treatment | Treated Duration (h) | Hardness (g) | Adhesiveness (mJ) |
|---|---|---|---|
| C | 3 | 8697 ± 2010 aA | 3.24 ± 0.56 aA |
| 6 | 7878 ± 2420 aAB | 2.30 ± 0.43 bB | |
| 12 | 9807 ± 1183 aA | 2.38 ± 0.83 bA | |
| B | 3 | 8213 ± 921 abA | 3.18 ± 0.30 bA |
| 6 | 7109 ± 828 bBC | 2.34 ± 0.26 bB | |
| 12 | 9144 ± 1626 aA | 2.37 ± 0.54 aA | |
| L | 3 | 6708 ± 731 aAB | 3.32 ± 0.52 aA |
| 6 | 9368 ± 518 aA | 1.80 ± 0.14 bC | |
| 12 | 8819 ± 2105 aAB | 2.75 ± 0.93 aA | |
| E | 3 | 6862 ± 1652 abAB | 3.18 ± 0.13 aA |
| 6 | 6618 ± 1550 bBC | 2.83 ± 0.44 aA | |
| 12 | 8882 ± 1622 aAB | 3.23 ± 0.40 aA | |
| BE | 3 | 7236 ± 316 aAB | 3.28 ± 0.15 aA |
| 6 | 5690 ± 781 aC | 2.86 ± 0.26 aA | |
| 12 | 6740 ± 1601 aC | 2.95 ± 0.50 aA | |
| LE | 3 | 6098 ± 1369 aB | 3.33 ± 1.27 aA |
| 6 | 6323 ± 874 aBC | 2.96 ± 0.44 aA | |
| 12 | 6816 ± 1579 aBC | 2.64 ± 0.70 aA |
| Treatment | Treatment Duration (h) | Particle Size (nm) | PdI | [-] ζ-Potential |
|---|---|---|---|---|
| B | 3 | 157.30 ± 0.61 aD | 0.20 ± 0.01 bB | 35.57 ± 0.84 aA |
| 6 | 160.20 ± 4.01 aB | 0.31 ± 0.05 aB | 36.63 ± 0.45 abA | |
| 12 | 156.43 ± 2.42 aC | 0.32 ± 0.03 aA | 34.87 ± 0.55 bB | |
| L | 3 | 180.10 ± 3.60 bC | 0.32 ± 0.00 bA | 33.00 ± 2.21 bB |
| 6 | 195.77 ± 9.07 aB | 0.39 ± 0.01 aA | 38.17 ± 4.19 ab | |
| 12 | 175.10 ± 1.80 bB | 0.32 ± 0.03 bA | 40.50 ± 3.55 aA | |
| BE | 3 | 280.47 ± 2.79 aA | 0.21 ± 0.04 aB | 11.60 ± 0.46 bC |
| 6 | 214.90 ± 3.95 bA | 0.13 ± 0.01 bD | 0.00 ± 0.81 cC | |
| 12 | 206.43 ± 2.50 cA | 0.19 ± 0.01 aB | 36.63 ± 0.82 aB | |
| LE | 3 | 248.97 ± 8.17 aB | 0.25 ± 0.05 aB | 9.63 ± 0.50 aC |
| 6 | 256.37 ± 5.80 aA | 0.20 ± 0.02 aC | 10.55 ± 1.33 bA | |
| 12 | 207.40 ± 3.24 bA | 0.13 ± 0.01 bC | 10.57 ± 0.32 aC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Kim, J.; Lee, J. Enzyme-Assisted Tenderization and Vitamin E-Loaded Liposome Coating for Garlic Scape Quality Enhancement. Foods 2026, 15, 8. https://doi.org/10.3390/foods15010008
Kim J, Lee J. Enzyme-Assisted Tenderization and Vitamin E-Loaded Liposome Coating for Garlic Scape Quality Enhancement. Foods. 2026; 15(1):8. https://doi.org/10.3390/foods15010008
Chicago/Turabian StyleKim, Juhyun, and Jiseon Lee. 2026. "Enzyme-Assisted Tenderization and Vitamin E-Loaded Liposome Coating for Garlic Scape Quality Enhancement" Foods 15, no. 1: 8. https://doi.org/10.3390/foods15010008
APA StyleKim, J., & Lee, J. (2026). Enzyme-Assisted Tenderization and Vitamin E-Loaded Liposome Coating for Garlic Scape Quality Enhancement. Foods, 15(1), 8. https://doi.org/10.3390/foods15010008






