The Role of Plant-Derived Bioactive Compounds in Mitigating Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
3. The Issue of Oxidative Stress
4. Antioxidants
5. Plant-Derived Bioactive Compounds (BACs)
5.1. Polyphenols as Antioxidants
5.2. Flavonoids and Oxidative Stress
5.3. Terpenes and Oxidative Stress
6. Health Benefits of Plant-Derived Bioactive Compounds
7. Encapsulation and Delivery Systems of Plant-Derived Bioactive Compounds
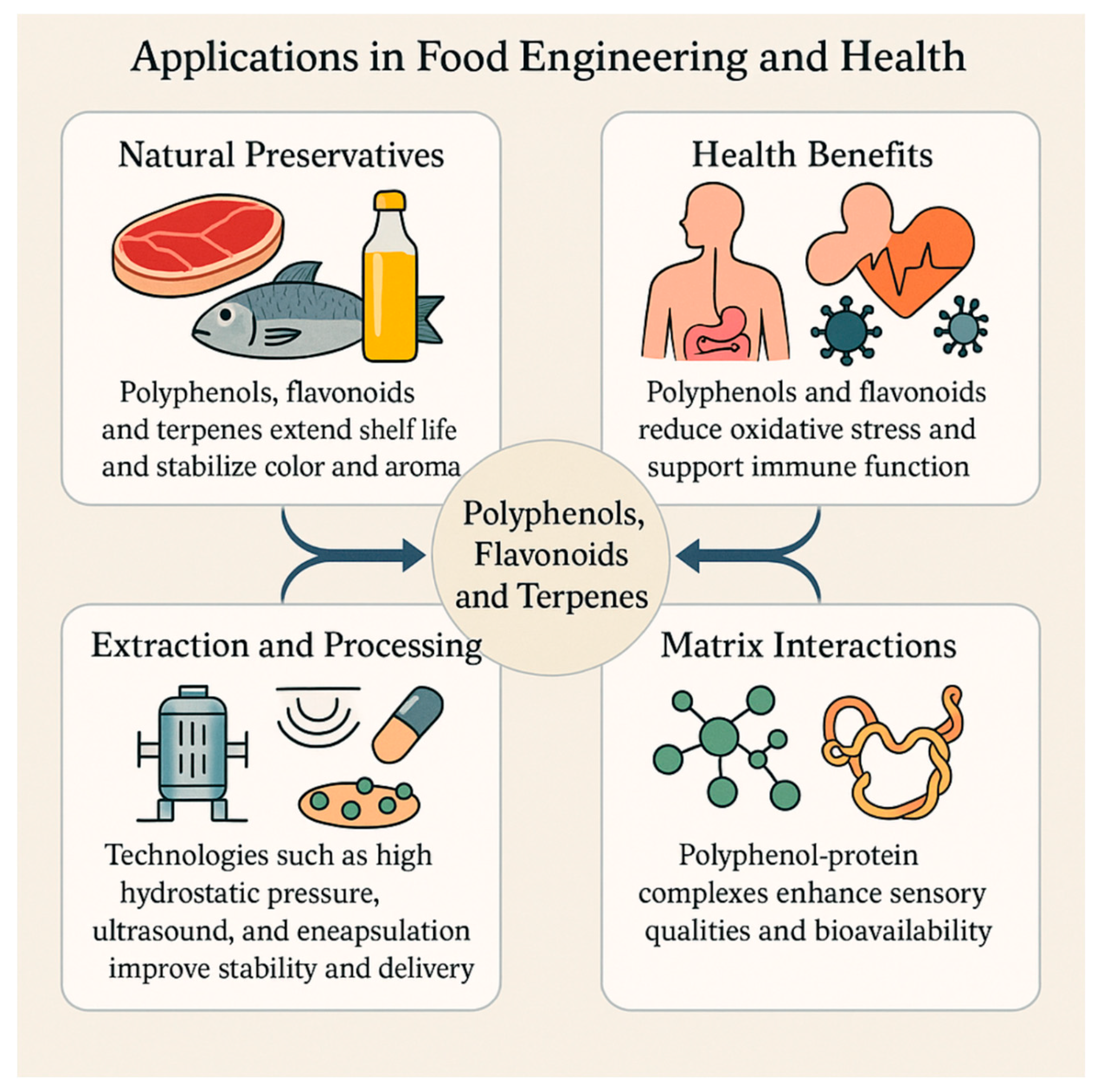
8. Applications in Food Engineering and Health
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Prak, S.-Y.; Lee, S.J. Free radicals and their impact on health and antioxidant defences: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguelez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Pruchniak, M.P.; Araźna, M.; Demkow, U. Biochemistry of oxidative stress. In Advances in Clinical Science; Springer: Cham, Switzerland, 2015; pp. 9–19. [Google Scholar]
- Garcia-Llorens, G.; El Ouardi, M.; Valls-Belles, V. Oxidative Stress Fundamentals: Unraveling the Pathophysiological Role of Redox Imbalance in Non-Communicable Diseases. Appl. Sci. 2025, 15, 10191. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and oxidative stress: An overview of basic concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef]
- Anwar, H.; Nawaz, L.; Saddiqa, A.; Shaheen, S.; Muzaffar, H.; Ijaz, M.U.; Mukhtaar, I. Oxidative Stress as a Triggering Mechanism of Various Diseases. In The Role of Natural Antioxidants in Brain Disorders; Springer International Publishing: Cham, Switzerland, 2023; pp. 71–87. [Google Scholar]
- Poljšak, B.; Jamnik, P.; Milisav, I. The importance of multifaceted approach for accurate and comprehensive evaluation of oxidative stress status in biological systems. Antioxidants 2025, 14, 1083. [Google Scholar] [CrossRef]
- Silva, J.P.; Coutinho, O.P. Free radicals in the regulation of damage and cell death—Basic mechanisms and prevention. Drug Discov. Ther. 2010, 4, 144–167. [Google Scholar]
- Skoryk, O.D.; Horila, M.V. Oxidative stress and disruption of the antioxidant defense system as triggers of diseases. Regul. Mech. Biosyst. 2023, 14, 665–672. [Google Scholar] [CrossRef]
- Corrales, L.C.; Muñoz Ariza, M.M. Oxidative Stress: Origin, evolution and consequences of oxygen toxicity. Nova 2012, 10, 213–225. [Google Scholar] [CrossRef]
- Camila Alcalde, M.; Edmo Atique, G.; de Mello, M.A.J. Oxidative Stress: Cause Mechanisms and Defense Agents. Cardiol. Vasc. Res. 2019, 3, 1–4. [Google Scholar] [CrossRef]
- Berakdar, N.O.U.R.A.; Alahmad, A. Review of oxidative stress and antioxidative. J. Clin. Diagn. Res. 2022, 16, BE01–BE06. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The role of oxidative stress in physiopathology and pharmacological treatment with pro-and antioxidant properties in chronic diseases. Oxidative Med. Cell. Longev. 2020, 1, 2082145. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, J.; Wang, Y.; Deng, F.; Deng, Z. Oxidative Stress: Signaling Pathways, Biological Functions, and Disease. MedComm 2025, 6, e70268. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 552535. [Google Scholar] [CrossRef] [PubMed]
- Al-Kufaishi, A.M.; Al-Musawi, N.J. Oxidative Stress and Related Diseases: A Comprehensive Review. JPDTSM 2025, 4, 65–70. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Souayah, N. Oxidative Stress: Pathological Driver in Chronic Neurodegenerative Diseases. Antioxidants 2025, 14, 696. [Google Scholar] [CrossRef]
- Reddy, V.P. Oxidative stress in health and disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef]
- Altanam, S.Y.; Darwish, N.; Bakillah, A. Exploring the Interplay of Antioxidants, Inflammation, and Oxidative Stress: Mechanisms, Therapeutic Potential, and Clinical Implications. Diseases 2025, 13, 309. [Google Scholar] [CrossRef]
- Francati, S.; Fiore, M.; Ferraguti, G. The janus face of oxidative stress in health and disease: The cause or the cure? Biomed. Rev. 2023, 34, 13–26. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Jawhara, S. How Do Polyphenol-Rich Foods Prevent Oxidative Stress and Maintain Gut Health? Microorganisms 2024, 12, 1570. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant compounds from microbial sources: A review. Food Res. Int. 2020, 129, 108849. [Google Scholar] [CrossRef] [PubMed]
- Hyży, A.; Rozenek, H.; Gondek, E.; Jaworski, M. Effect of Antioxidants on the Gut Microbiome Profile and Brain Functions: A Review of Randomized Controlled Trial Studies. Foods 2025, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef]
- Mohammadi, N.; Farrell, M.; O’Sullivan, L.; Langan, A.; Franchin, M.; Azevedo, L.; Granato, D. Effectiveness of anthocyanin-containing foods and nutraceuticals in mitigating oxidative stress, inflammation, and cardiovascular health-related biomarkers: A systematic review of animal and human interventions. Food Funct. 2024, 15, 3274. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Poljsak, B.; Kovac, V.; Milisav, I. Antioxidants, Food Processing and Health. Antioxidants 2021, 10, 433. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Sun, S.; Liu, Z.; Lin, M.; Gao, N.; Wang, X. Polyphenols in health and food processing: Antibacterial, anti-inflammatory, and antioxidant insights. Front. Nutr. 2024, 11, 1456730. [Google Scholar] [CrossRef]
- Bińkowska, W.; Szpicer, A.; Stelmasiak, A.; Wojtasik-Kalinowska, I.; Półtorak, A. Microencapsulation of Polyphenols and Their Application in Food Technology. Appl. Sci. 2024, 14, 11954. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Med. Cell. Longev. 2016, 1, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Kerimi, A.; Williamson, G. At the interface of antioxidant signalling and cellular function: Key polyphenol effects. Mol. Nutr. Food Res. 2016, 60, 1770–1788. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Luo, M.; Wei, S. The bioprotective effects of polyphenols on metabolic syndrome against oxidative stress: Evidences and perspectives. Oxidative Med. Cell. Longev. 2019, 2019, 6713194. [Google Scholar] [CrossRef]
- Villalobos-Delgado, L.H.; Nevárez-Moorillon, G.V.; Caro, I.; Quinto, E.J.; Mateo, J. Natural antimicrobial agents to improve foods shelf-life. In Food Quality and Shelf-Life; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 125–157. [Google Scholar]
- Markovic, Z.; Jeremic, S.; Dimitric Markovic, J.; Stanojevic Pirkovic, M.; Amic, D. Influence of structural characteristics of substituents on the antioxidant activity of some anthraquinone derivatives. Comput. Theor. Chem. 2016, 1077, 25–31. [Google Scholar] [CrossRef]
- Andriantsitohaina, R.; Auger, C.; Chataigneau, T.; Étienne-Selloum, N.; Li, H.; Martínez, M.C.; Laher, I. Molecular mechanisms of the cardiovascular protective effects of polyphenols. Br. J. Nutr. 2012, 108, 1532–1549. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Yu, Y.; Liu, X.; Shang, X.; Du, Z.; Xu, M.; Zhang, T. Recent advances in the inhibition of membrane lipid peroxidation by food-borne plant polyphenols via the nrf2/gpx4 pathway. J. Agric. Food Chem. 2024, 72, 12340–12355. [Google Scholar] [CrossRef]
- Wu, F.; Lin, B.; Chen, J.; Zheng, F.; Yang, Y.; Rasheed, U.; Chen, G. Mechanistic Insights into the Antioxidant Potential of Sugarcane Vinegar Polyphenols: A Combined Approach of DPPH-UPLC-MS, Network Pharmacology and Molecular Docking. Foods 2024, 13, 3379. [Google Scholar] [CrossRef]
- Gormaz, J.G.; Valls, N.; Sotomayor, C.; Turner, T.; Rodrigo, R. Potential role of polyphenols in the prevention of cardiovascular diseases: Molecular bases. Curr. Med. Chem. 2016, 23, 115–128. [Google Scholar] [CrossRef]
- García-Aguilar, A.; Palomino, O.; Benito, M.; Guillén, C. Dietary polyphenols in metabolic and neurodegenerative diseases: Molecular targets in autophagy and biological effects. Antioxidants 2021, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; San Feliciano, A. Flavonoids: From Structure to Health Issues. Molecules 2017, 22, 477. [Google Scholar] [CrossRef]
- Dixon, R.A.; Pasinetti, G.M. Flavonoids and Isoflavonoids: From Plant Biology to Agriculture and Neuroscience. Plant Physiol. 2010, 154, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.-B.; Li, Y.-H.; Shu, X.-C.; Pu, Y.-T.; Wang, X.-J.; Wang, T.; Wang, Z. The Classification, Molecular Structure and Biological Biosynthesis of Flavonoids, and Their Roles in Biotic and Abiotic Stresses. Molecules 2023, 28, 3599. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T. The Structure and Distribution of the Flavonoids in Plants. J. Plant Res. 2000, 113, 287–299. [Google Scholar] [CrossRef]
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Ogah, O.; Watkins, C.S.; Ubi, B.E.; Oraguzie, N.C. Phenolic Compounds in Rosaceae Fruit and Nut Crops. J. Agric. Food Chem. 2014, 62, 9369–9386. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Peter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Assalve, G.; Lunetti, P.; Zara, V.; Ferramosca, A. In vivo antioxidant activity of common dietary flavonoids: Insights from the yeast model Saccharomyces cerevisiae. Antioxidants 2024, 13, 1103. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef] [PubMed]
- Ahmadinejad, F.; Geir Møller, S.; Hashemzadeh-Chaleshtori, M.; Bidkhori, G.; Jami, M.S. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants 2017, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Treml, J.; Šmejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. CRFSFS 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defence against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Wójciak, M.; Feldo, M.; Borowski, G.; Kubrak, T.; Płachno, B.J.; Sowa, I. Antioxidant potential of diosmin and diosmetin against oxidative stress in endothelial cells. Molecules 2022, 27, 8232. [Google Scholar] [CrossRef]
- Kim, T.Y.; Leem, E.; Lee, J.M.; Kim, S.R. Control of reactive oxygen species for the prevention of Parkinson’s disease: The possible application of flavonoids. Antioxidants 2020, 9, 583. [Google Scholar] [CrossRef]
- Delgado, L.; González-Paramás, A.M.; González-Manzano, S.; Ayuda-Durán, B.; Santos-Buelga, C. Influence of flavonoids in ROS production and oxidative DNA damage in Caenorhabditis elegans submitted to thermal stress. Planta Med. 2014, 80, P2O5. [Google Scholar] [CrossRef]
- Zheng, Q.; Feng, K.; Zhong, W.; Tan, W.; Rengaowa, S.; Hu, W. Investigating the hepatoprotective properties of mulberry leaf flavonoids against oxidative stress in HepG2 cells. Molecules 2024, 29, 2597. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Keramat, M.; Ehsandoost, E.; Golmakani, M.T. Recent trends in improving the oxidative stability of oil-based food products by inhibiting oxidation at the interfacial region. Foods 2023, 12, 1191. [Google Scholar] [CrossRef] [PubMed]
- Cedeño-Pinos, C.; Martínez-Tomé, M.; Mercatante, D.; Rodríguez-Estrada, M.T.; Bañón, S. Assessment of a diterpene-rich rosemary (Rosmarinus officinalis L.) extract as a natural antioxidant for salmon pate formulated with linseed. Antioxidants 2022, 11, 1057. [Google Scholar] [CrossRef]
- Qi, Y.; Huang, Y.; Dong, Y.; Zhang, W.; Xia, F.; Bai, H.; Stevanovic, Z.D.; Li, H.; Shi, L. Effective improvement of the oxidative stability of Acer truncatum bunge seed oil, a new woody oil food resource, by rosemary extract. Antioxidants 2023, 12, 889. [Google Scholar] [CrossRef]
- López, P.L.; Enemark, G.K.G.; Grosso, N.R.; Olmedo, R.H. Antioxidant effectiveness between mechanisms of “Chain breaking antioxidant” and “Termination enhancing antioxidant” in a lipid model with essential oils. Food Biosci. 2024, 57, 103498. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Zhang, W.H.; Zhu, Q.M.; Ning, J.; Huo, X.K.; Xiao, H.T.; Sun, C.P. Total terpenoids of Inula japonica activated the Nrf2 receptor to alleviate the inflammation and oxidative stress in LPS-induced acute lung injury. Phytomedicine 2022, 104, 154307. [Google Scholar] [CrossRef]
- Juncos, N.S.; Ponso, C.F.C.; Grosso, N.R.; Olmedo, R.H. Oxidation protection efficiency of the combination of Minthostachys mollis K. and Origanum vulgare L. essential oils with “chain-breaking” and “termination- enhancing” antioxidant mechanisms. J. Food Sci. 2024, 89, 9166–9178. [Google Scholar] [CrossRef]
- Jin, Z.; Mollica, F.; Huang, Y.; Guernelli, S.; Baschieri, A.; Diquigiovanni, C.; Amorati, R. Pro-aromatic Natural Terpenes as Unusual “Slingshot” Antioxidants with Promising Ferroptosis Inhibition Activity. Chem. Eur. J. 2024, 30, e202403320. [Google Scholar] [CrossRef]
- Foti, M.C.; Ingold, K.U. Mechanism of inhibition of lipid peroxidation by γ-terpinene, an unusual and potentially useful hydrocarbon antioxidant. J. Agric. Food Chem. 2003, 51, 2758–2765. [Google Scholar] [CrossRef]
- Lee, M.H.; Do Kim, H.; Jang, Y.J. Delivery systems designed to enhance stability and suitability of lipophilic bioactive compounds in food processing: A review. Food Chem. 2024, 437, 137910. [Google Scholar] [CrossRef]
- Bravo-Díaz, C. Advances in the control of lipid peroxidation in oil-in-water emulsions: Kinetic approaches. Crit. Rev. Food Sci. Nutr. 2023, 63, 6252–6284. [Google Scholar] [CrossRef] [PubMed]
- Mirossay, L.; Varinska, L.; Mojzis, J. Antiangiogenic effect of flavonoids and chalcones: An update. Int. J. Mol. Sci. 2018, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Targhi, R.G.; Saba, V. Grape seed extract alleviates radiation-induced damages in human blood lymphocytes. Avicenna J. Phytomed. 2020, 10, 398–406. [Google Scholar]
- Ganesan, K.; Mickymaray, S.; Al Aboody, M.S.; Alfaiz, F.A.; Thatchinamoorthi, R.; Xu, B. Immunomodulatory and antineoplastic efficacy of common spices and their connection with phenolic antioxidants. BCHD 2020, 3, 15–31. [Google Scholar]
- Seidel, C.; Schnekenburger, M.; Dicato, M.; Diederich, M. Antiproliferative and proapoptotic activities of 4-hydroxybenzoic acid-based inhibitors of histone deacetylases. Cancer Lett. 2014, 343, 134–146. [Google Scholar] [CrossRef]
- Wang, L.C.; Chu, K.H.; Liang, Y.C.; Lin, Y.L.; Chiang, B.L. Caffeic acid phenethyl ester inhibits nuclear factor-kB and protein kinase B signalling pathways and induces caspase-3 expression in primary human CD4+ T cells. Clin. Exp. Immunol. 2010, 160, 223–232. [Google Scholar] [CrossRef]
- Fukuda, M.; Kobayashi, K.; Hirono, Y.; Miyagawa, M.; Ishida, T.; Ejiogu, E.C.; Sawai, M.; Pinkerton, K.E.; Takeuchi, M. Jungle honey enhances immune function and antitumor activity. Evid. Based Complement. Altern. Med. 2011, 8, 908743. [Google Scholar] [CrossRef]
- Yu, H.M.; Wang, B.S.; Chu, H.L.; Chang, L.W.; Yen, W.J.; Lin, C.J.; Duh, P.D. Napiergrass (Pennisetum purpureum S.) protects oxidative damage of biomolecules and modulates antioxidant enzyme activity. Food Chem. 2007, 105, 1364–1374. [Google Scholar] [CrossRef]
- Cheng, D.M.; Pogrebnyak, N.; Kuhn, P.; Poulev, A.; Waterman, C.; Rojas-Silva, P.; Johnson, W.D.; Raskin, I. Polyphenol-rich Rutgers Scarlet Lettuce improves glucose metabolism and liver lipid accumulation in diet-induced obese C57BL/6 mice. Nutrients 2014, 30, S52–S58. [Google Scholar] [CrossRef]
- Chacar, S.; Itani, T.; Hajal, J.; Saliba, Y.; Louka, N.; Faivre, J.-F.; Maroun, R.; Fares, N. The Impact of Long-Term Intake of Phenolic Compounds-Rich Grape Pomace on Rat Gut Microbiota. J. Food Sci. 2018, 83, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Nagata, R.; Echizen, M.; Yamaguchi, Y.; Kyu-Ho Han Shimada, K.; Ohba, K.; Kitano-Okada, T.; Nagura, T.; Uchino, H.; Fukushima, M. Effect of a combination of inulin and polyphenol-containing adzuki bean extract on intestinal fermentation in vitro and in vivo. Biosci. Biotechnol. Biochem. 2018, 82, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Presannakumar, G.; Vijayalakshmi, N.R. Investigations on the Effect of Flavonoids from Banana, Musa Paradisiaca L. on Lipid Metabolism in Rats. J. Diet. Suppl. 2009, 6, 111–123. [Google Scholar] [CrossRef]
- Li, N.; Sun, Y.-R.; He, L.-B.; Huang, L.; Li, T.-T.; Wang, T.-Y.; Tang, L. Amelioration by Idesia polycarpa Maxim. var. vestita Diels. of Oleic Acid-Induced Nonalcoholic Fatty Liver in HepG2 Cells through Antioxidant and Modulation of Lipid Metabolism. Oxidative Med. Cell. Longev. 2020, 2020, 120872. [Google Scholar] [CrossRef]
- Barona, J.; Aristizabal, J.C.; Blesso, C.N.; Volek, J.S.; Fernandez, M.L. Grape Polyphenols Reduce Blood Pressure and Increase Flow-Mediated Vasodilation in Men with Metabolic Syndrome. J. Nutr. 2012, 142, 1626–1632. [Google Scholar] [CrossRef]
- Sakthivel, R.; Devi, K.P. Antioxidant, anti-inflammatory and anticancer potential of natural bioactive compounds from seaweeds. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 5; Volume 63, pp. 113–160. ISBN 1572-5995. [Google Scholar]
- Yang, D.; Jiang, Y.; Wang, Y.; Lei, Q.; Zhao, X.; Yi, R.; Zhang, X. Improvement of Flavonoids in Lemon Seeds on Oxidative Damage of Human Embryonic Kidney 293T Cells Induced by H2O2. Oxidative Med. Cell. Longev. 2020, 2020, 3483519. [Google Scholar] [CrossRef]
- Regitz, C.; Fitzenberger, E.; Mahn, F.L.; Dußling, L.M.; Wenzel, U. Resveratrol reduces amyloid-beta (Aβ1–42)-induced paralysis through targeting proteostasis in an Alzheimer model of Caenorhabditis elegans. Eur. J. Nutr. 2016, 55, 741–747. [Google Scholar] [CrossRef]
- Parveen, B.; Rajinikanth, V.; Narayanan, M. Natural plant antioxidants for food preservation and emerging trends in nutraceutical applications. Discov. Appl. Sci. 2025, 7, 845. [Google Scholar] [CrossRef]
- Zhang, D.; Ivane, N.M.A.; Haruna, S.A.; Zekrumah, M.; Elysé, F.K.R.; Tahir, H.E.; Wang, G.; Wang, C.; Zou, X. Recent trends in the micro-encapsulation of plant-derived compounds and their specific application in meat as antioxidants and antimicrobials. Meat Sci. 2022, 191, 108842. [Google Scholar] [CrossRef]
- Lourenco, S.C.; Torres, C.A.; Nunes, D.; Duarte, P.; Freitas, F.; Reis, M.A.; Alves, V.D. Using a bacterial fucose-rich polysaccharide as encapsulation material of bioactive compounds. Int. J. Biol. Macromol. 2017, 104, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Vladić, J.; Ambrus, R.; Szabó-Révész, P.; Vasić, A.; Cvejin, A.; Pavlić, B.; Vidović, S. Recycling of filter tea industry by-products: Production of A. millefolium powder using spray drying technique. Ind. Crop Prod. 2015, 80, 197–206. [Google Scholar] [CrossRef]
- Weber, F.; Boch, K.; Schieber, A. Influence of copigmentation on the stability of spray dried anthocyanins from blackberry. LWT—Food Sci. Technol. 2017, 75, 72–77. [Google Scholar] [CrossRef]
- Ramakrishnan, Y.; Mohd, N.; Aniza, Y.; Muhammad, K. Effect of wall materials on the spray drying efficiency, powder properties and stability of bioactive compounds in tamarillo juice microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Lavelli, V.; Harsha, P.S.; Spigno, G. Modelling the stability of maltodextrin-encapsulated grape skin phenolics used as a new ingredient in apple puree. Food Chem. 2016, 209, 323–331. [Google Scholar] [CrossRef]
- Hoskin, R.T.; Xiong, J.; Esposito, D.A.; Lila, M.A. Blueberry polyphenol-protein food ingredients: The impact of spray drying on the in vitro antioxidant activity, anti-inflammatory markers, glucose metabolism and fibroblast migration. Food Chem. 2019, 280, 187–194. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef]
- Rocha-Parra, D.F.; Lanari, M.C.; Zamora, M.C.; Chirife, J. Influence of storage conditions on phenolic compounds stability, antioxidant capacity and colour of freeze-dried encapsulated red wine. LWT- Food Sci. Technol. 2016, 70, 162–170. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Marksa, M.; Sosnowska, K.; Winnicka, K.; Morkuniene, R.; Bernatoniene, J. Freeze-drying technique for microencapsulation of Elsholtzia ciliata ethanolic extract using different coating materials. Molecules 2020, 25, 2237. [Google Scholar] [CrossRef]
- Kelemen, V.; Pichler, A.; Ivic, I.; Buljeta, I.; Šimunovi’c, J.; Kopjar, M. Brown rice proteins as delivery system of phenolic and volatile compounds of raspberry juice. Int. J. Food Sci. Technol. 2022, 57, 1866–1874. [Google Scholar] [CrossRef]
- de Moura, S.; Schettini, G.N.; Garcia, A.O.; Gallina, D.A.; Alvim, I.D.; Hubinger, M.D. Stability of hibiscus extract encapsulated by ionic gelation incorporated in yogurt. Food Bioprocess Technol. 2019, 12, 1500–1515. [Google Scholar] [CrossRef]
- Mendoza-Wilson, A.M.; Balandrán-Quintana, R.R.; Valdés-Covarrubias, M.Á.; Cabellos, J.L. Potential of quercetin in combination with antioxidants of different polarity incorporated in oil-in-water nanoemulsions to control enzymatic browning of apples. J. Mol. Struct. 2022, 1254, 132372. [Google Scholar] [CrossRef]
- Hong, S.-C.; Park, K.-M.; Hong, C.R.; Kim, J.-C.; Yang, S.-H.; Yu, H.-S.; Paik, H.-D.; Pan, C.-H.; Chang, P.-S. Microfluidic assembly of liposomes dual-loaded with catechin and curcumin for enhancing bioavailability. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124670. [Google Scholar] [CrossRef]
- Ha, E.-S.; Park, H.; Lee, S.-K.; Sim, W.-Y.; Jeong, J.-S.; Baek, I.-H.; Kim, M.-S. Pure Trans-resveratrol nanoparticles prepared by a supercritical antisolvent process using alcohol and dichloromethane mixtures: Effect of particle size on dissolution and bioavailability in rats. Antioxidants 2020, 9, 342. [Google Scholar] [CrossRef]
- Lopez de Dicastillo, C.; Lopez-Carballo, G.; Gavara, R.; Galet, V.M.; Guarda, A.; Galotto, M.J. Improving polyphenolic thermal stability of Aristotelia Chilensis fruit extract by encapsulation within electrospun cyclodextrin capsules. J. Food Process Preserv. 2019, 43, 14044. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, D.; Liu, D.; Zhu, B. Food-grade encapsulated polyphenols: Recent advances as novel additives in foodstuffs. Crit. Rev. Food Sci. Nutr. 2023, 63, 11545–11560. [Google Scholar] [CrossRef]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.K.; Rocha, J.M. Recent developments in polyphenol applications on human health: A review with current knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef]
- Coşkun, N.; Sarıtaş, S.; Bechelany, M.; Karav, S. Polyphenols in Foods and Their Use in the Food Industry: Enhancing the Quality and Nutritional Value of Functional Foods. Int. J. Mol. Sci. 2025, 26, 5803. [Google Scholar] [CrossRef]
- Lyu, X.; Lee, J.; Chen, W.N. Potential natural food preservatives and their sustainable production in yeast: Terpenoids and polyphenols. J. Agric. Food Chem. 2019, 67, 4397–4417. [Google Scholar] [CrossRef]
- Khan, M.K.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C. Effect of novel technologies on polyphenols during food processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 361–381. [Google Scholar] [CrossRef]
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.; Barros, L.; Ferreira, I.C. Food bioactive compounds and emerging techniques for their extraction: Polyphenols as a case study. Foods 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Pop, O.L.; Suharoschi, R.; Socaci, S.A.; Berger Ceresino, E.; Weber, A.; Gruber-Traub, C.; Vodnar, D.C.; Fărcaș, A.C.; Johansson, E. Polyphenols-ensured accessibility from food to the human metabolism by chemical and biotechnological treatments. Antioxidants 2023, 12, 865. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Yang, T.; Saad, A.M.; Alkafaas, S.S.; Elkafas, S.S.; Eldeeb, G.S.; Mohammed, D.M.; Salem, H.M.; Korma, S.A.; Loutfy, S.A.; et al. Polyphenols: Chemistry, bioavailability, bioactivity, nutritional aspects and human health benefits: A review. Int. J. Biol. Macromol. 2024, 277, 134223. [Google Scholar] [CrossRef] [PubMed]
- Lila, M.A.; Schneider, M.; Devlin, A.; Plundrich, N.; Laster, S.; Foegeding, E.A. Polyphenol-enriched berry extracts naturally modulate reactive proteins in model foods. Food Funct. 2017, 8, 4760–4767. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, J.; Wang, D.; Wan, X.; Wang, Y. Covalent polyphenols-proteins interactions in food processing: Formation mechanisms, quantification methods, bioactive effects, and applications. Front. Nutr. 2024, 11, 1371401. [Google Scholar] [CrossRef]
- Li, Y.; He, D.; Li, B.; Lund, M.N.; Xing, Y.; Wang, Y.; Li, F.; Cao, X.; Liu, Y.; Chen, X.; et al. Engineering polyphenols with biological functions via polyphenol-protein interactions as additives for functional foods. Trends Food Sci. Technol. 2021, 110, 470–482. [Google Scholar] [CrossRef]
- Gervasi, T.; Calderaro, A.; Barreca, D.; Tellone, E.; Trombetta, D.; Ficarra, S.; Smeriglio, A.; Mandalari, G.; Gattuso, G. Biotechnological applications and health-promoting properties of flavonols: An updated view. Int. J. Mol. Sci. 2022, 23, 1710. [Google Scholar] [CrossRef]
- Scarano, A.; Chieppa, M.; Santino, A. Plant polyphenols-biofortified foods as a novel tool for the prevention of human gut diseases. Antioxidants 2020, 9, 1225. [Google Scholar] [CrossRef]
- Polia, F.; Pastor-Belda, M.; Martínez-Blázquez, A.; Horcajada, M.N.; Tomás-Barberán, F.A.; García-Villalba, R. Technological and biotechnological processes to enhance the bioavailability of dietary (poly)phenols in humans. J. Agric. Food Chem. 2022, 70, 2092–2107. [Google Scholar] [CrossRef]
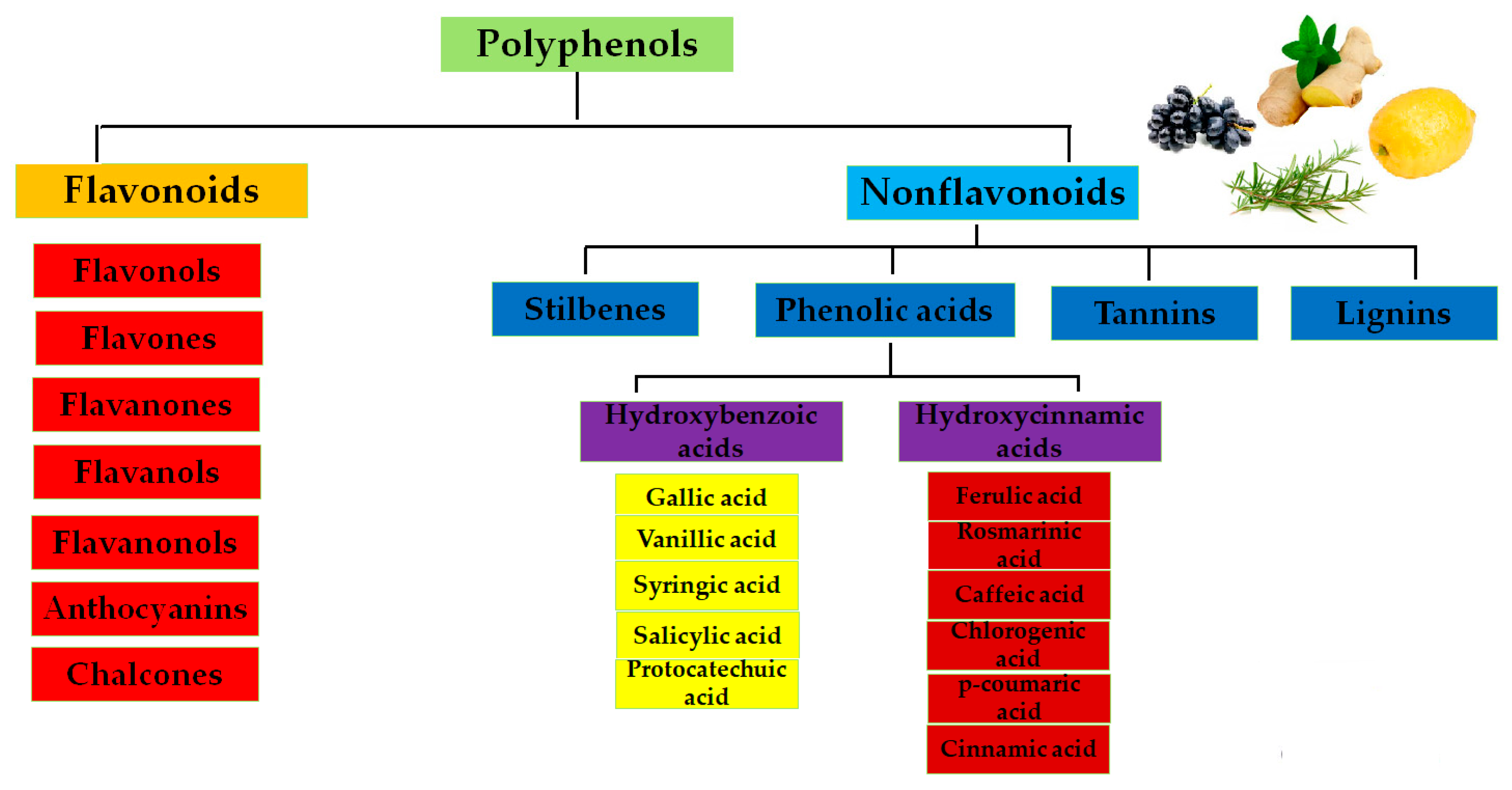
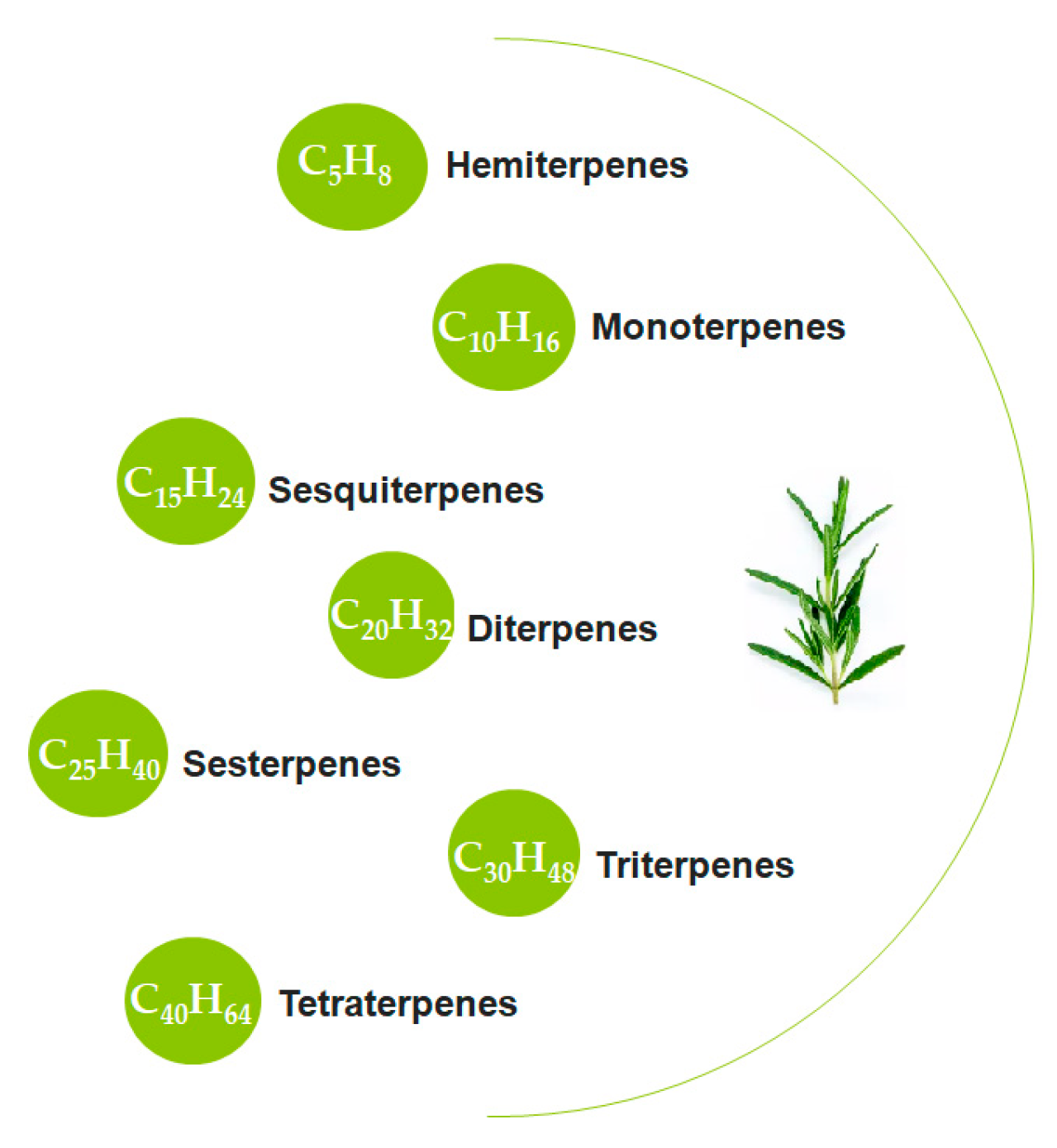
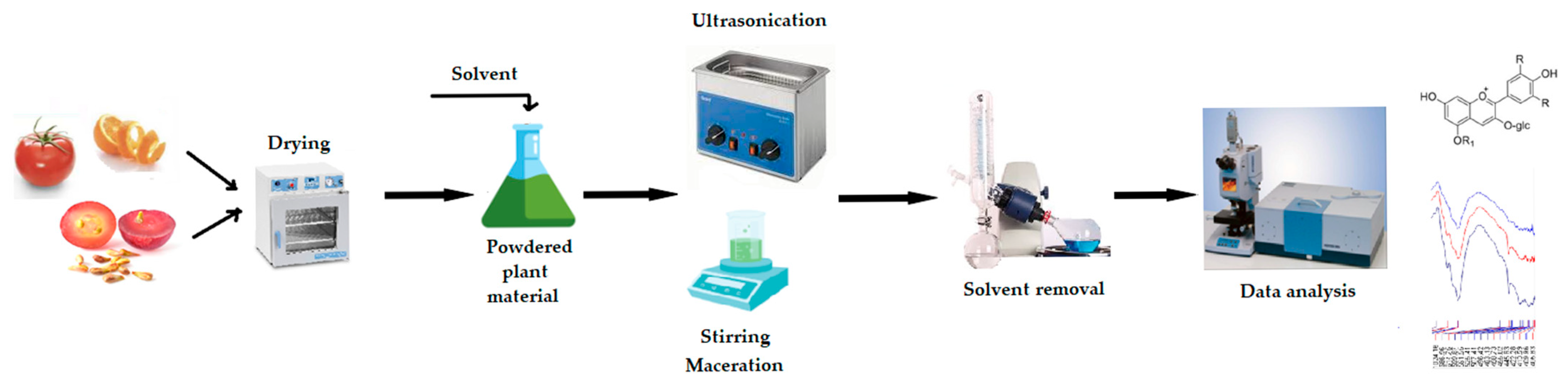
| Category | Representatives | Specific Action |
|---|---|---|
| Preventative antioxidant enzymes | Superoxide dismutase (SOD) | Converts the superoxide anion radical into hydrogen peroxide |
| Catalase | Scavenges hydrogen peroxide, yielding water and molecular oxygen by decomposing H2O2 | |
| Glutathione peroxidase | Decomposes hydrogen peroxide and hydroperoxides at the expense of glutathione | |
| Primary antioxidants (chain-breaking antioxidants) | α-Tocopherol Catechins Phenolic antioxidants | Retards initiation or interrupts propagation Converts radical species into more stable radicals or non-radical species |
| Secondary antioxidants | Metal chelators Oxidative enzyme inhibitors | Decomposes peroxides, yielding non-radical species Chelates prooxidative metal ions Inhibits oxidative enzymes Absorbs UV radiation |
| Complementary agents | Ascorbic acid β-carotene Retinoids | Scavenges active oxygen and free radicals |
| Mechanism | Main Effect | Key References |
|---|---|---|
| Direct scavenging of ROS/RNS | Neutralizes hydroxyl radicals and superoxide anions to prevent cellular damage | [33,34] |
| Metal ion chelation | Reduces free radical formation by binding to metal ions | [34] |
| Suppression of oxidative enzymes | Decreases ROS production by inhibiting oxidative enzymes such as xanthine oxidoreductase | [36] |
| Activation of the Nrf2–Keap1 pathway | Increases synthesis of antioxidant enzymes like SOD, catalase, and GPx4 | [35,40] |
| Inhibition of NF-κB pathway | Reduces inflammatory cytokine synthesis by blocking gene transcription | [34,36] |
| Regulation of PI3K–Akt and IL-17 signaling | Regulates stress response proteins, leading to anti-inflammatory effects | [42,43] |
| Maintenance of mitochondrial function | Protects mitochondrial DNA and enhances energy production | [37,44] |
| Induction of autophagy | Clears damaged organelles and promotes cellular renewal | [4] |
| Regulation of nitric oxide homeostasis | Balances eNOS activity and enhances vascular function | [36,43] |
| Mechanism | Main Effect | Key References |
|---|---|---|
| Direct scavenging of reactive oxygen species (ROS) | Interrupts free radical chain reactions by donating electrons or hydrogen atoms | [55,56,57] |
| Structural determinants of antioxidant activity | Hydroxyl and catechol groups enable strong binding to hydroxyl radicals; gallate groups enhance activity | [58,59] |
| Regulation of enzymatic antioxidant systems | Increases the activity of SOD, CAT, and GPx enzymes to detoxify reactive oxygen species | [56,60,61] |
| Activation of the Nrf2 signaling pathway | Stimulates the transcription factor Nrf2, leading to upregulation of antioxidant genes and enzyme synthesis | [60,62] |
| Restoration of cellular redox balance and mitochondrial protection | Reduces intracellular ROS and MDA levels, restores mitochondrial membrane integrity, and enhances cell resistance | [55,63,64] |
| Mechanism | Main Effect | Key References |
|---|---|---|
| Chain-breaking antioxidant action | Stops lipid oxidation chain reactions by neutralizing free radicals in oils (e.g., carnosic acid, carnosol) | [67,69] |
| Singlet oxygen quenching | Converts high-energy singlet oxygen into stable molecular oxygen, preventing oxidative degradation | [65] |
| Termination enhancing mechanism | Accelerates the end of oxidation chain reactions by promoting stable product formation | [71] |
| Slingshot mechanism (γ-terpinene) | Releases water-soluble HOO• radicals that halt oxidation externally without pro-oxidant effect | [72,73] |
| Application in food preservation | Prevents MDA and cholesterol oxidation products formation, maintaining food color and quality | [67,69] |
| Encapsulation in biopolymer carrier systems | Enhances terpene stability by forming biopolymer barriers that limit oxygen access and prolong shelf life | [66,74,75] |
| BACs | Health Benefits | Mechanism of Action | References |
|---|---|---|---|
| Cardamonin | Antiangiogenic and anticancer effects | Suppressing mammalian target of rapamycin as well as vascular endothelial growth factor (VEGF)-induced angiogenesis via microRNAs | [76] |
| Flavonoids from grape seed extract | Protects human blood lymphocytes against oxidative stress induced by ionizing radiation | Significant decrease in the incidence of micronuclei protection | [77] |
| Flavonoid and total polyphenols | Anticarcinogenic potential | Inhibition of the production of NO and TNF-α | [78] |
| Gallic acid | Induces cancer cell death | Inhibition of histone deacetylase | [79] |
| Caffeic acid | Cell cycle arrest in oral cavity, neck, and tongue carcinoma cell lines | Modulates key signaling pathways such as NF-kβ, MAPK, and AKT (protein kinase B) | [80,81] |
| Phenolic-rich water extract of napiergrass | Protection to BNL cells from H2O2-induced cytotoxicity | Upregulates the levels of GSH and that of antioxidant enzymes | [82] |
| Lettuce polyphenols | Antidiabetic effects | Inhibition of hepatic glucose-6-phosphate translocase, lower fasting blood glucose in db/db mice | [83] |
| Grape pomace extract (phenolic compounds) | Gut health | Selective modulation of the rat gut microbiota to a healthier phenotype | [84] |
| Cranberry polyphenols | Gut health | Improves high fat/high sucrose diet-induced features of the metabolic syndrome, with a proportional increase in the Akkermansia spp. population | [85] |
| Adzuki bean polyphenols | Gut health | Reduces butyrate production | [86] |
| Flavonoids from M. paradisiaca | Reduction of cholesterol levels | Reduces the activities of lipogenic enzymes Increases turnover of cholesterol into bile acids and neutral sterols | [87] |
| Idesia polycarpa polyphenols | Lipid-lowering effect | Activates PPARα in association with decreased expression of NF-κB, and IL-1 | [88] |
| Grape polyphenols | Improves vascular function | Potentiates vasorelaxation and reduces BP and circulating cell adhesion molecules | [89] |
| Brown algae (Ecklonia cava) polyphenols | Prevents tumor progression in vivo | Inhibits the activity of cyclooxygenase-2 and cell proliferation | [90] |
| Lemon seeds flavonoids | Improvement of oxidative stress system | Activation of the Nrf2 antioxidant signal pathways | [91] |
| Resveratrol from red wine | Improving brain health | Inhibition of beta amyloid protein aggregation | [92] |
| Diterpene from red alga Gracilaria edulis | Antiproliferative activity against the human lung adenocarcinoma cell line A549 | Several mechanisms of action, such as apoptosis | [90] |
| BACs | Wall Material | Advantages/Disadvantages | Impact in Food Matrix/Food Application | Ref. |
|---|---|---|---|---|
| Spray drying technique | ||||
| Gallic acid | Bacterial exopolysaccharide | No antioxidant activity was lost during the encapsulation process/different release profiles of the bioactive compounds in simulated gastric and intestinal fluids | To be investigated | [95] |
| Chlorogenic acid | Maltodextrin | Powders with good chemical and physical properties/- | To be investigated | [96] |
| Anthocyanins | Maltodextrin | The shelf life and stability of spray-dried blackberry anthocyanins was greatly enhanced by the presence of copigments/poor stability and rapid loss of coloring properties | Formation of colored derivatives and polymers during storage due to the degradation of anthocyanins | [97] |
| Anthocyanins | Gum arabic, n-octenyl succinic anhydride-modified starch | Highest encapsulation efficiency/- | No data | [98] |
| Anthocyanins | n-Octenyl succinic anhydride modified starch, low-viscosity gum arabic alternative | Greatest storage stability/- | No data | [98] |
| Grape skin phenolics | Maltodextrin | Good processing and storage stability/- | Potential sustainable, beneficial coloring agents and health promoting compounds | [99] |
| Blueberry polyphenols | Whole wheat flour, soy protein isolate, chickpea flour, coconut flour | Very positive performance on all in vitro biological assays/- | Novel environmentally-friendly anthocyanin-rich dietary sources with promising application as healthy ingredient | [100] |
| Freeze-drying technique | ||||
| Phenolic compounds from spent coffee grounds | Maltodextrin, gum arabic | Maltodextrin as good option for encapsulation of antioxidant phenolic compounds/detrimental effect of gum arabic on the retention of phenolics, as well as on the antioxidant activity | No data | [101] |
| Polyphenols from red wine | Maltodextrin, gum arabic | Phenolic concentration of the dealcoholized wine powder is 7.1 times higher than the original liquid red wine; good stability of wine powder over 145 days under accelerated storage conditions; protection to phenolics against oxidation/an important decrease in malvidin 3-G compared to caffeic acid and resveratrol | Polyphenol enrichment of healthy powder drinks with wine powder | [102] |
| Polyphenols from Elsholtzia ciliate herb extract | Skim milk, maltodextrin, resistant-maltodextrin, sodium caseinate, gum arabic, beta-cyclodextrin | High encapsulation efficiency of polyphenols in sodium caseinate and in mixture with resistant-maltodextrin and maltodextrin, respectively/lower encapsulation efficiency of polyphenols with maltodextrin | Freeze-dried powders could be incorporated in food | [103] |
| Phenolic and volatile compounds of raspberry juice | Brown rice proteins | Highest phenolic content, anthocyanin content, and antioxidant activity in complexes with the lowest amount of protein (2%)/changes in the denaturation temperature of complexes | Novel food ingredient with health benefits and sensory attributes, potential to be used as food colorant and flavoring | [104] |
| Extrusion | ||||
| Extract of Hibiscus sabdariffa L. | Double emulsion (hibiscus extract/rapeseed oil/pectin) and a cross-linked solution (CaCl2) | Microparticles with greater stability of anthocyanins and color; higher bioactive compound retention in yogurt matrix/less homogeneous color distribution | Microparticles incorporated in the yogurt matrix promote lactic acid bacteria viability, color, and functionality | [105] |
| Emulsification | ||||
| Quercetin in combination with other antioxidants from natural sources | Oil-in-water (O/W) nanoemulsions | Inhibition of polyphenol oxidase/turbidity of nanoemulsions, without affecting their stability | Controlling of enzymatic browning of apples | [106] |
| Microfluidic technology | ||||
| Curcumin and catechin | Dipalmitoylphosphatidylcholine | Encapsulation efficiencies of curcumin and catechin in the dual-loaded liposomes were 100% and 16.77%, respectively/- | Liposomal encapsulation of functional compounds | [107] |
| Supercritical antisolvent technology | ||||
| Trans-resveratrol | Ethanol/dichloromethane mixture | Enhanced oral bioavailability of trans-resveratrol; nanoparticles with particle size controlled by solvent composition/- | Pure trans-resveratrol nanoparticles for the development of health supplements | [108] |
| Electrostatic-encapsulation technologies/electrospraying | ||||
| Polyphenols from maqui fruit extract | Cyclodextrin | Improved thermal stability of polyphenols; preserved total phenolic content at high-temperature treatments/- | Food formulations involving high temperatures, such as bakery and dairy products | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Tüğen, A.; Buruleanu, C.L. The Role of Plant-Derived Bioactive Compounds in Mitigating Oxidative Stress. Foods 2026, 15, 108. https://doi.org/10.3390/foods15010108
Tüğen A, Buruleanu CL. The Role of Plant-Derived Bioactive Compounds in Mitigating Oxidative Stress. Foods. 2026; 15(1):108. https://doi.org/10.3390/foods15010108
Chicago/Turabian StyleTüğen, Aslıhan, and Claudia Lavinia Buruleanu. 2026. "The Role of Plant-Derived Bioactive Compounds in Mitigating Oxidative Stress" Foods 15, no. 1: 108. https://doi.org/10.3390/foods15010108
APA StyleTüğen, A., & Buruleanu, C. L. (2026). The Role of Plant-Derived Bioactive Compounds in Mitigating Oxidative Stress. Foods, 15(1), 108. https://doi.org/10.3390/foods15010108





