Age-Related Meat Flavor Precursors of Naturally Grazed Sunit Sheep: Metabolomics and Transcriptomics Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Metabolomics Profiling
2.2.1. Sample Preparation and Extraction
2.2.2. Chromatography Mass Spectrometry Acquisition Conditions
T3 and Amide UPLC Conditions
ESI-QTRAP-MS/MS
2.2.3. Metabolomics Analysis
2.3. Transcriptomics Analysis
2.3.1. RNA Extraction, cDNA Preparation, and Sequencing
2.3.2. Transcript Assembly
2.3.3. Transcriptomics Data Analysis
2.3.4. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.4. Integrative Analysis of Metabolomics and Transcriptomics Data
3. Results
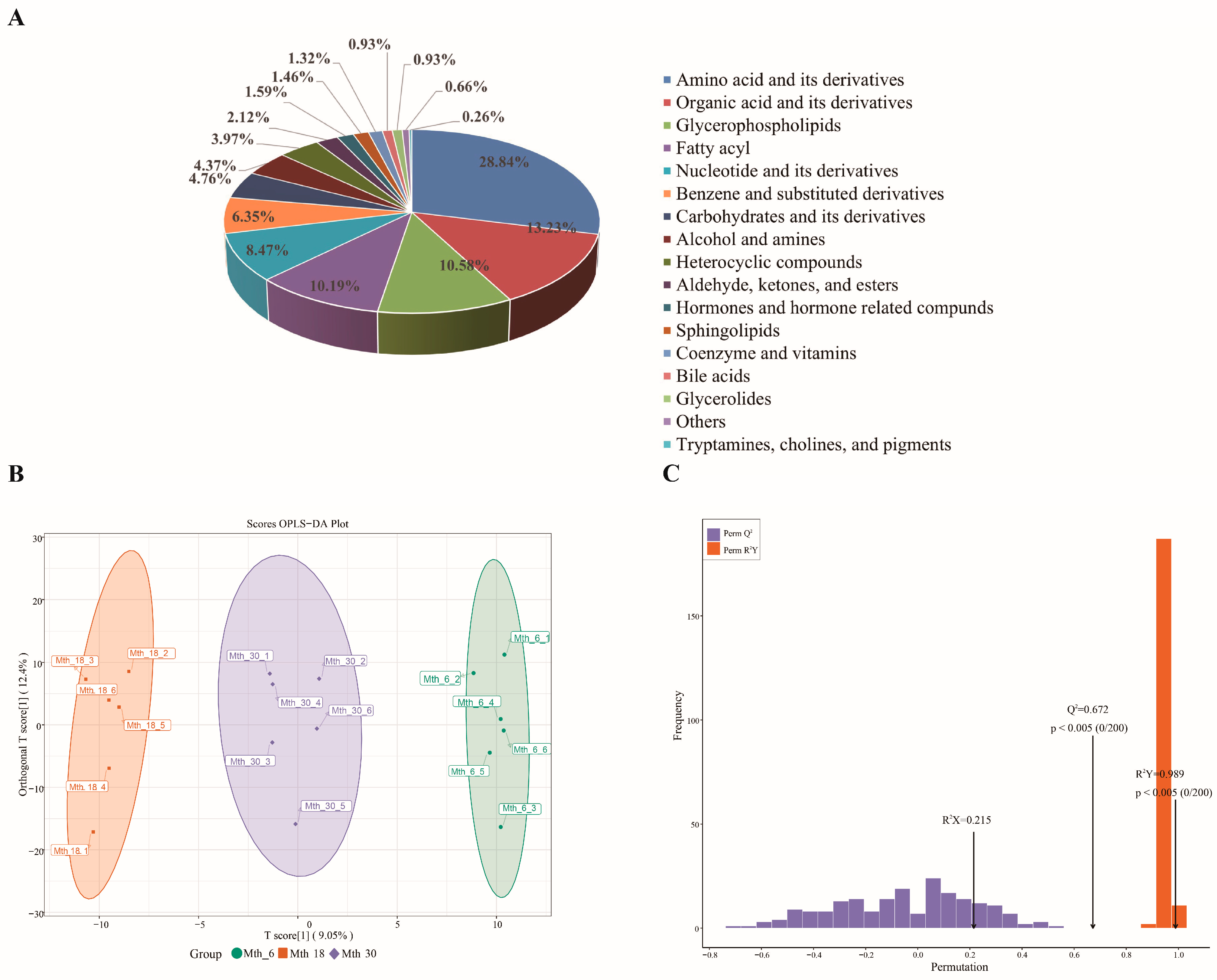
3.1. Metabolomics Analysis
3.1.1. Metabolomics Profiling and OPLS-DA Results
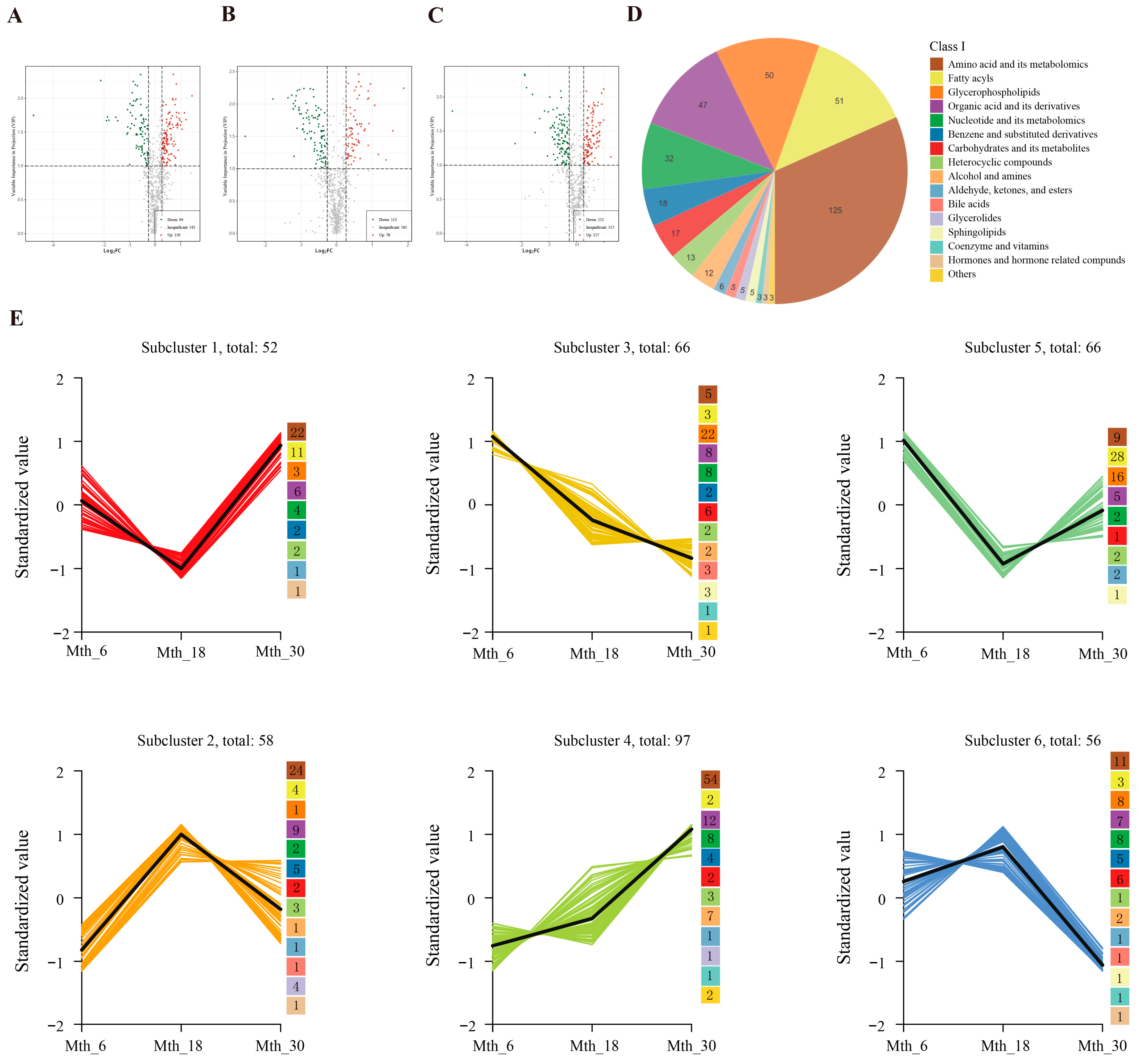
3.1.2. Differential Metabolites (DMs) Analysis
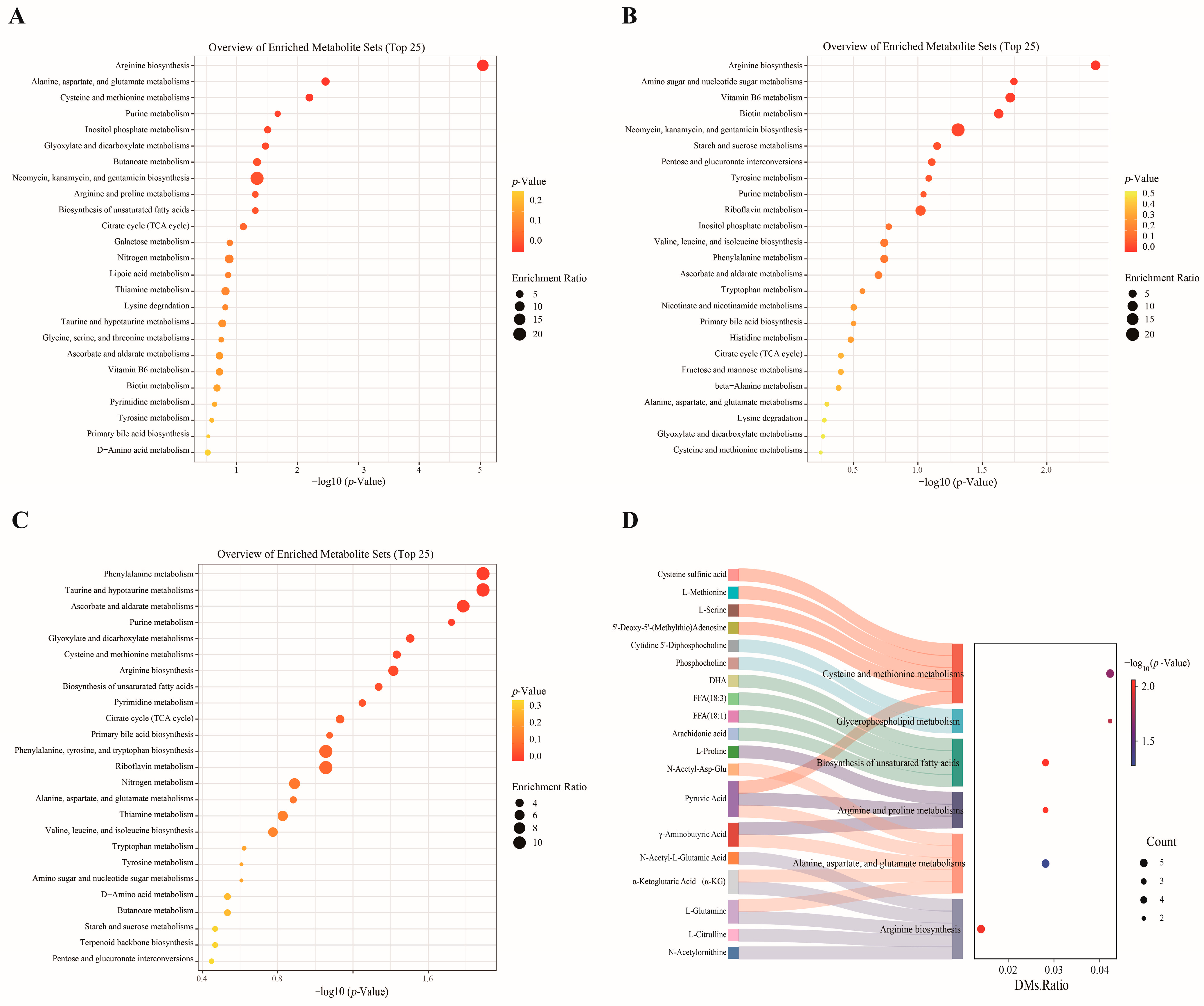
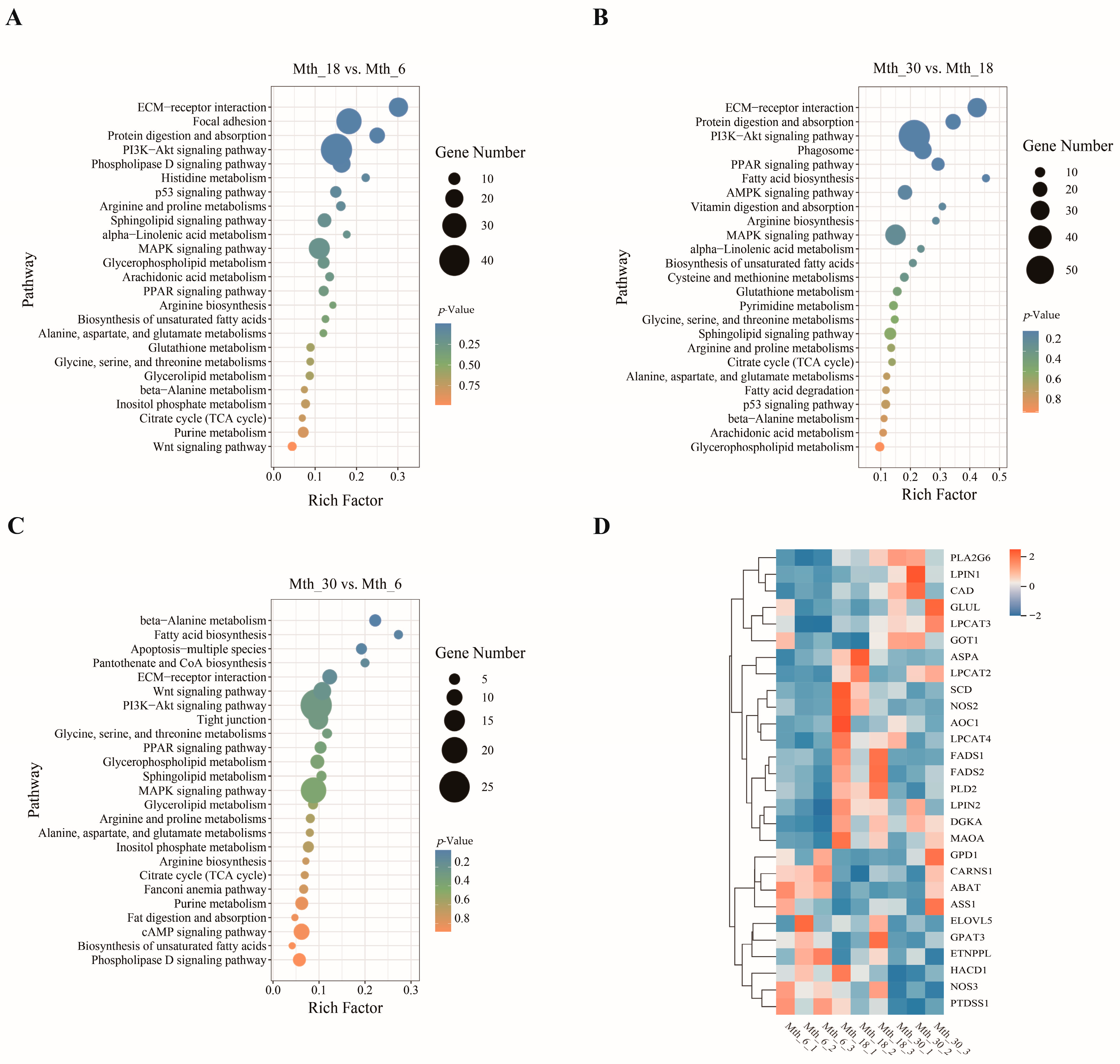
3.1.3. KEGG Pathway Enrichment Analysis of DMs
3.2. Transcriptomics Analysis
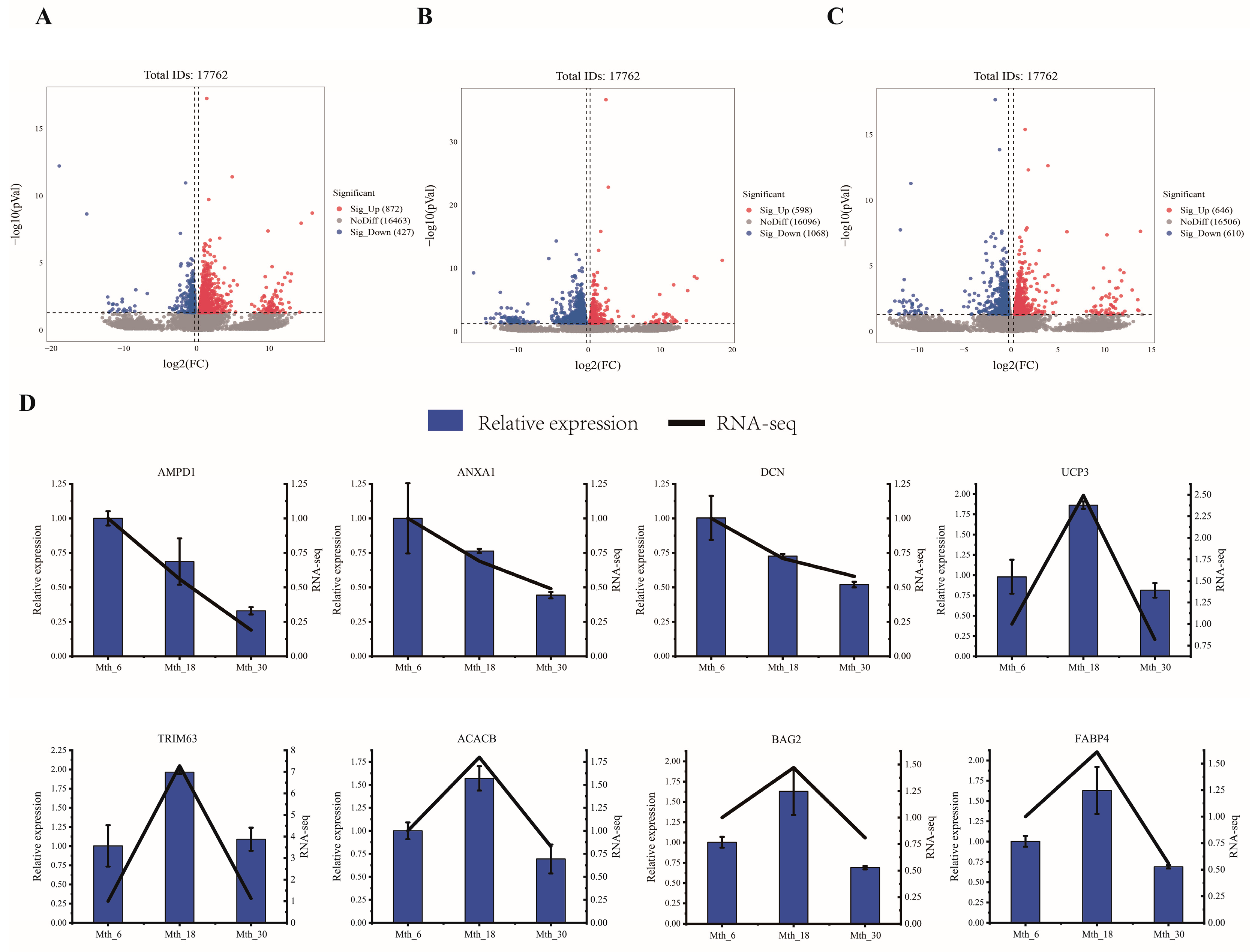
3.2.1. Analysis of RNA-Seq and Differential Expression
3.2.2. RT-qPCR Verification of DEGs
3.2.3. KEGG Pathway of DEGs
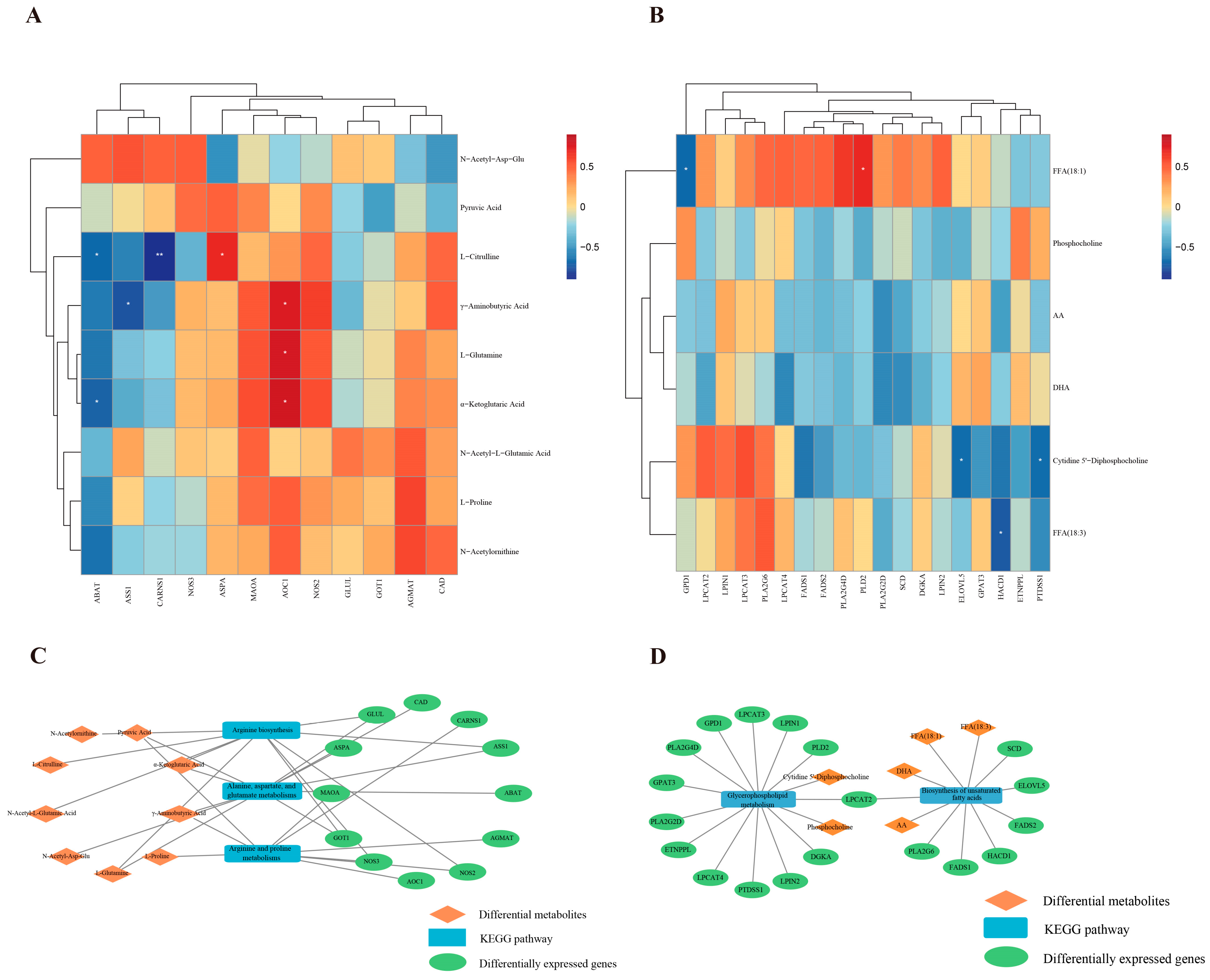
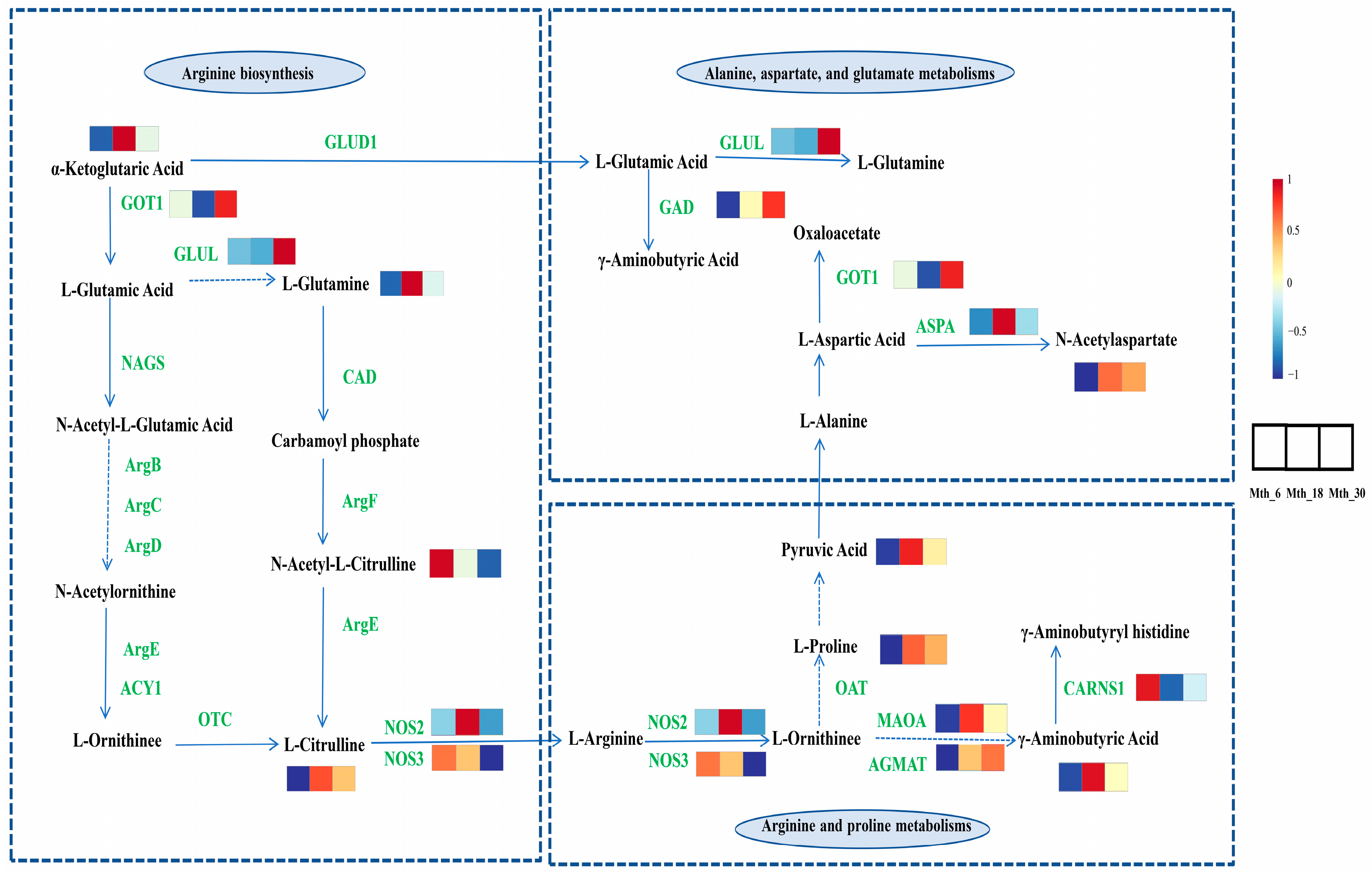
3.3. Integrated Analysis of Metabolomics and Transcriptomics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABAT | 4-aminobutyrate aminotransferase |
| ASS1 | argininosuccinate synthase 1 |
| CARNS1 | carnosine synthase 1 |
| NOS3 | nitric oxide synthase 3 |
| ASPA | aspartoacylase |
| MAOA | monoamine oxidase A |
| AOC1 | amine oxidase, copper containing 1 |
| GLUL | glutamate–ammonia ligase |
| GOT1 | glutamic-oxaloacetic transaminase 1 |
| AGMAT | agmatinase |
| CAD | carbamoyl-phosphate synthetase 2 |
| GPAT3 | glycerol-3-phosphate acyltransferase 3 |
| PTDSS1 | phosphatidylserine synthase |
| DGKA | diacylglycerol kinase alpha |
| ETNPPL | diacylglycerol kinase alpha |
| LPIN1 | lipin 1 |
| LPIN2 | lipin 2 |
| LPCAT3 | lysophosphatidylcholine acyltransferase 3 |
| LPCAT4 | lysophosphatidylcholine acyltransferase 4 |
| PLA2G4D | phospholipase A2 group IVD |
| PLA2G2D | phospholipase A2 group IID |
| PLD2 | phospholipase D2 |
| LPCAT2 | lysophosphatidylcholine acyltransferase 2 |
| FADS1 | fatty acid desaturase 1 |
| FADS2 | fatty acid desaturase 2 |
| HACD1 | 3-hydroxyacyl-CoA dehydratase 1 |
| SCD | stearoyl-CoA desaturase |
| PLA2G6 | phospholipase A2 group VI |
| NOS2 | nitric oxide synthase 2, aspartate transcarbamylase, and dihydroorotase |
References
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat Flavor Precursors and Factors Influencing Flavor Precursors—A Systematic Review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Herrera, N.J.; Calkins, C.R. Developments in Meat Flavor. In New Aspects of Meat Quality; Elsevier: Amsterdam, The Netherlands, 2022; Volume 9, pp. 195–235. ISBN 978-0-323-85879-3. [Google Scholar]
- Jacondino, L.R.; Poli, C.H.E.C.; Tontini, J.F.; Corrêa, G.F.; Somacal, S.; Mello, R.O.; Leal, M.L.R.; Raimondo, R.F.S.; Riet-Correa, B.; Muir, J.P. Acacia Mearnsii Tannin Extract and α-Tocopherol Supplementation in Lamb Diet: Effects on Growth Performance, Serum Lipid Peroxidation and Meat Quality. Anim. Feed. Sci. Technol. 2022, 294, 115483. [Google Scholar] [CrossRef]
- Wang, W.; Sun, B.; Hu, P.; Zhou, M.; Sun, S.; Du, P.; Ru, Y.; Suvorov, A.; Li, Y.; Liu, Y.; et al. Comparison of Differential Flavor Metabolites in Meat of Lubei White Goat, Jining Gray Goat and Boer Goat. Metabolites 2019, 9, 176. [Google Scholar] [CrossRef]
- Cafferky, J.; Hamill, R.M.; Allen, P.; O’Doherty, J.V.; Cromie, A.; Sweeney, T. Effect of Breed and Gender on Meat Quality of M. Longissimus Thoracis et Lumborum Muscle from Crossbred Beef Bulls and Steers. Foods 2019, 8, 173. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, H.-Y. Analyses of the Physicochemical and Sensory Characteristics of Black Goat Triceps Brachii Muscle Based on Slaughter Age. Food Chem. X 2024, 24, 101905. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Ying, Y.; Zhang, Y.; Huang, Y.; Wu, H.; Lin, K. Non-Genetic Factors Affecting the Meat Quality and Flavor of Inner Mongolian Lambs: A Review. Front. Vet. Sci. 2022, 9, 1067880. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.; Zuo, S.; Peng, S.; Wang, Z.; Zhang, Y.; Luo, H. Untargeted and Targeted Metabolomics Profiling of Muscle Reveals Enhanced Meat Quality in Artificial Pasture Grazing Tan Lambs via Rescheduling the Rumen Bacterial Community. J. Agric. Food Chem. 2021, 69, 846–858. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, M.; Feng, T.; Liu, H.; Lin, Y.; Bai, B. Comparative Characterization of Flavor Precursors and Volatiles in Chongming White Goat of Different Ages by UPLC-MS/MS and GC–MS. Food Chem. X 2024, 24, 101929. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, Y.; Wang, H.; Xi, B.; He, X.; Yang, X.; Li, W. Analysis of Volatile Compounds and Flavor Fingerprint in Jingyuan Lamb of Different Ages Using Gas Chromatography–Ion Mobility Spectrometry (GC–IMS). Meat Sci. 2021, 175, 108449. [Google Scholar] [CrossRef]
- Zhang, R.; Yoo, M.J.; Gathercole, J.; Reis, M.G.; Farouk, M.M. Effect of Animal Age on the Nutritional and Physicochemical Qualities of Ovine Bresaola. Food Chem. 2018, 254, 317–325. [Google Scholar] [CrossRef]
- Echegaray, N.; Domínguez, R.; Cadavez, V.A.P.; Bermúdez, R.; Purriños, L.; Gonzales-Barron, U.; Hoffman, E.; Lorenzo, J.M. Influence of the Production System (Intensive vs. Extensive) at Farm Level on Proximate Composition and Volatile Compounds of Portuguese Lamb Meat. Foods 2021, 10, 1450. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, C.; Dou, L.; Chen, X.; Zhao, L.; Su, L.; Jin, Y. Effects of Feeding Regimes and Postmortem Aging on Meat Quality, Fatty Acid Composition, and Volatile Flavor of Longissimus Thoracis Muscle in Sunit Sheep. Animals 2022, 12, 3081. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ding, H.; Erdene, K.; Chen, R.; Mu, Q.; Ao, C. Effects of Flavonoids from Allium Mongolicum Regel as a Dietary Additive on Meat Quality and Composition of Fatty Acids Related to Flavor in Lambs. Can. J. Anim. Sci. 2019, 99, 15–23. [Google Scholar] [CrossRef]
- Chen, L.; He, X.; Han, Y.; Huang, Y.; Li, J.; Li, J.; Yu, X.; Yun, X.; Wu, J.; Sha, R.; et al. Lipidomics Analysis of Adipose Depots at Differently Aged Sunit Sheep. Food Chem. 2024, 12, 142243. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; He, X.; Yun, Y.; Chen, L.; Huang, Y.; Wu, Q.; Qin, X.; Wu, H.; Wu, J.; Sha, R.; et al. The Characterization of Subcutaneous Adipose Tissue in Sunit Sheep at Different Growth Stages: A Comprehensive Analysis of the Morphology, Fatty Acid Profile, and Metabolite Profile. Foods 2024, 13, 544. [Google Scholar] [CrossRef]
- Kiran, M.; Naveena, B.M.; Smrutirekha, M.; Baswa Reddy, P.; Rituparna, B.; Praveen Kumar, Y.; Venkatesh, C.; Rapole, S. Traditional Halal Slaughter without Stunning versus Slaughter with Electrical Stunning of Sheep (Ovis Aries). Meat Sci. 2019, 148, 127–136. [Google Scholar] [CrossRef]
- Fan, D.; Liu, C.; Guo, Z.; Huang, K.; Peng, M.; Li, N.; Luo, H.; Wang, T.; Cen, Z.; Cai, W.; et al. Resveratrol Promotes Angiogenesis in a FoxO1-Dependent Manner in Hind Limb Ischemia in Mice. Molecules 2021, 26, 7528. [Google Scholar] [CrossRef]
- Yao, L.; Li, Y.; Knapp, J.; Smith, P. Exploration of Molecular Pathways Mediating Electric Field-Directed Schwann Cell Migration by RNA-Seq: Regulation of Ef-Directed Cell Migration. J. Cell. Physiol. 2015, 230, 1515–1524. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Prockop, D.J.; Juva, K. Synthesis of Hydroxyproline in Vitro by the Hydroxylation of Proline in a Precursor of Collagen. Proc. Natl. Acad. Sci. USA 1965, 53, 661–668. [Google Scholar] [CrossRef]
- Miller, R.A.; Shi, Y.; Lu, W.; Pirman, D.A.; Jatkar, A.; Blatnik, M.; Wu, H.; Cárdenas, C.; Wan, M.; Foskett, J.K.; et al. Targeting Hepatic Glutaminase Activity to Ameliorate Hyperglycemia. Nat. Med. 2018, 24, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, X.; Huang, Y.; Qin, R.; Xiong, P.; Qiu, Y. L-Serine Metabolic Regulation and Host Respiratory Homeostasis. Front. Cell. Infect. Microbiol. 2025, 15, 1518659. [Google Scholar] [CrossRef] [PubMed]
- Bachmanov, A.A.; Bosak, N.P.; Glendinning, J.I.; Inoue, M.; Li, X.; Manita, S.; McCaughey, S.A.; Murata, Y.; Reed, D.R.; Tordoff, M.G.; et al. Genetics of Amino Acid Taste and Appetite. Adv. Nutr. 2016, 7, 806S–822S. [Google Scholar] [CrossRef]
- Ge, Y.; Gai, K.; Li, Z.; Chen, Y.; Wang, L.; Qi, X.; Xing, K.; Wang, X.; Xiao, L.; Ni, H.; et al. HPLC-QTRAP-MS-Based Metabolomics Approach Investigates the Formation Mechanisms of Meat Quality and Flavor of Beijing You Chicken. Food Chem. X 2023, 17, 100550. [Google Scholar] [CrossRef]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of Fatty Acids on Meat Quality: A Review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Zhang, X.; Wang, D.; Jin, G.; Li, B.; Xu, F.; Cheng, J.; Zhang, F.; Wu, S.; et al. The Comprehensive Liver Transcriptome of Two Cattle Breeds with Different Intramuscular Fat Content. Biochem. Biophys. Res. Commun. 2017, 490, 1018–1025. [Google Scholar] [CrossRef]
- Lu, K.; Wang, X.; Wan, J.; Zhou, Y.; Li, H.; Zhu, Q. Correlation and Difference between Core Micro-Organisms and Volatile Compounds of Suan Rou from Six Regions of China. Foods 2022, 11, 2708. [Google Scholar] [CrossRef]
- Bao, G.; Liu, X.; Wang, J.; Hu, J.; Shi, B.; Li, S.; Luo, Y. Effects of Slaughter Age on Myosin Heavy Chain Isoforms, Muscle Fibers, Fatty Acids, and Meat Quality in Longissimus Thoracis Muscle of Tibetan Sheep. Front. Vet. Sci. 2021, 8, 689589. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yuan, Z.H.; Li, F.D.; Yue, X.P. Integrating Transcriptome and Metabolome to Identify Key Genes Regulating Important Muscular Flavour Precursors in Sheep. Animal 2022, 16, 100679. [Google Scholar] [CrossRef]
- Bleicher, J.; Ebner, E.E.; Bak, K.H. Formation and Analysis of Volatile and Odor Compounds in Meat—A Review. Molecules 2022, 27, 6703. [Google Scholar] [CrossRef]
- Jaborek, J.R.; Zerby, H.N.; Wick, M.P.; Fluharty, F.L.; Moeller, S.J. Effect of Energy Source and Level, Animal Age, and Sex on the Flavor Profile of Sheep Meat12. Transl. Anim. Sci. 2020, 4, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Margari, A.; Monteduro, A.G.; Rizzato, S.; Capobianco, L.; Crestini, A.; Rivabene, R.; Piscopo, P.; D’Onofrio, M.; Manzini, V.; Trapani, G.; et al. The Encapsulation of Citicoline within Solid Lipid Nanoparticles Enhances Its Capability to Counteract the 6-Hydroxydopamine-Induced Cytotoxicity in Human Neuroblastoma SH-SY5Y Cells. Pharmaceutics 2022, 14, 1827. [Google Scholar] [CrossRef]
- Li, J.; Tang, C.; Zhao, Q.; Yang, Y.; Li, F.; Qin, Y.; Liu, X.; Yue, X.; Zhang, J. Integrated Lipidomics and Targeted Metabolomics Analyses Reveal Changes in Flavor Precursors in Psoas Major Muscle of Castrated Lambs. Food Chem. 2020, 333, 127451. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.-Z.; Luo, Y.-L.; Luo, R.-M.; Ji, C.; Gao, S.; Bai, S.; Wang, Y.-R.; Dong, F.-J.; Hu, X.-L.; Guo, J.-J. High Freezing Rate Improves Flavor Fidelity Effect of Hand Grab Mutton after Short-Term Frozen Storage. Front. Nutr. 2022, 9, 959824. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Chen, C.-W.; Chen, H.-C.; Chen, T.-L.; Yang, K.-M. Developing the Procedure-Enhanced Model of Ginger-Infused Sesame Oil Based on Its Flavor and Functional Properties. Food Chem. X 2024, 21, 101227. [Google Scholar] [CrossRef]
- Kaczmarska, K.; Taylor, M.; Piyasiri, U.; Frank, D. Flavor and Metabolite Profiles of Meat, Meat Substitutes, and Traditional Plant-Based High-Protein Food Products Available in Australia. Foods 2021, 10, 801. [Google Scholar] [CrossRef] [PubMed]
- Meinert, L.; Schäfer, A.; Bjergegaard, C.; Aaslyng, M.D.; Bredie, W.L.P. Comparison of Glucose, Glucose 6-Phosphate, Ribose, and Mannose as Flavour Precursors in Pork; the Effect of Monosaccharide Addition on Flavour Generation. Meat Sci. 2009, 81, 419–425. [Google Scholar] [CrossRef]
- Watkins, P.J.; Jaborek, J.R.; Teng, F.; Day, L.; Castada, H.Z.; Baringer, S.; Wick, M. Branched Chain Fatty Acids in the Flavour of Sheep and Goat Milk and Meat: A Review. Small Rumin. Res. 2021, 200, 106398. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Ma, Q.; Wang, Q.; Wu, J.; Yu, H.; Li, K.; Li, Y.; Wang, J.; Zhang, Q.; et al. Glutamine Metabolism Is Required for Alveolar Regeneration during Lung Injury. Biomolecules 2022, 12, 728. [Google Scholar] [CrossRef]
- Song, X.; Gong, Z.; Liu, K.; Kou, J.; Liu, B.; Liu, K. Baicalin Combats Glutamate Excitotoxicity via Protecting Glutamine Synthetase from ROS-Induced 20S Proteasomal Degradation. Redox Biol. 2020, 34, 101559. [Google Scholar] [CrossRef]
- Sakusic, A.; Sabov, M.; McCambridge, A.J.; Rabinstein, A.A.; Singh, T.D.; Mukesh, K.; Kashani, K.B.; Cook, D.; Gajic, O. Features of Adult Hyperammonemia Not Due to Liver Failure in the ICU. Crit. Care Med. 2018, 46, e897–e903. [Google Scholar] [CrossRef]
- Shanely, R.; Nieman, D.; Perkins-Veazie, P.; Henson, D.; Meaney, M.; Knab, A.; Cialdell-Kam, L. Comparison of Watermelon and Carbohydrate Beverage on Exercise-Induced Alterations in Systemic Inflammation, Immune Dysfunction, and Plasma Antioxidant Capacity. Nutrients 2016, 8, 518. [Google Scholar] [CrossRef]
- Shosha, E.; Fouda, A.Y.; Lemtalsi, T.; Haigh, S.; Fulton, D.; Ibrahim, A.; Al-Shabrawey, M.; Caldwell, R.W.; Caldwell, R.B. Endothelial Arginase 2 Mediates Retinal Ischemia/Reperfusion Injury by Inducing Mitochondrial Dysfunction. Mol. Metab. 2021, 53, 101273. [Google Scholar] [CrossRef] [PubMed]
- Bergin, D.; Jing, Y.; Mockett, B.; Zhang, H.; Abraham, W.; Liu, P. Altered Plasma Arginine Metabolome Precedes Behavioural and Brain Arginine Metabolomic Profile Changes in the APPswe/PS1ΔE9 Mouse Model of Alzheimer’s Disease. Transl. Psychiatry 2018, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Kardos, G.R.; Wastyk, H.C.; Robertson, G.P. Disruption of Proline Synthesis in Melanoma Inhibits Protein Production Mediated by the GCN2 Pathway. Mol. Cancer Res. 2015, 13, 1408–1420. [Google Scholar] [CrossRef]
- Navratil, A.R.; Vozenilek, A.E.; Cardelli, J.A.; Green, J.M.; Thomas, M.J.; Sorci-Thomas, M.G.; Orr, A.W.; Woolard, M.D. Lipin-1 Contributes to Modified Low-Density Lipoprotein-Elicited Macrophage pro-Inflammatory Responses. Atherosclerosis 2015, 242, 424–432. [Google Scholar] [CrossRef][Green Version]
- Segarra-Mondejar, M.; Casellas-Díaz, S.; Ramiro-Pareta, M.; Müller-Sánchez, C.; Martorell-Riera, A.; Hermelo, I.; Reina, M.; Aragonés, J.; Martínez-Estrada, O.M.; Soriano, F.X. Synaptic Activity-induced Glycolysis Facilitates Membrane Lipid Provision and Neurite Outgrowth. EMBO J. 2018, 37, e97368. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, D.L.; Mortimer, S.I. Effect of Genotype, Gender and Age on Sheep Meat Quality and a Case Study Illustrating Integration of Knowledge. Meat Sci. 2014, 98, 544–555. [Google Scholar] [CrossRef]
- Palladini, G.; Di Pasqua, L.G.; Berardo, C.; Siciliano, V.; Richelmi, P.; Mannucci, B.; Croce, A.C.; Rizzo, V.; Perlini, S.; Vairetti, M.; et al. Fatty Acid Desaturase Involvement in Non-Alcoholic Fatty Liver Disease Rat Models: Oxidative Stress versus Metalloproteinases. Nutrients 2019, 11, 799. [Google Scholar] [CrossRef]
- Korbecki, J.; Kojder, K.; Jeżewski, D.; Simińska, D.; Tarnowski, M.; Kopytko, P.; Safranow, K.; Gutowska, I.; Goschorska, M.; Kolasa-Wołosiuk, A.; et al. Expression of SCD and FADS2 Is Lower in the Necrotic Core and Growing Tumor Area than in the Peritumoral Area of Glioblastoma Multiforme. Biomolecules 2020, 10, 727. [Google Scholar] [CrossRef]
- Roke, K.; Walton, K.; Klingel, S.; Harnett, A.; Subedi, S.; Haines, J.; Mutch, D. Evaluating Changes in Omega-3 Fatty Acid Intake after Receiving Personal FADS1 Genetic Information: A Randomized Nutrigenetic Intervention. Nutrients 2017, 9, 240. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; He, X.; Han, Y.; Chen, L.; Yu, X.; Li, J.; Yun, X.; Sha, R.; Borjigin, G. Age-Related Meat Flavor Precursors of Naturally Grazed Sunit Sheep: Metabolomics and Transcriptomics Approaches. Foods 2025, 14, 1616. https://doi.org/10.3390/foods14091616
Huang Y, He X, Han Y, Chen L, Yu X, Li J, Yun X, Sha R, Borjigin G. Age-Related Meat Flavor Precursors of Naturally Grazed Sunit Sheep: Metabolomics and Transcriptomics Approaches. Foods. 2025; 14(9):1616. https://doi.org/10.3390/foods14091616
Chicago/Turabian StyleHuang, Yajuan, Xige He, Yunfei Han, Lu Chen, Xueting Yu, Jin Li, Xueyan Yun, Rina Sha, and Gerelt Borjigin. 2025. "Age-Related Meat Flavor Precursors of Naturally Grazed Sunit Sheep: Metabolomics and Transcriptomics Approaches" Foods 14, no. 9: 1616. https://doi.org/10.3390/foods14091616
APA StyleHuang, Y., He, X., Han, Y., Chen, L., Yu, X., Li, J., Yun, X., Sha, R., & Borjigin, G. (2025). Age-Related Meat Flavor Precursors of Naturally Grazed Sunit Sheep: Metabolomics and Transcriptomics Approaches. Foods, 14(9), 1616. https://doi.org/10.3390/foods14091616




