Preparation of Epigallocatechin Gallate-Enriched Antioxidant Edible Films Based on Konjac Glucomannan and Sodium Alginate: Impact on Storage Stability of Mandarin Fish
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of SA/KGM Edible Films
2.3. Characterizations of Films
2.3.1. Rheological Test
2.3.2. Fourier Transformation Infrared Analysis (FT-IR)
2.3.3. XRD Experiment
2.3.4. Scanning Electron Microscope (SEM)
2.3.5. Thermal Weight Analysis (TGA)
2.3.6. Mechanical Properties
2.3.7. Color and Opacity
2.3.8. Oxygen Permeability (OP)
2.3.9. Moisture Content and Contact Angle
2.3.10. Antioxidant Ability
2.4. Application in Mandarin Fish Preservation
2.4.1. Treatment and Storage of Mandarin Fish Meat
2.4.2. pH Value
2.4.3. TBARS
2.4.4. TVB-N
2.5. Data Analysis
3. Results and Discussion
3.1. Characterizations of Films
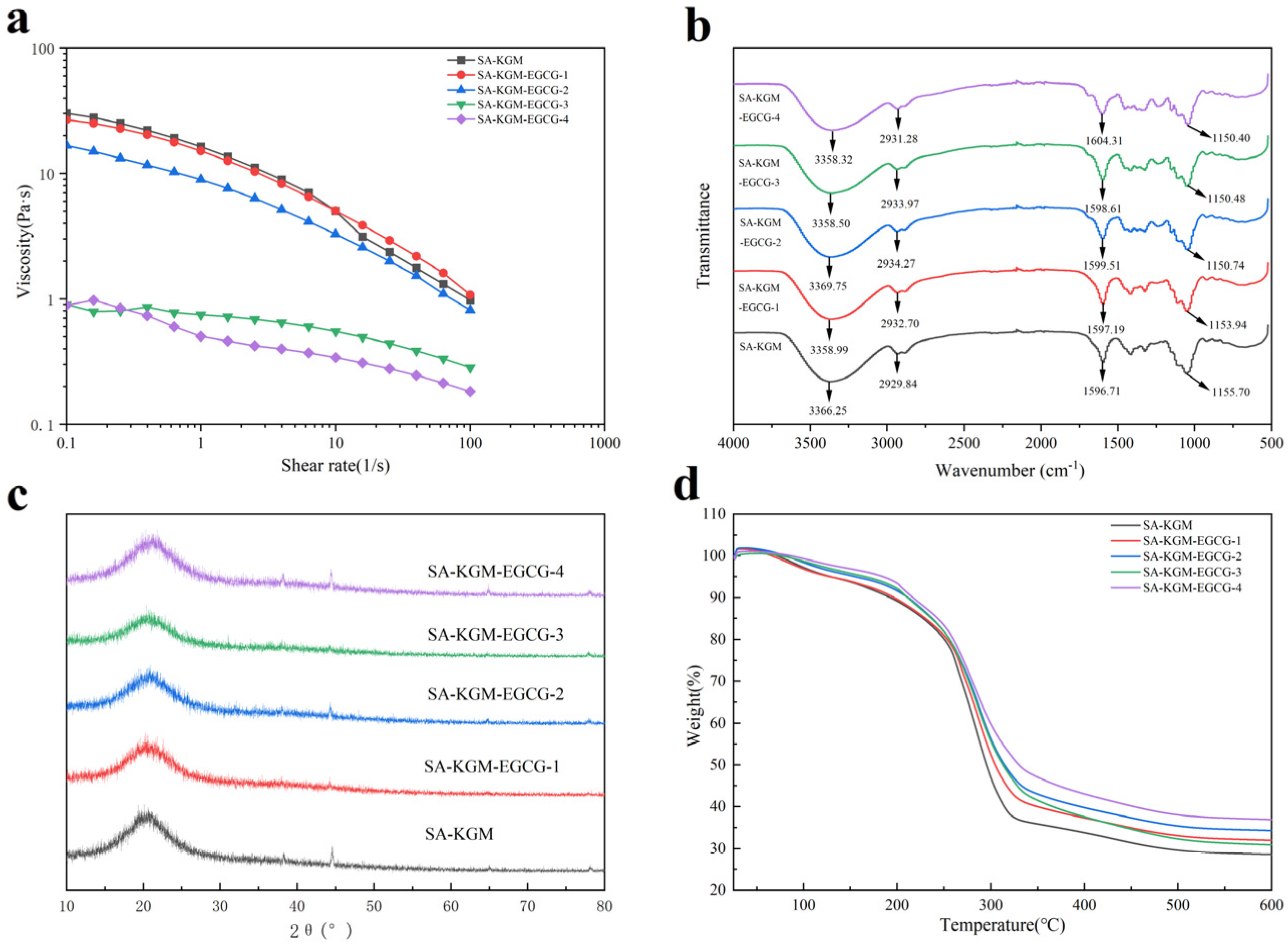
3.1.1. Rheological Properties
3.1.2. Fourier Transformation Infrared Analysis (FT-IR)
3.1.3. XRD Result
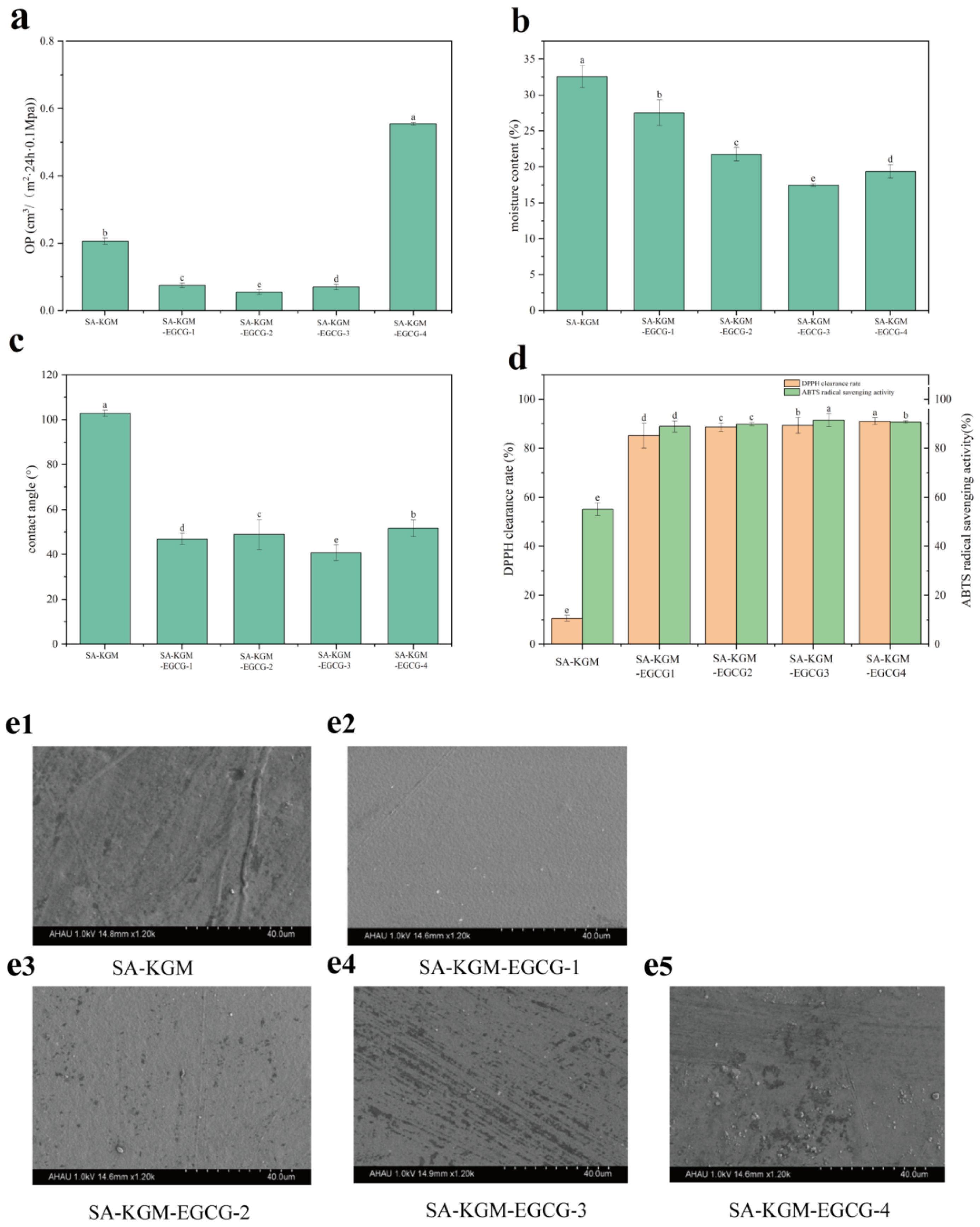
3.1.4. SEM
3.1.5. Thermal Weight Analysis (TGA)
3.1.6. Mechanical Properties
3.1.7. Color and Opacity
3.1.8. Oxygen Permeability (OP)
3.1.9. Moisture Content and Contact Angle
3.1.10. Antioxidant Properties
3.2. Application in Mandarin Fish Preservation
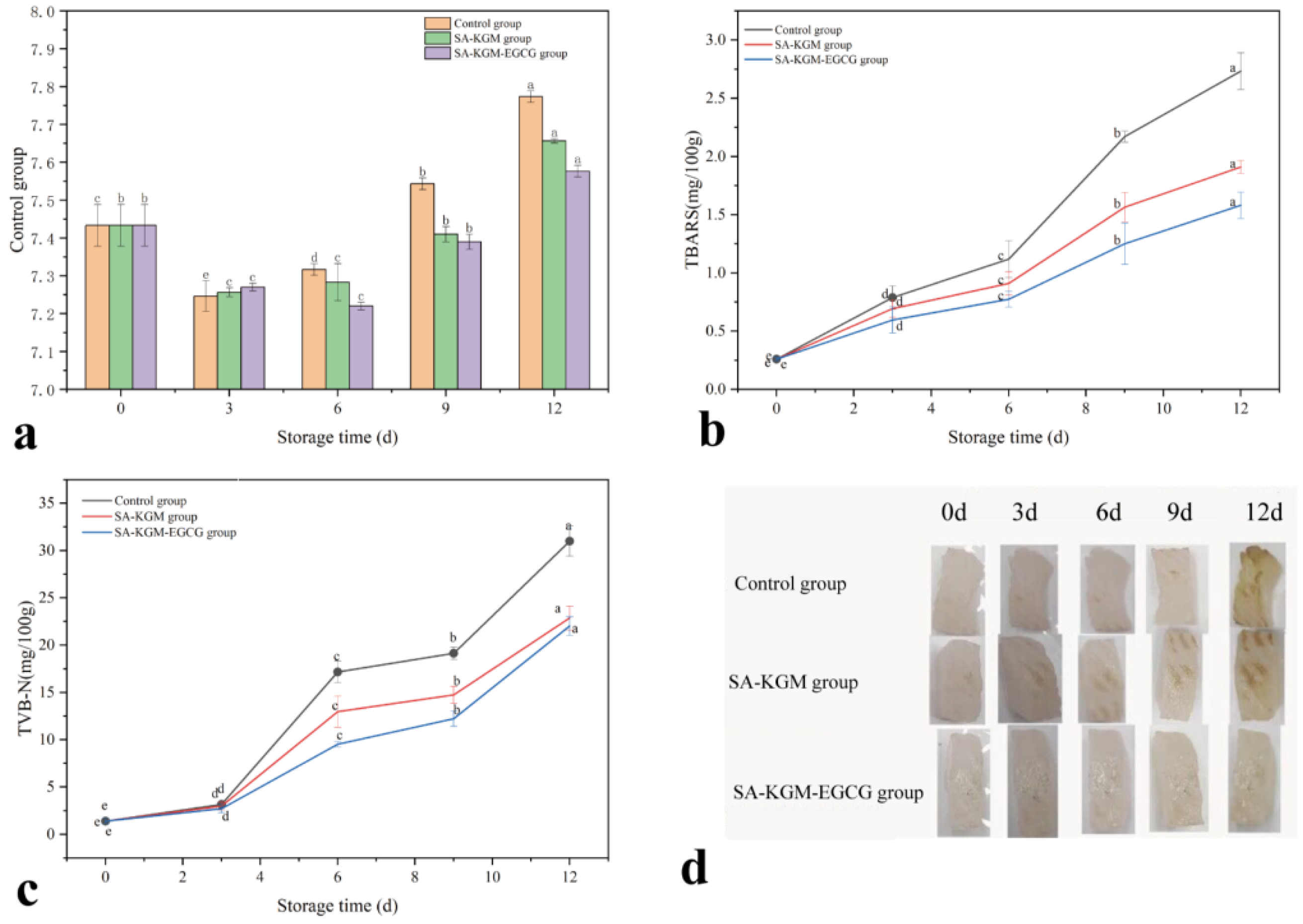
3.2.1. pH Value
3.2.2. TBARS
3.2.3. TVB-N
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EGCG | epigallocatechin gallate |
| KGM | konjac glucomannan |
| SA | sodium alginate |
| OP | oxygen permeability |
References
- Chuesiang, P.; Sanguandeekul, R.; Siripatrawan, U. Phase inversion temperature-fabricated cinnamon oil nanoemulsion as a natural preservative for prolonging shelf-life of chilled Asian seabass (Lates calcarifer) fillets. LWT 2020, 125, 109122. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Use of tea extracts for inhibition of polyphenoloxidase and retardation of quality loss of Pacific white shrimp during iced storage. LWT—Food Sci. Technol. 2011, 44, 924–932. [Google Scholar] [CrossRef]
- Zawani, C.J.; Nor-Khaizura, M.A.; Mahyudin, N.A.; Ismail-Fitry, M.R.; Nirmal, N.P. Microbiological and Sensorial Quality of Beef Meat (Longissimus dorsi) Marinated with Cinnamon Extract and Stored at Various Temperatures. Foods 2022, 11, 3971. [Google Scholar] [CrossRef] [PubMed]
- Koirala, P.; Nirmal, N.P.; Woraprayote, W.; Visessanguan, W.; Bhandari, Y.; Karim, N.U.; Nor-Khaizura, M.A.R.; Saricaoğlu, F.T. Nano-engineered edible films and coatings for seafood products. Food Packag. Shelf Life 2023, 38, 101135. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef]
- Luo, X.; He, P.; Lin, X. The mechanism of sodium hydroxide solution promoting the gelation of Konjac glucomannan (KGM). Food Hydrocoll. 2013, 30, 92–99. [Google Scholar] [CrossRef]
- Wu, H.; Wu, H.; Qing, Y.; Wu, C.; Pang, J. KGM/chitosan bio-nanocomposite films reinforced with ZNPs: Colloidal, physical, mechanical and structural attributes. Food Packag. Shelf Life 2022, 33, 100870. [Google Scholar] [CrossRef]
- Ranjbar, M.; Azizi Tabrizzad, M.H.; Asadi, G.; Ahari, H. Investigating the microbial properties of sodium alginate/chitosan edible film containing red beetroot anthocyanin extract for smart packaging in chicken fillet as a pH indicator. Heliyon 2023, 9, e18879. [Google Scholar] [CrossRef]
- Li, K.; Zhu, J.; Guan, G.; Wu, H. Preparation of chitosan-sodium alginate films through layer-by-layer assembly and ferulic acid crosslinking: Film properties, characterization, and formation mechanism. Int. J. Biol. Macromol. 2019, 122, 485–492. [Google Scholar] [CrossRef]
- Yang, X.; Gong, T.; Lu, Y.-h.; Li, A.; Sun, L.; Guo, Y. Compatibility of sodium alginate and konjac glucomannan and their applications in fabricating low-fat mayonnaise-like emulsion gels. Carbohydr. Polym. 2020, 229, 115468. [Google Scholar] [CrossRef]
- Mao, S.; Ren, Y.; Wei, C.; Chen, S.; Ye, X.; Jinhu, T. Development of novel EGCG/Fe loaded sodium alginate-based packaging films with antibacterial and slow-release properties. Food Hydrocoll. 2023, 145, 109032. [Google Scholar] [CrossRef]
- Ruan, C.; Zhang, Y.; Wang, J.; Sun, Y.; Gao, X.; Xiong, G.; Liang, J. Preparation and antioxidant activity of sodium alginate and carboxymethyl cellulose edible films with epigallocatechin gallate. Int. J. Biol. Macromol. 2019, 134, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Lin, S.; Wu, Y.; Shen, J.; Wu, J.; Zhu, W.; Yu, S.; Li, J.; Wang, S. Emulsifier free fish gelatin based films with excellent antioxidative and antibacterial activity: Preparation, characterization and application in coating preservation of fish fillets. J. Food Eng. 2023, 343, 111362. [Google Scholar] [CrossRef]
- Zeng, J.; Ren, X.; Zhu, S.; Gao, Y. Fabrication and characterization of an economical active packaging film based on chitosan incorporated with pomegranate peel. Int. J. Biol. Macromol. 2021, 192, 1160–1168. [Google Scholar] [CrossRef]
- de Menezes, B.B.; Frescura, L.M.; Duarte, R.; Villetti, M.A.; da Rosa, M.B. A critical examination of the DPPH method: Mistakes and inconsistencies in stoichiometry and IC50 determination by UV–Vis spectroscopy. Anal. Chim. Acta 2021, 1157, 338398. [Google Scholar] [CrossRef]
- Subbuvel, M.; Kavan, P. Preparation and characterization of polylactic acid/fenugreek essential oil/curcumin composite films for food packaging applications. Int. J. Biol. Macromol. 2022, 194, 470–483. [Google Scholar] [CrossRef]
- Hao, Y.; Chu, Y.; Zhang, M.; Shi, W.; Chen, Y.; Li, D.; Li, L. Preparation of functional degradable antibacterial film and application in fresh-keeping of grass carp. J. Agric. Food Res. 2022, 9, 100341. [Google Scholar] [CrossRef]
- Song, S.; Ji, R.; Xu, J.; Yang, X.; An, Q.; Zhang, X.; Zhang, W. Preparation and characterization of highly stable pH-sensitive multifunctional films based on co-pigment-anthocyanin conjugate system for pork monitoring and preservation. Food Hydrocoll. 2025, 164, 115–128. [Google Scholar] [CrossRef]
- Fathi, M.; Mohebbi, M.; Koocheki, A. Introducing Prunus cerasus gum exudates: Chemical structure, molecular weight, and rheological properties. Food Hydrocoll. 2016, 61, 946–955. [Google Scholar] [CrossRef]
- Alghooneh, A.; Razavi, S.M.A.; Behrouzian, F. Rheological characterization of hydrocolloids interaction: A case study on sage seed gum-xanthan blends. Food Hydrocoll. 2017, 66, 206–215. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T. Properties and antioxidative activity of fish gelatin-based film incorporated with epigallocatechin gallate. Food Hydrocoll. 2018, 80, 212–221. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.-C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bai, H.; Wang, S.; Wang, R.; Wang, Z. Casein phosphopeptide-calcium chelate: Preparation, calcium holding capacity and simulated digestion in vitro. Food Chem. 2023, 401, 134218. [Google Scholar] [CrossRef]
- Ning, H.; Lu, L.; Xu, J.; Lu, L.; Pan, L.; Lin, Z. Development of sodium alginate-based antioxidant and antibacterial bioactive films added with IRMOF-3/Carvacrol. Carbohydr. Polym. 2022, 292, 119682. [Google Scholar] [CrossRef]
- Gabriel, E.F.M.; do Lago, C.L.; Gobbi, A.L.; Carrilho, E.; Coltro, W.K.T. Characterization of microchip electrophoresis devices fabricated by direct-printing process with colored toner. Electrophoresis 2013, 34, 2169–2176. [Google Scholar] [CrossRef]
- Karimi Khorrami, N.; Radi, M.; Amiri, S.; McClements, D.J. Fabrication and characterization of alginate-based films functionalized with nanostructured lipid carriers. Int. J. Biol. Macromol. 2021, 182, 373–384. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, H.; Jiao, C.; Jiang, Y.; Liu, R.; Xiao, D.; Lu, J.; Zhang, Z.; Shen, G.; Li, S. Investigation of the structural and physical properties, antioxidant and antimicrobial activity of pectin-konjac glucomannan composite edible films incorporated with tea polyphenol. Food Hydrocoll. 2019, 94, 128–135. [Google Scholar] [CrossRef]
- Tanwar, R.; Gupta, V.; Kumar, P.; Kumar, A.; Singh, S.; Gaikwad, K.K. Development and characterization of PVA-starch incorporated with coconut shell extract and sepiolite clay as an antioxidant film for active food packaging applications. Int. J. Biol. Macromol. 2021, 185, 451–461. [Google Scholar] [CrossRef]
- Moghadaskhou, F.; Tadjarodi, A.; Mollahosseini, A.; Maleki, A. Synthesis of UiO-66-Sal-Cu(OH)2 by a Simple and Novel Method: MOF-Based Metal Thin Film as a Heterogeneous Catalyst for Olefin Oxidation. ACS Appl. Mater. Interfaces 2023, 15, 4021–4032. [Google Scholar] [CrossRef]
- Zhao, P.; Yan, X.; Cheng, M.; Wang, Y.; Wang, Y.; Wang, K.; Wang, X.; Wang, J. Effect of Pickering emulsion on the physical properties, microstructure and bioactivity of corn starch/cassia gum composite films. Food Hydrocoll. 2023, 141, 108713. [Google Scholar] [CrossRef]
- Luo, X.; Li, J.; Lin, X. Effect of gelatinization and additives on morphology and thermal behavior of corn starch/PVA blend films. Carbohydr. Polym. 2012, 90, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Al-Harrasi, A.; Shah, Y.; Jawad, M.; Anwer, M.K.; Aldawsari, M.; Koca, E.; Aydemir, L.; Ullah, S. The Effect of Sage (Salvia sclarea) Essential Oil on the Physiochemical and Antioxidant Properties of Sodium Alginate and Casein-Based Composite Edible Films. Gels 2023, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Abutalib, M.M.; Rajeh, A. Influence of ZnO/Ag nanoparticles doping on the structural, thermal, optical and electrical properties of PAM/PEO composite. Phys. B Condens. Matter 2020, 578, 411796. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.; Li, M.; Lu, Y.; Du, Y.; Tong, C.; Pang, J.; Wu, C. Preparation and characterization of multifunctional konjac glucomannan/carboxymethyl chitosan biocomposite films incorporated with epigallocatechin gallate. Food Hydrocoll. 2020, 105, 105756. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Karbowiak, T.; Brachais, C.-H.; Debeaufort, F. Coupling tyrosol, quercetin or ferulic acid and electron beam irradiation to cross-link chitosan–gelatin films: A structure–function approach. Eur. Polym. J. 2015, 67, 113–127. [Google Scholar] [CrossRef]
- Tang, C.; Zhao, B.; Zhu, J.; Lu, X.; Jiang, G. Preparation and characterization of chitosan/sodium cellulose sulfate/silver nanoparticles composite films for wound dressing. Mater. Today Commun. 2022, 33, 104192. [Google Scholar] [CrossRef]
- Biao, Y.; Yuxuan, C.; Qi, T.; Ziqi, Y.; Yourong, Z.; McClements, D.J.; Chongjiang, C. Enhanced performance and functionality of active edible films by incorporating tea polyphenols into thin calcium alginate hydrogels. Food Hydrocoll. 2019, 97, 105197. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Tang, J.; Zhang, H.; Dai, J.; Li, S.; Yan, J.; Qin, W.; Liu, Y. Preparation of sodium alginate/konjac glucomannan active films containing lycopene microcapsules and the effects of these films on sweet cherry preservation. Int. J. Biol. Macromol. 2022, 215, 67–78. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Vitchayakitti, W. Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
- Hiremani, V.D.; Khanapure, S.; Gasti, T.; Goudar, N.; Vootla, S.K.; Masti, S.P.; Malabadi, R.B.; Mudigoudra, B.S.; Chougale, R.B. Preparation and physicochemical assessment of bioactive films based on chitosan and starchy powder of white turmeric rhizomes (Curcuma Zedoaria) for green packaging applications. Int. J. Biol. Macromol. 2021, 193, 2192–2201. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, W.; Ma, C.; Chen, S.; Liu, X.; Liu, F. Enzymatic synthesis of sodium caseinate-EGCG-carboxymethyl chitosan ternary film: Structure, physical properties, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2022, 222, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, Y.; Bai, R.; Zhang, X.; Yuan, L.; Liu, J. Preparation of pH-sensitive and antioxidant packaging films based on κ-carrageenan and mulberry polyphenolic extract. Int. J. Biol. Macromol. 2019, 134, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Kiselevsky, D.B.; Samuilova, O.V.; Samuilov, V.D. Epigallocatechin Gallate: pH-Dependent Redox Properties and Effect on Respiration, Photosynthesis, and Cell Death in Pea Plants. Biochemistry 2023, 88, 211–220. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Luo, H.; Wang, Z.; Chen, D.; Feng, Q.; Cao, X. Injectable and tissue adhesive EGCG-laden hyaluronic acid hydrogel depot for treating oxidative stress and inflammation. Carbohydr. Polym. 2023, 299, 120180. [Google Scholar] [CrossRef]
- Zeng, Z.; Yang, Y.-J.; Tu, Q.; Jian, Y.-Y.; Xie, D.-M.; Bai, T.; Li, S.-S.; Liu, Y.-T.; Li, C.; Wang, C.-X.; et al. Preparation and characterization of carboxymethyl chitosan/pullulan composite film incorporated with eugenol and its application in the preservation of chilled meat. Meat Sci. 2023, 198, 109085. [Google Scholar] [CrossRef]
- Muniandy, P.; Shori, A.B.; Baba, A.S. Influence of green, white and black tea addition on the antioxidant activity of probiotic yogurt during refrigerated storage. Food Packag. Shelf Life 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Vatsa, S.; Srivastav, P.P.; Pathak, S.S. A comprehensive review on freshness of fish and assessment: Analytical methods and recent innovations. Food Res. Int. 2020, 133, 109157. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
| Film Samples | Thickness (mm) | TS (MPa) | EAB (%) |
|---|---|---|---|
| SA-KGM | 0.16 ± 0.04 b | 6.94 ± 1.33 b | 47.12 ± 3.07 a |
| SA-KGM-EGCG1 | 0.15 ± 0.01 c | 9.44 ± 2.06 b | 39.21 ± 2.61 a |
| SA-KGM-EGCG2 | 0.18 ± 0.01 a | 12.34 ± 1.44 ab | 38.46 ± 0.08 a |
| SA-KGM-EGCG3 | 0.18 ± 0.01 a | 12.68 ± 5.12 ab | 28.08 ± 5.46 b |
| SA-KGM-EGCG4 | 0.15 ± 0.01 c | 17.68 ± 5.62 a | 26.25 ± 10.45 b |
| Film Samples | L* | a* | b* | Opacity |
|---|---|---|---|---|
| SA-KGM | 72.67 ± 1.21 a | 0.68 ± 0.01 e | −1.96 ± 0.09 e | 1.12 ± 0.13 d |
| SA-KGM-EGCG1 | 63.57 ± 1.57 c | 2.20 ± 0.01 a | 14.88 ± 0.36 a | 2.60 ± 0.45 a |
| SA-KGM-EGCG2 | 64.18 ± 1.19 bc | 2.17 ± 0.19 b | 11.16 ± 0.73 b | 1.96 ± 0.15 b |
| SA-KGM-EGCG3 | 65.59 ± 1.92 b | 1.71 ± 0.12 c | 7.32 ± 1.00 c | 1.68 ± 0.13 c |
| SA-KGM-EGCG4 | 64.69 ± 1.83 b | 1.59 ± 0.28 d | 6.55 ± 0.81 d | 1.09 ± 0.10 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Wang, Y.; Zhang, X.; Gao, Y.; Wu, X.; Li, X.; Liu, Z.; Sun, Y.; Liang, J. Preparation of Epigallocatechin Gallate-Enriched Antioxidant Edible Films Based on Konjac Glucomannan and Sodium Alginate: Impact on Storage Stability of Mandarin Fish. Foods 2025, 14, 1570. https://doi.org/10.3390/foods14091570
Wang R, Wang Y, Zhang X, Gao Y, Wu X, Li X, Liu Z, Sun Y, Liang J. Preparation of Epigallocatechin Gallate-Enriched Antioxidant Edible Films Based on Konjac Glucomannan and Sodium Alginate: Impact on Storage Stability of Mandarin Fish. Foods. 2025; 14(9):1570. https://doi.org/10.3390/foods14091570
Chicago/Turabian StyleWang, Ran, Yuqi Wang, Xinzhen Zhang, Yang Gao, Xian Wu, Xueling Li, Zhengquan Liu, Yue Sun, and Jin Liang. 2025. "Preparation of Epigallocatechin Gallate-Enriched Antioxidant Edible Films Based on Konjac Glucomannan and Sodium Alginate: Impact on Storage Stability of Mandarin Fish" Foods 14, no. 9: 1570. https://doi.org/10.3390/foods14091570
APA StyleWang, R., Wang, Y., Zhang, X., Gao, Y., Wu, X., Li, X., Liu, Z., Sun, Y., & Liang, J. (2025). Preparation of Epigallocatechin Gallate-Enriched Antioxidant Edible Films Based on Konjac Glucomannan and Sodium Alginate: Impact on Storage Stability of Mandarin Fish. Foods, 14(9), 1570. https://doi.org/10.3390/foods14091570








