Structural Elucidation of Heteropolysaccharides from the Peach-Shaped Dictyophora indusiata and Its Anti-Inflammatory Activity
Abstract
1. Introduction
2. Materials and Methods
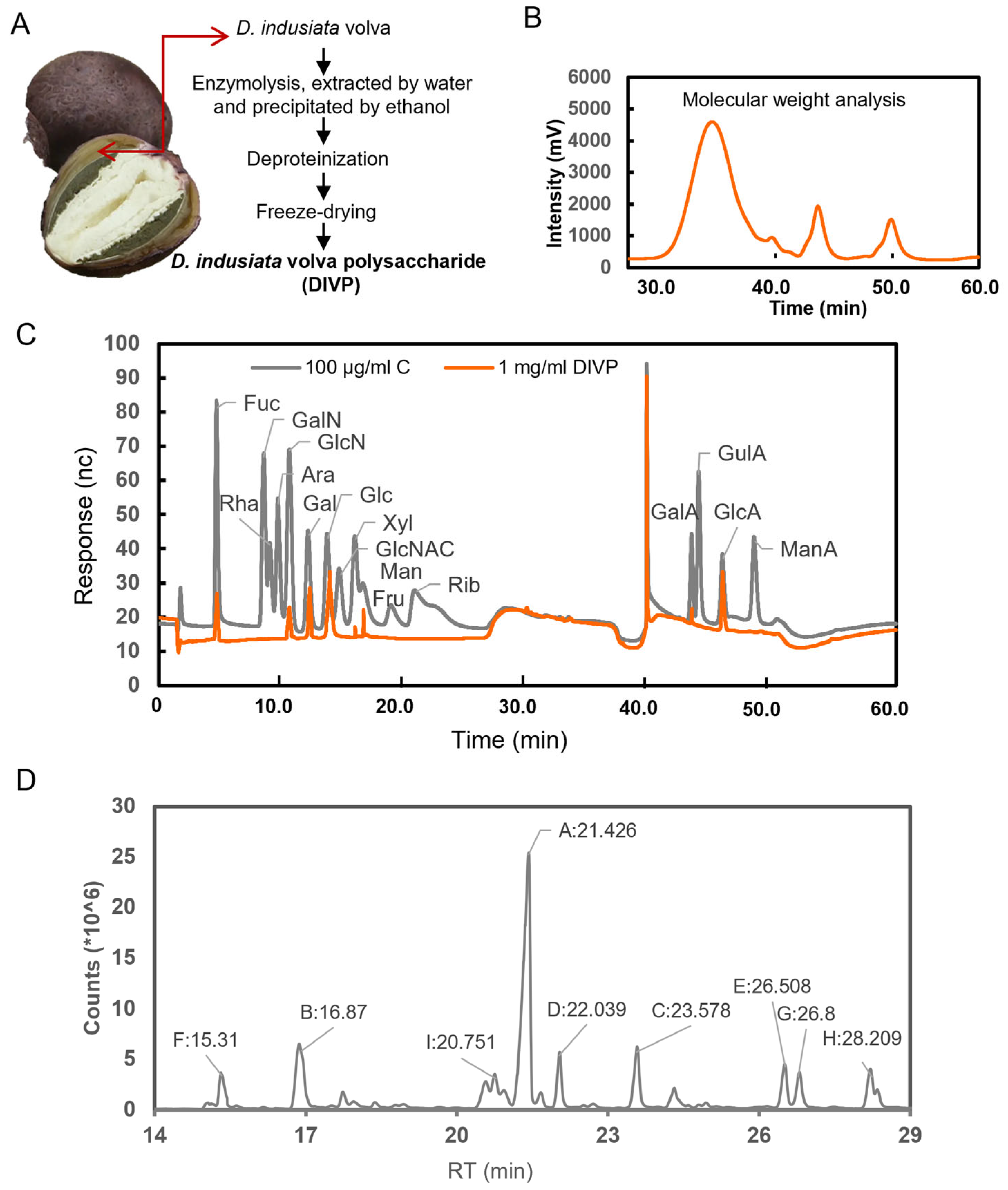
2.1. Preparation of DIVP
2.2. Characteristics of DIVP
2.2.1. Molecular Weight (Mw) and Monosaccharide Composition Analysis
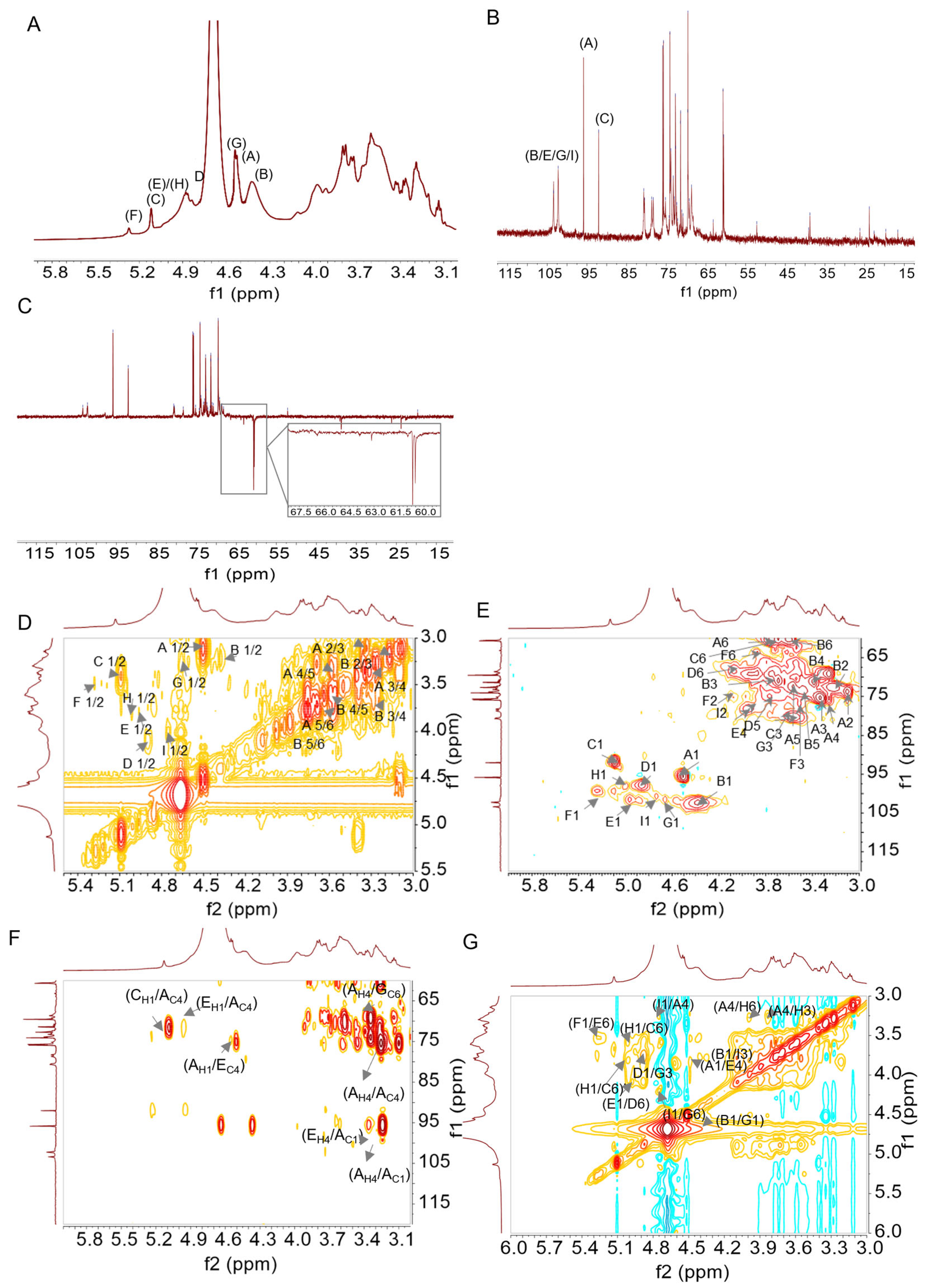
2.2.2. Methylation Analysis
2.2.3. Nuclear Magnetic Resonance Spectroscopy (NMR) Analysis
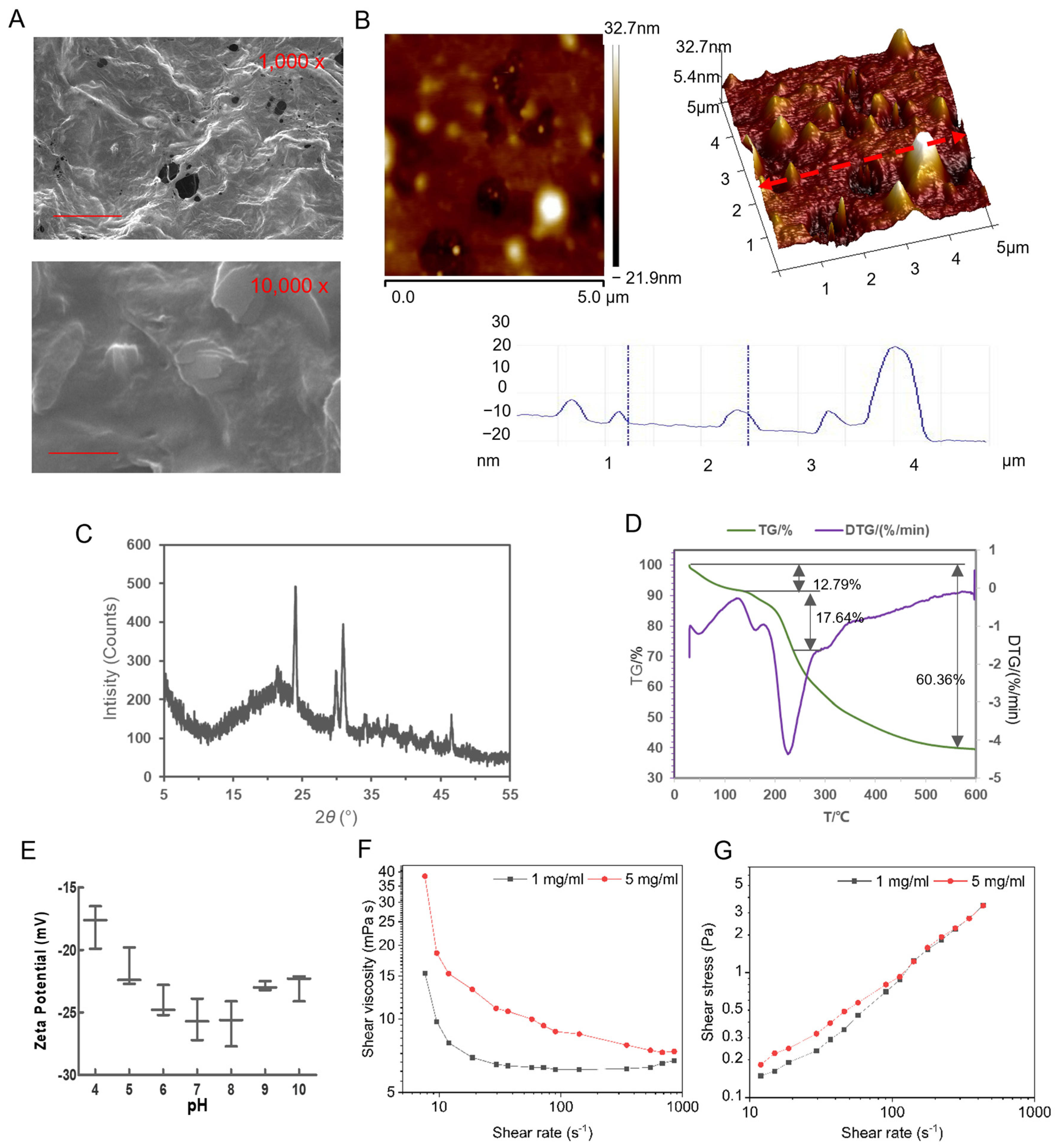
2.2.4. Observation of Morphology and Microstructure
2.2.5. X-Ray Diffraction (XRD) Analysis
2.2.6. Thermal Analysis
2.2.7. Zeta Potential Analysis
2.2.8. Viscosity Characterization Analysis
2.3. Anti-Inflammatory Activity of DIVP in Human U937 Cells
2.3.1. Cell Culture and Differentiation Induction
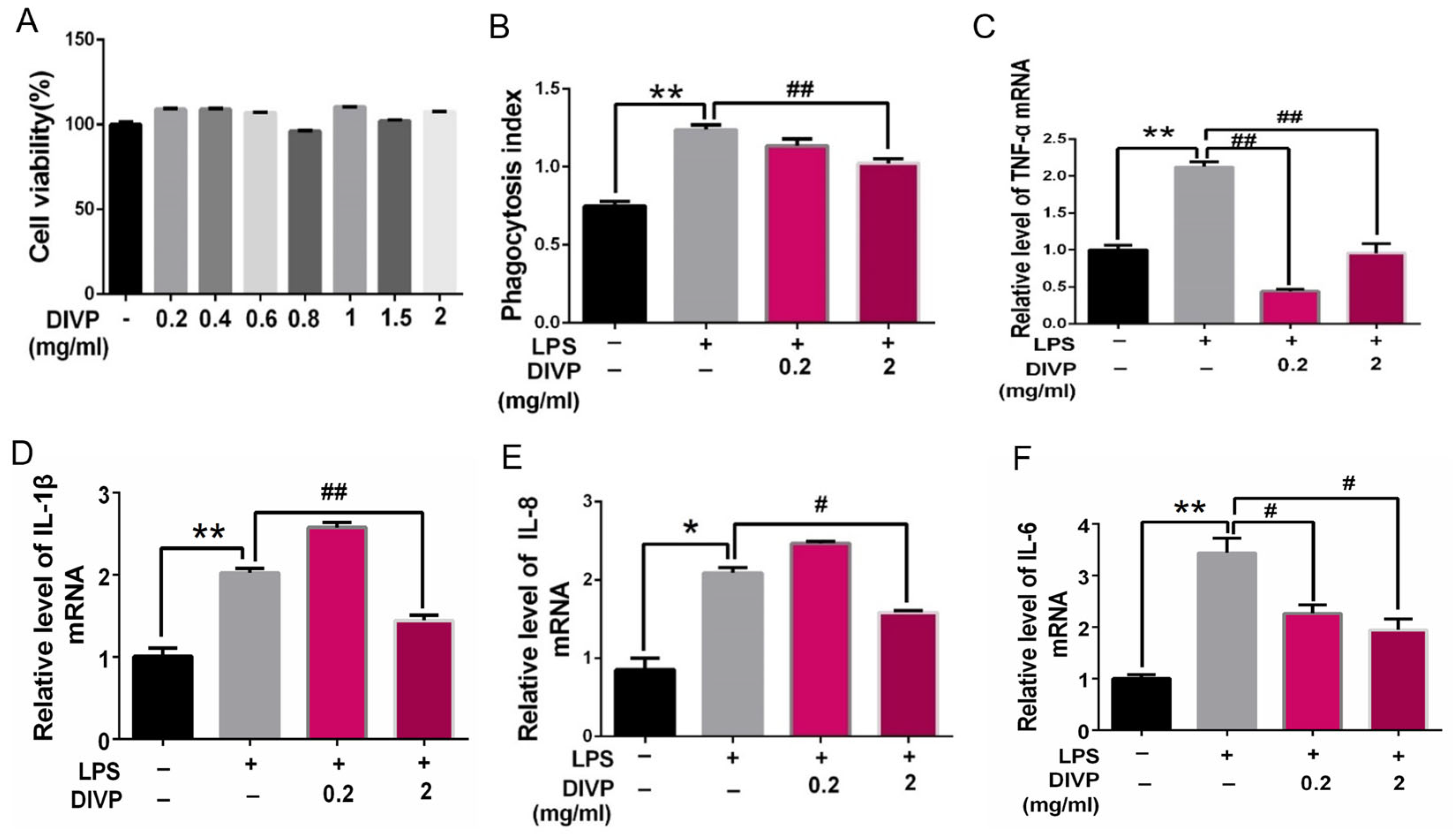
2.3.2. Cell Viability Analysis
2.3.3. Assay of Phagocytic Activity
2.3.4. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.3.5. Dot Blot Array for the Detection of Inflammatory Cytokines
2.3.6. Phosphokinase Antibody Array
2.4. Statistical Analysis
3. Results and Discussion
3.1. Structural Characteristics of DIVP
3.2. Physiochemical Characteristics of DIVP
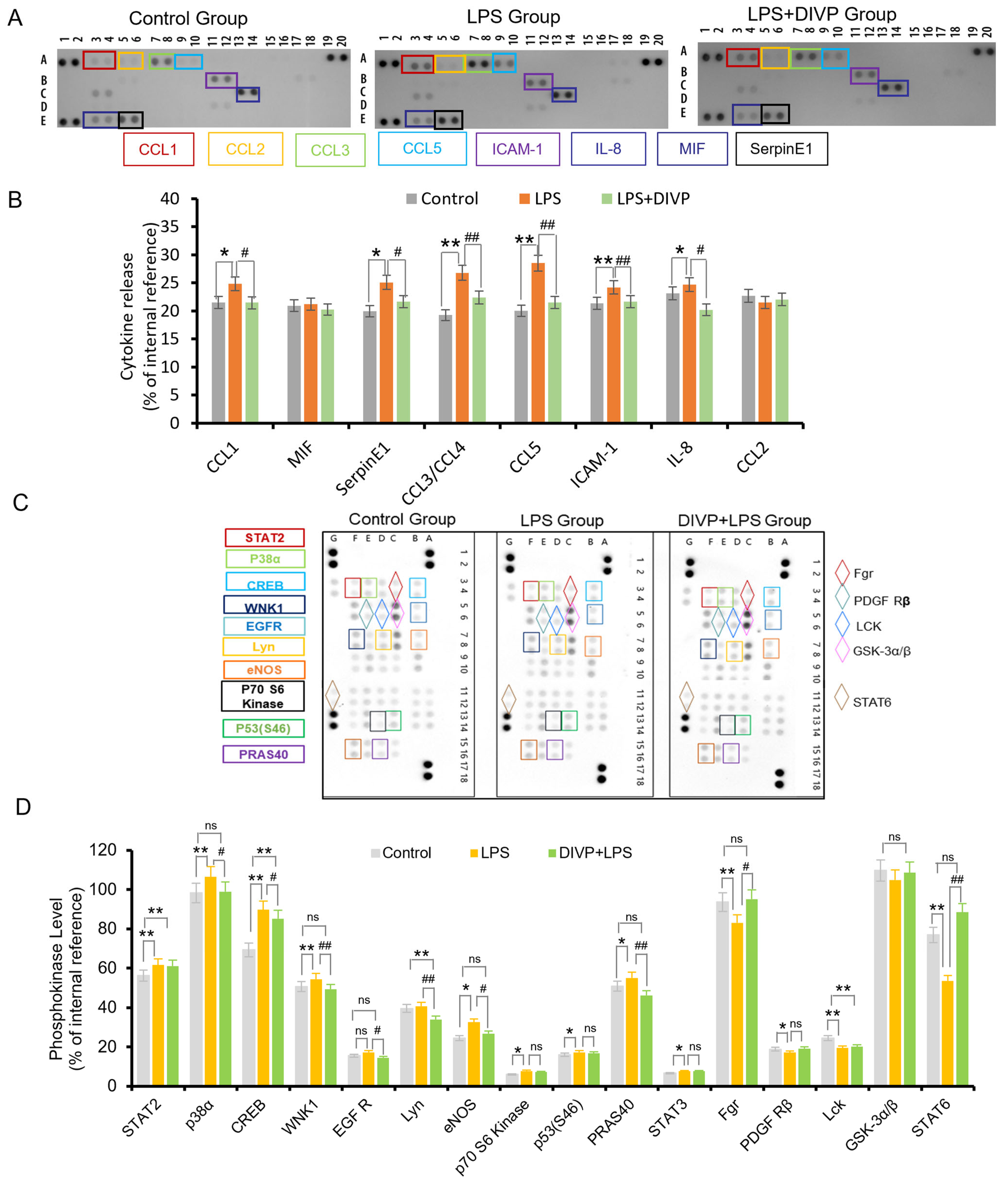
3.3. Inhibitory Activity on the Inflammatory Response in Human U937 Macrophages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Wang, P. Regulatory Effects Mediated by Enteromorpha prolifera Polysaccharide and Its Zn(II) Complex on Hypoglycemic Activity in High-Sugar High-Fat Diet-Fed Mice. Foods 2023, 12, 2854. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Fan, S.; Zeng, R.; Liu, D.; Wang, X.; Wang, J.; Geng, F. Effects of Refrigerated Storage on Restarted Morphological Development of Dictyophora indusiata Fruiting Bodies. Agronomy 2024, 14, 1539. [Google Scholar] [CrossRef]
- Habtemariam, S. The Chemistry, Pharmacology and Therapeutic Potential of the Edible Mushroom Dictyophora indusiata (Vent ex. Pers.) Fischer (Synn. Phallus indusiatus). Biomedicines 2019, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wen, X.; Yang, B.; Liu, D.; Li, X.; Geng, F. De novo transcriptome and proteome analysis of Dictyophora indusiata fruiting bodies provides insights into the changes during morphological development. Int. J. Biol. Macromol. 2020, 146, 875–886. [Google Scholar] [CrossRef]
- Sun, L.; Bao, C.; Chang, W.; Zhuang, Y. Preparation, characterisation, antioxidant and antiglycation activities of the novel polysaccharides from the pileus of Dictyophora rubrovolvata. Int. J. Food Sci. Technol. 2016, 52, 161–170. [Google Scholar] [CrossRef]
- Fu, H.; Deng, C.; Yang, C.; Teng, L.; Yu, L.; Su, T.; Xu, X.; Chen, J. Immunomodulatory Activities on RAW 264.7 Macrophages of a Polysaccharide from Veiled Lady Mushroom, Dictyophora indusiata (Higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 151–160. [Google Scholar] [CrossRef]
- Yuan, Q.; Liu, W.; Hao, W.; Wang, S. Glycosidic linkages of fungus polysaccharides influence the anti-inflammatory activity in mice. J. Adv. Res. 2024, 67, 161–172. [Google Scholar] [CrossRef]
- Liao, W.; Luo, Z.; Liu, D.; Ning, Z.; Yang, J.; Ren, J. Structure Characterization of a Novel Polysaccharide from Dictyophora indusiata and Its Macrophage Immunomodulatory Activities. J. Agric. Food Chem. 2015, 63, 535–544. [Google Scholar] [CrossRef]
- Duan, M.; Long, S.; Wu, X.; Feng, B.; Qin, S.; Li, Y.; Li, X.; Li, C.; Zhao, C.; Wang, L.; et al. Genome, transcriptome, and metabolome analyses provide new insights into the resource development in an edible fungus Dictyophora indusiata. Front. Microbiol. 2023, 14, 1137159. [Google Scholar] [CrossRef]
- Wang, J.; Wen, X.; Zhang, Y.; Zou, P.; Cheng, L.; Gan, R.; Li, X.; Liu, D.; Geng, F. Quantitative proteomic and metabolomic analysis of Dictyophora indusiata fruiting bodies during post-harvest morphological development. Food Chem. 2021, 339, 127884. [Google Scholar] [CrossRef] [PubMed]
- Nazir, Y.; Linsaenkart, P.; Khantham, C.; Chaitep, T.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; et al. High Efficiency In Vitro Wound Healing of Dictyophora indusiata Extracts via Anti-Inflammatory and Collagen Stimulating (MMP-2 Inhibition) Mechanisms. J. Fungi 2021, 7, 1100. [Google Scholar] [CrossRef]
- Srisuk, N.; Jirasatid, S. Characteristics Co-Encapsulation of Lactobacillus Acidophilus with Dictyophora indusiata. Curr. Res. Nutr. Food Sci. J. 2020, 8, 1013–1024. [Google Scholar] [CrossRef]
- Elkhateeb, W.A. Trametes Versicolor and Dictyophora indusiata Champions of Medicinal Mushrooms. Open Access J. Pharm. Res. 2020, 4, 000192. [Google Scholar] [CrossRef]
- He, Y.; Gao, W.; Zhang, Y.; Sun, M.; Kuang, H.; Sun, Y. Progress in the preparation, structure and bio-functionality of Dictyophora indusiata polysaccharides: A review. Int. J. Biol. Macromol. 2024, 283, 137519. [Google Scholar] [CrossRef]
- Wu, D.-T.; Zhao, Y.-X.; Yuan, Q.; Wang, S.; Gan, R.-Y.; Hu, Y.-C.; Zou, L. Influence of ultrasound assisted metal-free Fenton reaction on the structural characteristic and immunostimulatory activity of a β-D-glucan isolated from Dictyophora indusiata. Int. J. Biol. Macromol. 2022, 220, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.R.; Fernandes, N.A.R.; Maldonado, L.A.G.; Junior, C.R. Comparison of monocytic cell lines U937 and THP-1 as macrophage models for in vitro studies. Biochem. Biophys. Rep. 2022, 32, 101383. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, Z.; Chen, W.; Zhao, S.; Pi, Y.; Yue, X. Structural characterization, α-glucosidase inhibitory activity and antioxidant activity of neutral polysaccharide from apricot (Armeniaca sibirica L. Lam) kernels. Int. J. Biol. Macromol. 2023, 238, 124109. [Google Scholar] [CrossRef]
- Zhu, Z.-Y.; Liu, X.-C.; Fang, X.-N.; Sun, H.-Q.; Yang, X.-Y.; Zhang, Y.-M. Structural characterization and anti-tumor activity of polysaccharide produced by Hirsutella sinensis. Int. J. Biol. Macromol. 2016, 82, 959–966. [Google Scholar] [CrossRef]
- Yang, C.; Hu, C.; Zhang, H.; Chen, W.; Deng, Q.; Tang, H.; Huang, F. Optimation for preparation of oligosaccharides from flaxseed gum and evaluation of antioxidant and antitumor activities in vitro. Int. J. Biol. Macromol. 2020, 153, 1107–1116. [Google Scholar] [CrossRef]
- Wu, Q.; Luo, M.; Yao, X.; Yu, L. Purification, structural characterization, and antioxidant activity of the COP-W1 polysaccharide from Codonopsis tangshen Oliv. Carbohydr. Polym. 2020, 236, 116020. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.; Berezhnoy, G.; Flores, A.; Cannet, C.; Schäfer, H.; Dahlke, M.H.; Michl, P.; Löffler, M.W.; Königsrainer, A.; Trautwein, C. Quantitative Metabolomics and Lipoprotein Analysis of PDAC Patients Suggests Serum Marker Categories for Pancreatic Function, Pancreatectomy, Cancer Metabolism, and Systemic Disturbances. J. Proteome Res. 2024, 23, 1249–1262. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Zhang, T.; Wang, J.; Huang, X.; Hu, J.; Yin, J.; Nie, S. Fractionation, physicochemical and structural characterization of polysaccharides from barley water-soluble fiber. Food Hydrocoll. 2021, 113, 106539. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Lin, L.; Li, E.; Cao, Q.; Wei, C. Relationships between X-ray Diffraction Peaks, Molecular Components, and Heat Properties of C-Type Starches from Different Sweet Potato Varieties. Molecules 2022, 27, 3385. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, C.; Roggenbuck, J.; Hanss, J.; Tiemann, M. Tiemann. Synthesis of Mesoporous Metal Oxides by Structure Replication: Thermal Analysis of Metal Nitrates in Porous Carbon Matrices. Nanomaterials 2015, 5, 1431–1441. [Google Scholar] [CrossRef]
- Shen, H.-H.; Peng, J.-F.; Wang, R.-R.; Wang, P.-Y.; Zhang, J.-X.; Sun, H.-F.; Liang, Y.; Li, Y.-M.; Xue, J.-N.; Li, Y.-J.; et al. IL-12-Overexpressed Nanoparticles Suppress the Proliferation of Melanoma Through Inducing ICD and Activating DC, CD8+ T, and CD4+ T Cells. Int. J. Nanomed. 2024, 19, 2755–2772. [Google Scholar] [CrossRef]
- Ji, Y.-H.; Liao, A.-M.; Huang, J.-H.; Thakur, K.; Li, X.-L.; Hu, F.; Wei, Z.-J. The rheological properties and emulsifying behavior of polysaccharides sequentially extracted from Amana edulis. Int. J. Biol. Macromol. 2019, 137, 160–168. [Google Scholar] [CrossRef]
- Córdova-Dávalos, L.E.; Cervantes-García, D.; Ballona-Alba, M.F.; Santos-López, A.; Esquivel-Basaldúa, A.S.; Gallegos-Alcalá, P.; Jiménez, M.; Salinas, E. Protective Effect of Glycomacropeptide on the Inflammatory Response of U937 Macrophages. Foods 2023, 12, 1528. [Google Scholar] [CrossRef]
- Tafreshi, M.G.K.; Mazaheri, Z.; Heidari, M.; Babaei, N.; Doosti, A. Induction of Apoptosis in the U937 Cell Line Co-cultured with Adipose-derived Stem Cells Secreting Bone Morphogenetic Protein. Int. J. Mol. Cell. Med. 2021, 10, 265–276. [Google Scholar] [CrossRef]
- Alkorashy, A.I.; Doghish, A.S.; Abulsoud, A.I.; Ewees, M.G.; Abdelghany, T.M.; Elshafey, M.M.; Elkhatib, W.F. Effect of scopoletin on phagocytic activity of U937-derived human macrophages: Insights from transcriptomic analysis. Genomics 2020, 112, 3518–3524. [Google Scholar] [CrossRef]
- Kokoulin, M.S.; Kuzmich, A.S.; Kalinovsky, A.I.; Tomshich, S.V.; Romanenko, L.A.; Mikhailov, V.V.; Komandrova, N.A. Structure and anticancer activity of sulfated O-polysaccharide from marine bacterium Cobetia litoralis KMM 3880 T. Carbohydr. Polym. 2016, 154, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Chen, W.; He, P.; Zhan, Q.; Wang, Q.; Wu, H.; Zhang, M. Structural characterization of polysaccharides with potential antioxidant and immunomodulatory activities from Chinese water chestnut peels. Carbohydr. Polym. 2020, 246, 116551. [Google Scholar] [CrossRef]
- Ren, Y.; Li, L.; Mu, B.; Li, C.; Huang, R. Electrocatalytic properties of three new POMs-based inorganic–organic frameworks with flexible zwitterionic dicarboxylate ligands. J. Solid State Chem. 2017, 249, 1–8. [Google Scholar] [CrossRef]
- Colodel, C.; Vriesmann, L.C.; de Oliveira Petkowicz, C.L. Cell wall polysaccharides from Ponkan mandarin (Citrus reticulata Blanco cv. Ponkan) peel. Carbohydr. Polym. 2018, 195, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wen, L.; Zhao, Y.; Jiang, Y. Structure identification of an arabinogalacturonan in Citrus reticulata Blanco ‘Chachiensis’ peel. Food Hydrocoll. 2018, 84, 481–488. [Google Scholar] [CrossRef]
- Zaghloul, E.H.; Ibrahim, M.I.A. Production and Characterization of Exopolysaccharide From Newly Isolated Marine Probiotic Lactiplantibacillus plantarum EI6 with in vitro Wound Healing Activity. Front. Microbiol. 2022, 13, 903363. [Google Scholar] [CrossRef]
- Li, R.; Zhou, J.; He, C.; Chen, H. Isolation, structural characterization and cholesterol-lowering effects of a novel polysaccharide from mulberry (Morus alba L.) leaf. Ind. Crops Prod. 2023, 202, 117010. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Tao, P.; Wang, Y. Attenuated Structural Transformation of Aconitine during Sand Frying Process and Antiarrhythmic Effect of Its Converted Products. Evid.-Based Complement. Altern. Med. 2021, 2021, 7243052. [Google Scholar] [CrossRef]
- Zhou, R.; Qian, Y.; Lei, Z.; Tang, Y.; Li, Y. Production and characterization of exopolysaccharides from salinity-induced auxenochlorella protothecoides and the analysis of anti-inflammatory activity. Int. J. Biol. Macromol. 2023, 240, 124217. [Google Scholar] [CrossRef]
- Huang, X.; Yang, Q.; Chang, S.; Liu, Y.; Wang, X.; Liu, Z.; Ren, J. Potential osteoporosis-blocker Sparassis crispa polysaccharide: Isolation, purification and structure elucidation. Int. J. Biol. Macromol. 2024, 261, 129879. [Google Scholar] [CrossRef]
- Li, W.; Wu, D.; Min, W. Structure-activity relationship of walnut peptide in gastrointestinal digestion, absorption and antioxidant activity. LWT 2023, 189, 115521. [Google Scholar] [CrossRef]
- Fu, C.; Ren, L.; Liu, W.; Sui, Y.; Nong, Q. Structural characteristics of a hypoglycemic polysaccharide from Fructus Corni. Carbohydr. Res. 2021, 506, 108358. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Yang, X.; Lin, P. Purification, structural characterization, and PCSK9 secretion inhibitory effect of the novel alkali-extracted polysaccharide from Cordyceps militaris. Int. J. Biol. Macromol. 2021, 179, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. In vivo immunological activity of chitosan-derived nanoparticles. Int. J. Biol. Macromol. 2024, 262, 130105. [Google Scholar] [CrossRef] [PubMed]
- Casillo, A.; Fabozzi, A.; Krauss, I.R.; Parrilli, E.; Biggs, C.I.; Gibson, M.I.; Corsaro, M.M. Physicochemical Approach to Understanding the Structure, Conformation, and Activity of Mannan Polysaccharides. Biomacromolecules 2021, 22, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Qi, J.; Ho, C.; Li, B.; Wu, Q. Purification, Physicochemical Properties, and Antioxidant Activities of Two Low-Molecular-Weight Polysaccharides from Ganoderma leucocontextum Fruiting Bodies. Antioxidants 2021, 10, 1145. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, C.; Shi, H.; Li, X. Structural studies of a mannoglucan from Cremastra appendiculata (Orchidaceae) by chemical and enzymatic methods. Carbohydr. Polym. 2021, 272, 118524. [Google Scholar] [CrossRef]
- Wang, J.; Pan, J.; Zou, J.; Shi, Y.; Guo, D.; Zhai, B.; Zhao, C.; Luan, F.; Zhang, X.; Sun, J. Isolation, structures, bioactivities, and utilizations of polysaccharides from Dictyophora species: A review. Int. J. Biol. Macromol. 2024, 278, 134566. [Google Scholar] [CrossRef]
- Song, J.; Geng, X.; Su, Y.; Zhang, X.; Tu, L.; Zheng, Y.; Wang, M. Structure feature and antidepressant-like activity of a novel exopolysaccharide isolated from Marasmius androsaceus fermentation broth. Int. J. Biol. Macromol. 2020, 165, 1646–1655. [Google Scholar] [CrossRef]
- Yang, B.; Luo, Y.; Sang, Y.; Kan, J. Isolation, purification, structural characterization, and hypoglycemic activity assessment of polysaccharides from Hovenia dulcis (Guai Zao). Int. J. Biol. Macromol. 2022, 208, 1106–1115. [Google Scholar] [CrossRef]
- Abbout, S.; Hsissou, R.; Chebabe, D.; Erramli, H.; Hajjaji, N. Investigation of the anti-corrosion properties of Galactomannan as additive in epoxy coatings for carbon steel: Rheological and electrochemical study. Inorg. Chem. Commun. 2021, 134, 108971. [Google Scholar] [CrossRef]
- Alpizar-Reyes, E.; Carrillo-Navas, H.; Gallardo-Rivera, R.; Varela-Guerrero, V.; Alvarez-Ramirez, J.; Pérez-Alonso, C. Functional properties and physicochemical characteristics of tamarind (Tamarindus indica L.) seed mucilage powder as a novel hydrocolloid. J. Food Eng. 2017, 209, 68–75. [Google Scholar] [CrossRef]
- Sadiq, B.N.; Mousa, E.F. Production of Nano Beta-Glucans From Baking Yeast Saccharomyces cerevisiae and Its Comparison with Extracted Beta-Glucans. Third Virtual Int. Conf. Environ. Sci. 2024, 1325, 012049. [Google Scholar] [CrossRef]
- Zou, D.; Li, X.; Wu, M.; Yang, J.; Qin, W.; Zhou, Z.; Yang, J. Schiff base synergized with protonation of PEI to achieve smart antibacteria of nanocellulose packaging films. Carbohydr. Polym. 2023, 318, 121136. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Ikeda, A.; Kawasaki, T.; Miyazaki, K. Universal mechanism of shear thinning in supercooled liquids. Commun. Phys. 2024, 7, 199. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, J.; Zhang, X.; Liu, S.; Zhao, C. Antitumor effect of soluble β-glucan as an immune stimulant. Int. J. Biol. Macromol. 2021, 179, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Liu, Y.; Yuan, K.; Lin, Y.; Qian, X.; Pei, H.; Weng, L.; Fan, K.; Hu, Y.; et al. A low-molecular-weight α-glucan from edible fungus Agaricus blazei Murrill activates macrophage TFEB-mediated antibacterial defense to combat implant-associated infection. Carbohydr. Polym. 2024, 346, 122659. [Google Scholar] [CrossRef]
- Mei, X.; Tang, Q.; Huang, G.; Long, R.; Huang, H. Preparation, structural analysis and antioxidant activities of phosphorylated (1 → 3)-β-d-glucan. Food Chem. 2020, 309, 125791. [Google Scholar] [CrossRef]
- No, H.; Kim, J.; Seo, C.-R.; Lee, D.E.; Kim, J.H.; Kuge, T.; Mori, T.; Kimoto, H.; Kim, J.-K. Anti-inflammatory effects of β-1,3-1,6-glucan derived from black yeast Aureobasidium pullulans in RAW264.7 cells. Int. J. Biol. Macromol. 2021, 193, 592–600. [Google Scholar] [CrossRef]
- Fang, J.; Wang, Y.; Lv, X.; Shen, X.; Ni, X.; Ding, K. Structure of a β-glucan from Grifola frondosa and its antitumor effect by activating Dectin-1/Syk/NF-κB signaling. Glycoconj. J. 2012, 29, 365–377. [Google Scholar] [CrossRef]
- Chae, J.S.; Shin, H.; Song, Y.; Kang, H.; Yeom, C.-H.; Lee, S.; Choi, Y.S. Yeast (1 → 3)-(1 → 6)-β-d-glucan alleviates immunosuppression in gemcitabine-treated mice. Int. J. Biol. Macromol. 2019, 136, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Yin, L.; Shen, X.; Dai, Y.; Wang, J.; Yin, D.; Zhang, D.; Pan, X. β-Glucans from Trametes versicolor (L.) Lloyd Is Effective for Prevention of Influenza Virus Infection. Viruses 2022, 14, 237. [Google Scholar] [CrossRef] [PubMed]

| Target | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| β-actin (human) | ATGTGCAAAAAGCTGGCTTTG | AGATAGCAAATCGGCTGACG |
| TNF-α (human) | ACGGCATGGATCTCAAAGAC | CGTCAGCCGATTTGCTATCT |
| IL-1β (human) | ATGGCAACTGTTCCTGAACTCAACT | CAGGACAGGTATAGATTCTTTCCTT |
| IL-6 (human) | TTCCTCTCTGCAAGAGACT | TGTATCTCTCTGAAGGACT |
| IL-8 (human) | CTCCAAACCTTTCCACCCC | TCCACAACCCTCTGCACCC |
| Molar Ratio | RT | Methylated Sugar | Mass Fragments (m/z) | Type of Linkage | |
|---|---|---|---|---|---|
| A | 0.456 | 21.426 | 2,4,6-Me3-Glcp | 45,87, 99, 101, 117, 129, 161, 173, 233 | →3)-Glcp-(1→ |
| B | 0.112 | 16.87 | 2,3,4,6-Me4-Glcp | 45, 71, 87, 101, 117, 129, 145, 161, 205 | Glcp-(1→ |
| C | 0.070 | 23.578 | 2,3,4-Me3-Galp | 45, 87, 99, 101, 117, 129, 161, 189, 233 | →6)-Galp-(1→ |
| D | 0.056 | 22.039 | 2,3,6-Me3-Glcp | 45, 87, 99, 101, 113, 117, 129, 131, 161, 173, 233 | →4)-Glcp-(1→ |
| E | 0.054 | 26.508 | 2,3-Me2-Glcp | 45, 71, 85, 87, 99, 101, 117, 127, 159, 161, 201, 261 | →4,6)-Glcp-(1→ |
| F | 0.053 | 15.311 | 2,3,4,6-Me4-Manp | 45, 75, 87, 101, 117, 129, 145, 161, 205 | Manp-(1→ |
| G | 0.051 | 26.8 | 2,4-Me2-Glcp | 45, 87, 117, 129, 159, 189, 233 | →3,6)-Glcp-(1→ |
| H | 0.051 | 28.209 | 2,4-Me2-Galp | 45, 87, 117, 129, 159, 189, 233 | →3,6)-Galp-(1→ |
| I | 0.041 | 20.751 | 3,4,6-Me3-Glcp | 45, 71, 87, 99, 101, 129, 145, 161, 189 | →2)-Glcp-(1→ |
| Chemical Shifts (ppm) | |||||||
|---|---|---|---|---|---|---|---|
| Glycosyl Residues | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | |
| A | →3)-β-D-Glcp-(1→ | 4.51/95.84 | 3.12/73.77 | 3.32/75.23 | 3.26/77.34 | 3.56/72.50 | 3.75/60.44 |
| B | β-D-Glcp-(1→ | 4.39/102.24 | 3.22/72.61 | 3.69/70.87 | 3.39/71.16 | 3.48/73.52 | 3.60/60.51 |
| C | →6)-α-D-Galp-(1→ | 5.09/91.76 | 3.42/72.98 | 3.56/80.13 | 4.07/68.43 | 3.26/75.70 | 3.69/60.44 |
| D | →4)-β-D-Glcp-(1→ | 4.86/97.87 | 3.54/70.43 | 3.70/73.52 | 3.31/71.16 | 3.77/74.79 | 4.09/68.43 |
| E | →4,6)-β-D-Glcp-(1→ | 4.97/101.58 | 3.41/72.56 | 3.64/74.57 | 3.75/78.06 | 3.76/73.66 | 3.64/69.34 |
| F | α-D-Manp(1→ | 5.24/99.32 | 4.10/74.61 | 3.52/76.24 | 3.49/68.73 | 3.74/76.35 | 3.86/63.89 |
| G | →3,6)-β-D-Glcp-(1→ | 4.66/101.58 | 3.34/77.88 | 3.76/77.52 | 3.29/70.38 | 3.41/77.23 | 4.12/70.61 |
| H | →3,6)-α-D-Galp-(1→ | 4.99/98.05 | 3.79/72.79 | 3.53/79.35 | 4.01/68.55 | 3.79/74.81 | 3.98/71.06 |
| I | →2)-β-D-Glcp-(1→ | 4.73/100.67 | 3.96/75.16 | 3.76/79.70 | 3.43/70.54 | 3.52/77.14 | 3.79/62.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Yang, H.; Liu, Y.; Sun, Y.; Feng, Z.; Zheng, X.; Wang, F.; Ma, L.; Zhang, J.; Xu, D.; et al. Structural Elucidation of Heteropolysaccharides from the Peach-Shaped Dictyophora indusiata and Its Anti-Inflammatory Activity. Foods 2025, 14, 1536. https://doi.org/10.3390/foods14091536
He Y, Yang H, Liu Y, Sun Y, Feng Z, Zheng X, Wang F, Ma L, Zhang J, Xu D, et al. Structural Elucidation of Heteropolysaccharides from the Peach-Shaped Dictyophora indusiata and Its Anti-Inflammatory Activity. Foods. 2025; 14(9):1536. https://doi.org/10.3390/foods14091536
Chicago/Turabian StyleHe, Ying, Hao Yang, Yaxin Liu, Yanting Sun, Zeguo Feng, Xueying Zheng, Fei Wang, Lei Ma, Jianbao Zhang, Dan Xu, and et al. 2025. "Structural Elucidation of Heteropolysaccharides from the Peach-Shaped Dictyophora indusiata and Its Anti-Inflammatory Activity" Foods 14, no. 9: 1536. https://doi.org/10.3390/foods14091536
APA StyleHe, Y., Yang, H., Liu, Y., Sun, Y., Feng, Z., Zheng, X., Wang, F., Ma, L., Zhang, J., Xu, D., Guo, H., Qin, L., & Zhang, Y. (2025). Structural Elucidation of Heteropolysaccharides from the Peach-Shaped Dictyophora indusiata and Its Anti-Inflammatory Activity. Foods, 14(9), 1536. https://doi.org/10.3390/foods14091536








