In Vitro, Ex Vivo, and In Vivo Evidence of Nitrate-Reducing Activity in Levilactobacillus brevis CD2: A Potential Tool for Oral and Systemic Health Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bacterial Lysate
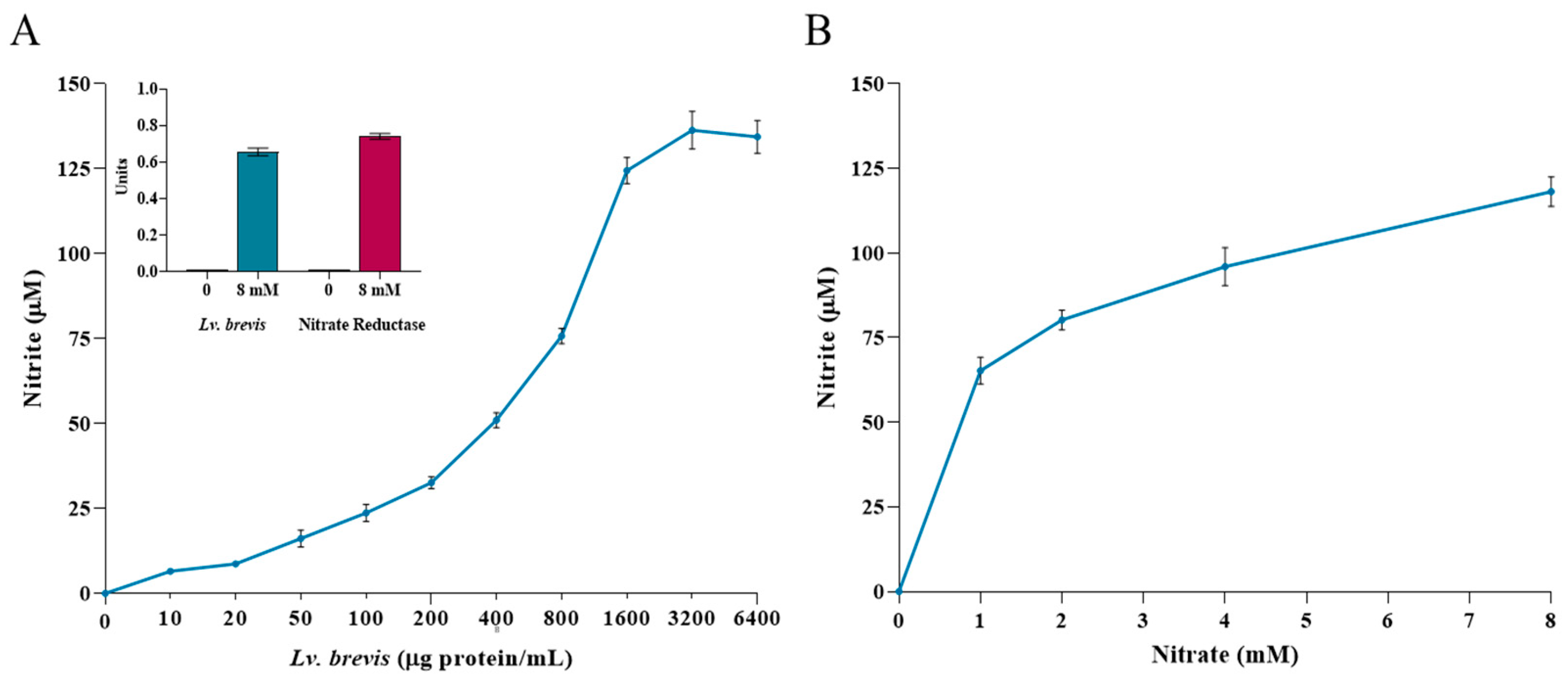
2.2. Nitrate-Reducing Activity in Lv. brevis CD2 Lysate
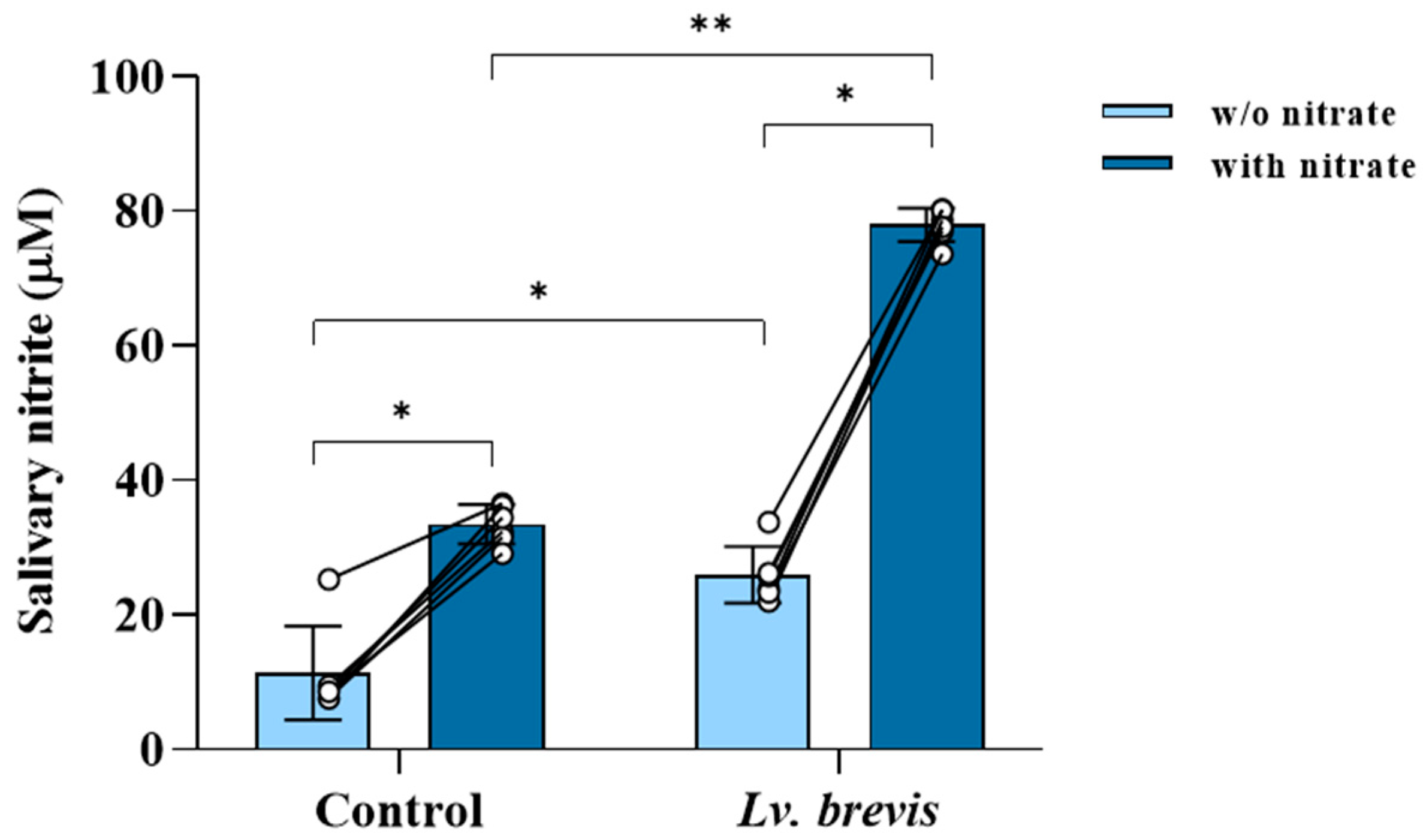
2.3. Nitrate-Reducing Activity in Human Saliva Samples Ex Vivo
2.4. Salivary Nitrate Reduction Test
2.5. Nitrite Level Assay
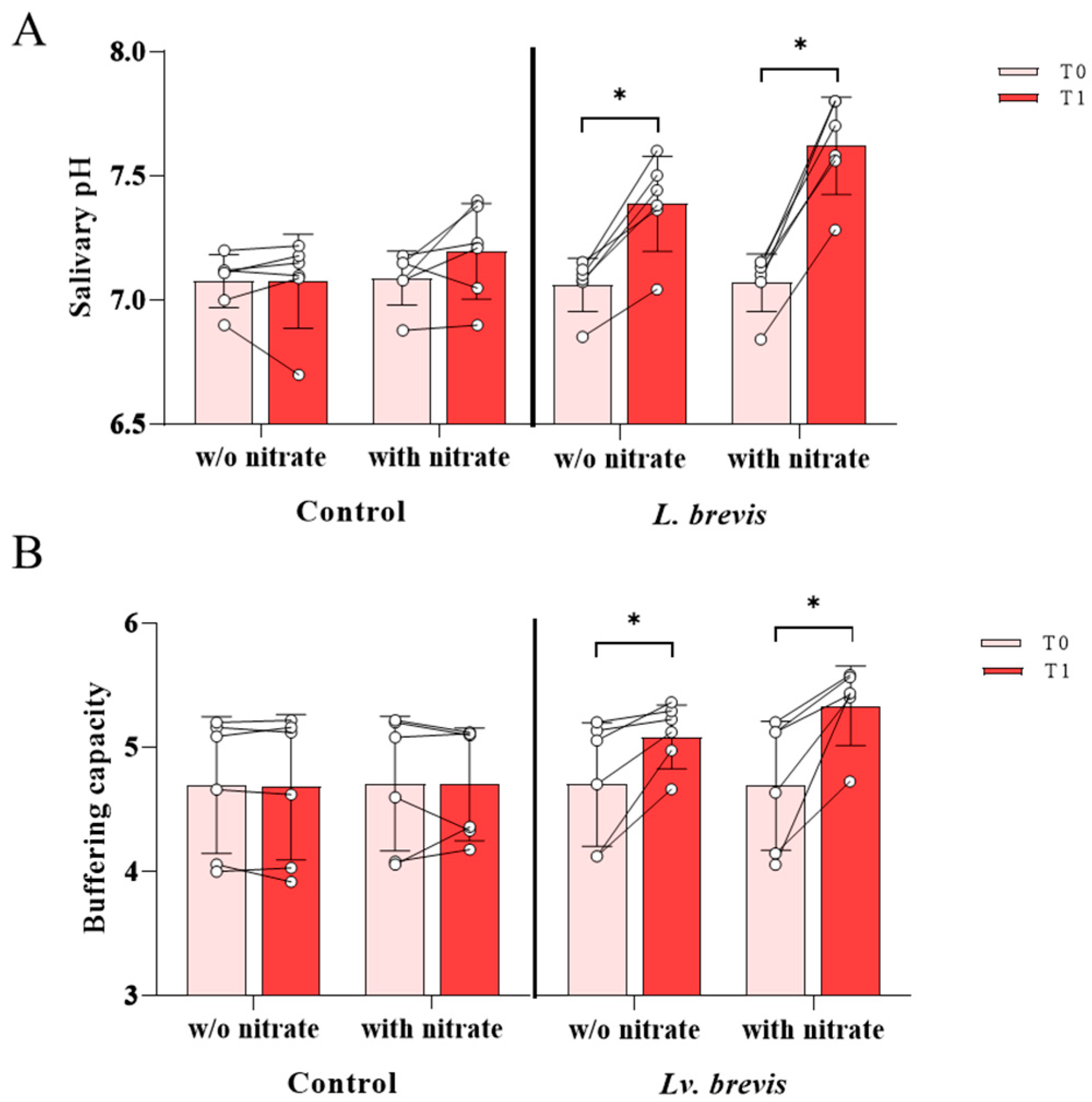
2.6. pH Measurements and Buffering Capacity of Saliva Samples
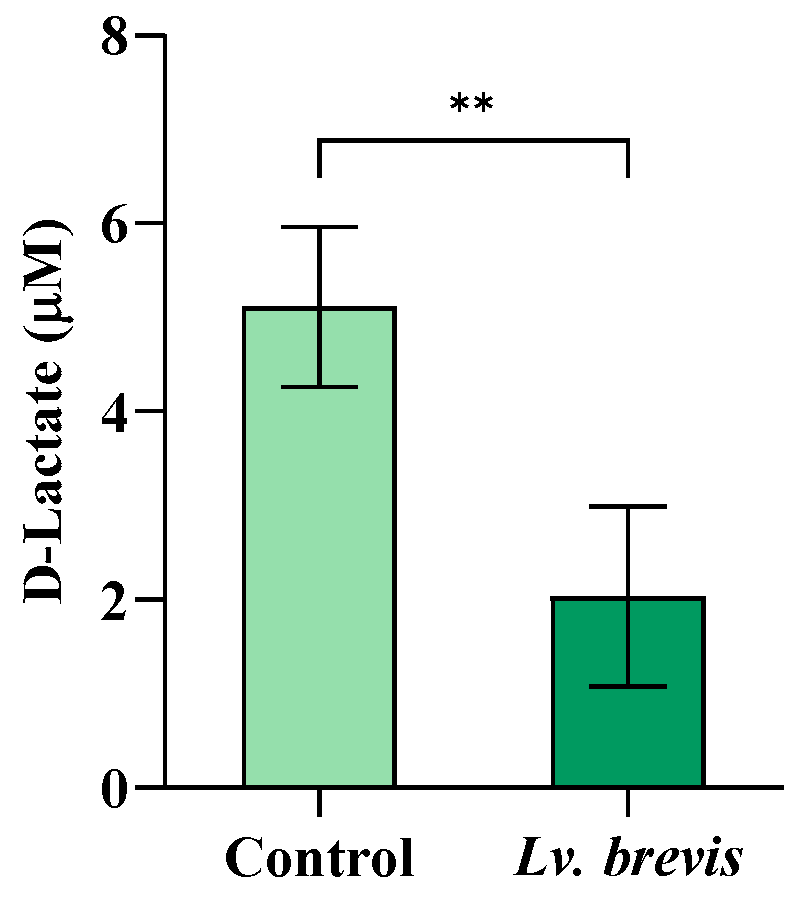
2.7. Salivary D-Lactate Levels
2.8. In Vivo Study Design
2.9. Whole-Mouth Nitrate Reduction
2.10. Statistical Analysis
3. Results
3.1. Nitrate-Reducing Activity in Lv. brevis CD2 Lysate
3.2. Nitrate-Reducing Activity of Lv. brevis CD2 in Human Salivary Samples
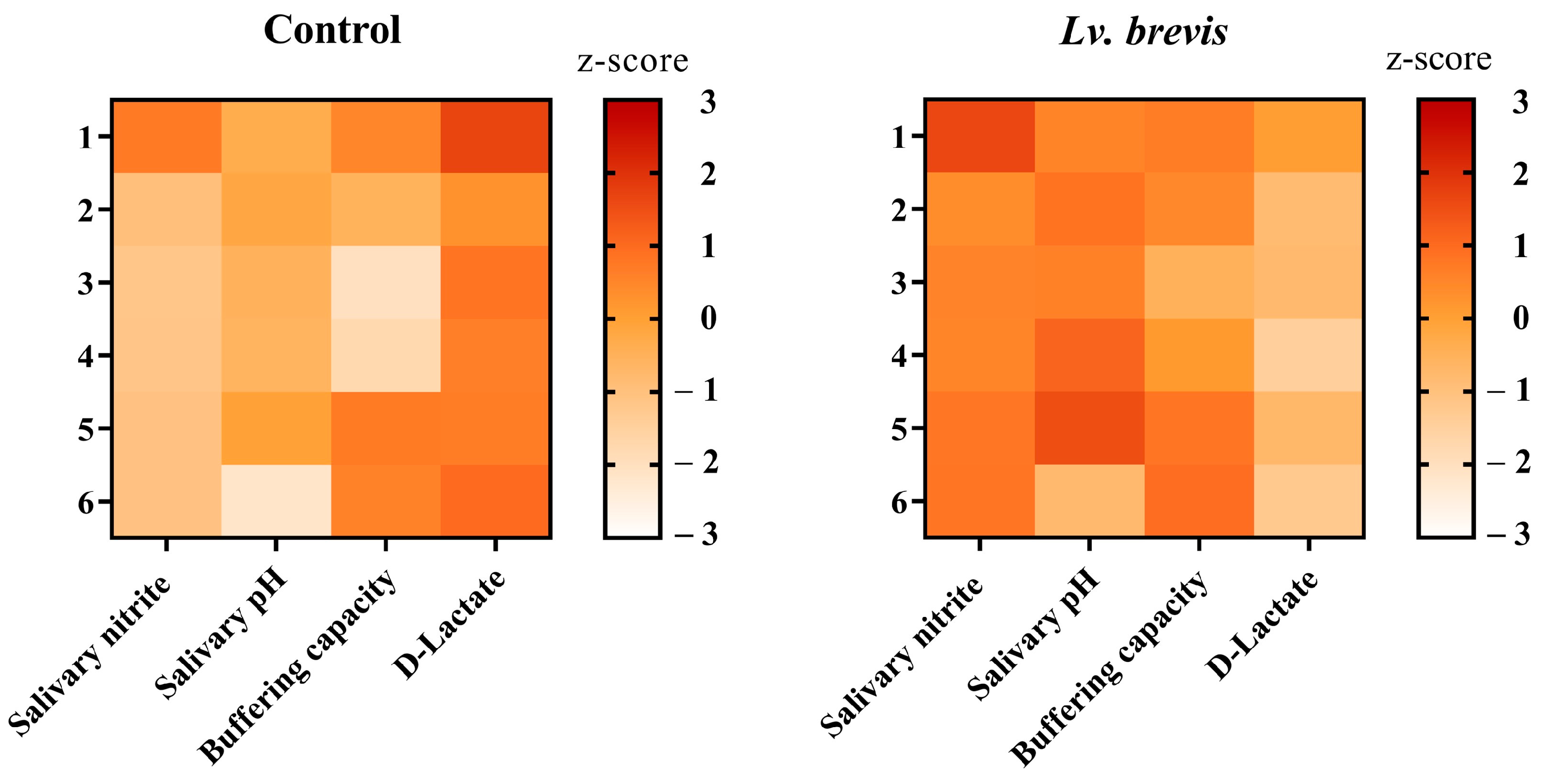
3.3. Effect of Lv. brevis CD2 on Salivary pH and Buffering Capacity
3.4. Effect of Lv. brevis CD2 on D-Lactate Salivary Levels
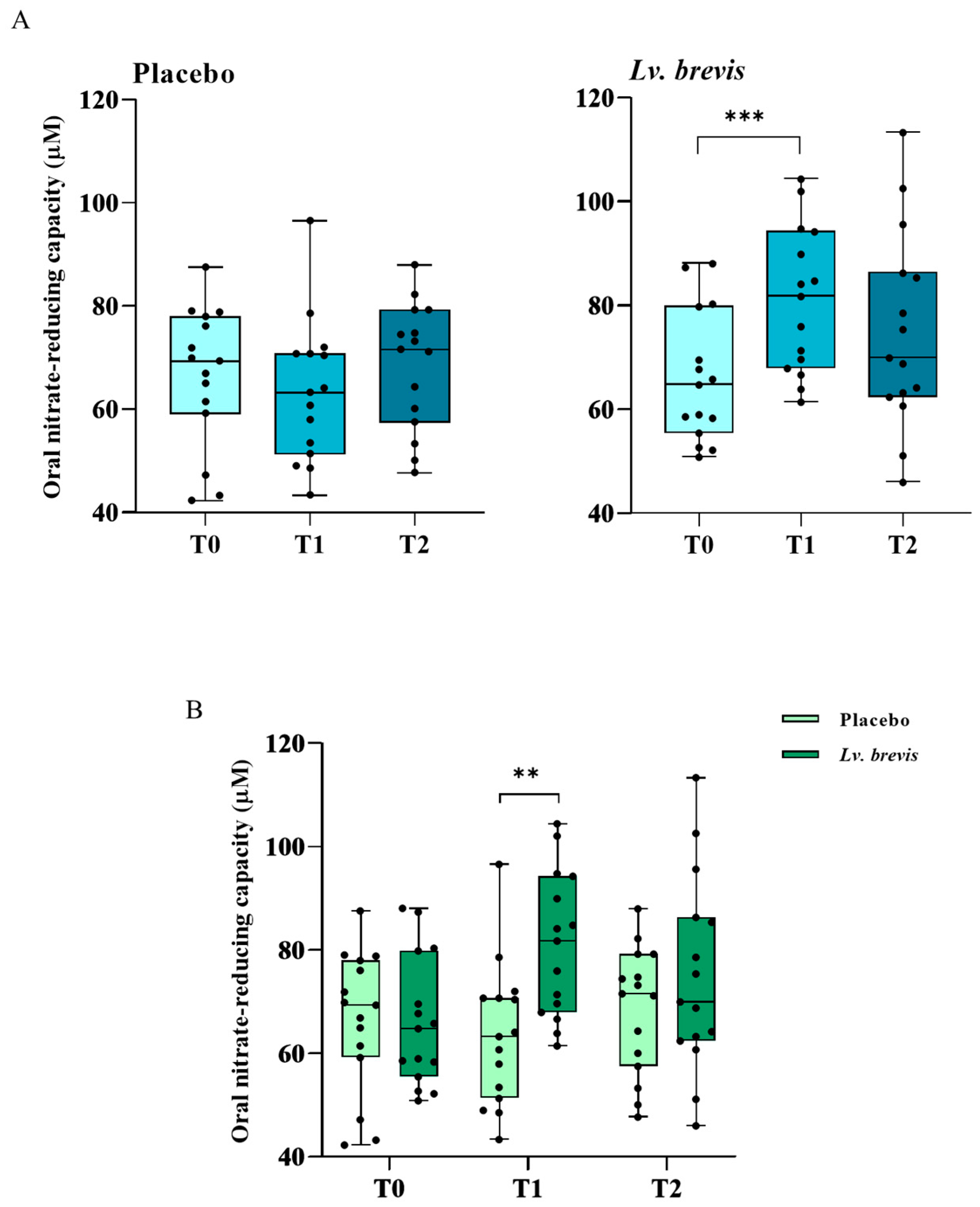
3.5. Effects of Lv. brevis CD2-Containing Lozenges on Oral Nitrate-Reducing Capacity In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duar, R.M.; Lin, X.B.; Zheng, J.; Martino, M.E.; Grenier, T.; Pérez-Muñoz, M.E.; Leulier, F.; Gänzle, M.; Walter, J. Lifestyles in transition: Evolution and natural history of the genus Lactobacillus. FEMS Microbiol. Rev. 2017, 41, S27–S48. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pasolli, E.; Ercolini, D. The food-gut axis: Lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiol. Rev. 2020, 44, 454–489. [Google Scholar] [CrossRef]
- Yang, H.; Hao, L.; Jin, Y.; Huang, J.; Zhou, R.; Wu, C. Functional roles and engineering strategies to improve the industrial functionalities of lactic acid bacteria during food fermentation. Biotechnol. Adv. 2024, 74, 108397. [Google Scholar] [CrossRef]
- Özogul, F.; Hamed, I. The importance of lactic acid bacteria for the prevention of bacterial growth and their biogenic amines formation: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic Acid Bacteria as Antifungal and Anti-Mycotoxigenic Agents: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.F.; Xu, B.C.; Chen, C.G.; Li, P.J.; Luo, H.T. The function and mechanism of lactic acid bacteria in the reduction of toxic substances in food: A review. Crit. Rev. Food Sci. 2022, 62, 5950–5963. [Google Scholar] [CrossRef]
- Martín, R.; Langella, P. Emerging Health Concepts in the Probiotics Field: Streamlining the Definitions. Front. Microbiol. 2019, 10, 1047. [Google Scholar] [CrossRef]
- Žuntar, I.; Petric, Z.; Bursac Kovačević, D.; Putnik, P. Safety of Probiotics: Functional Fruit Beverages and Nutraceuticals. Foods 2020, 9, 947. [Google Scholar] [CrossRef]
- Roe, A.L.; Boyte, M.E.; Elkins, C.A.; Goldman, V.S.; Heimbach, J.; Madden, E.; Oketch-Rabah, H.; Sanders, M.E.; Sirois, J.; Smith, A. Considerations for determining safety of probiotics: A USP perspective. Regul. Toxicol. Pharmacol. 2022, 136, 105266. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Abhari, K.; Eş, I.; Soares, M.B.; Oliveira, R.B.A.; Hosseini, H.; Rezaei, M.; Balthazar, C.F.; Silva, R.; Cruz, A.G.; et al. Interactions between probiotics and pathogenic microorganisms in hosts and foods: A review. Trends Food Sci. Technol. 2020, 95, 205–218. [Google Scholar] [CrossRef]
- Choksket, S.; Sharma, S.; Harshvardhan; Pal, V.; Jain, A.; Patil, P.B.; Korpole, S.; Grover, V. Evaluation of Human Dental Plaque Lactic Acid Bacilli for Probiotic Potential and Functional Analysis in Relevance to Oral Health. Indian J. Microbiol. 2023, 63, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Minj, J.; Chandra, P.; Paul, C.; Sharma, R.K. Bio-functional properties of probiotic Lactobacillus: Current applications and research perspectives. Crit. Rev. Food Sci. Nutr. 2021, 61, 2207–2224. [Google Scholar] [CrossRef]
- Bisht, V.; Das, B.; Hussain, A.; Kumar, V.; Navani, N.K. Understanding of probiotic origin antimicrobial peptides: A sustainable approach ensuring food safety. NPJ Sci. Food 2024, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Ammor, M.S.; Mayo, B. Selection criteria for lactic acid bacteria to be used as functional starter cultures in dry sausage production: An update. Meat Sci. 2007, 76, 138–146. [Google Scholar] [CrossRef]
- Laranjo, M.; Potes, M.E.; Elias, M. Role of Starter Cultures on the Safety of Fermented Meat Products. Front. Microbiol. 2019, 10, 853. [Google Scholar] [CrossRef]
- Xia, C.; Tian, Q.; Kong, L.; Sun, X.; Shi, J.; Zeng, X.; Pan, D. Metabolomics Analysis for Nitrite Degradation by the Metabolites of Limosilactobacillus fermentum RC4. Foods 2022, 11, 1009. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Y.; Huang, M.; Wang, M.; Ming, Y.; Chen, W.; Chen, Y.; Tang, Z.; Jia, B. From nitrate to NO: Potential effects of nitrate-reducing bacteria on systemic health and disease. Eur. J. Med. Res. 2023, 28, 425. [Google Scholar] [CrossRef]
- Yuan, J.W.; Zeng, X.Q.; Zhang, P.; Leng, L.L.; Du, Q.W.; Pan, D.D. Nitrite reductases of lactic acid bacteria: Regulation of enzyme synthesis and activity, and different applications. Food Biosci. 2024, 59, 103833. [Google Scholar] [CrossRef]
- Tan, X.; Cui, F.; Wang, D.; Lv, X.; Li, X.; Li, J. Fermented Vegetables: Health Benefits, Defects, and Current Technological Solutions. Foods 2023, 13, 38. [Google Scholar] [CrossRef]
- Altamura, S.; Pietropaoli, D.; Lombardi, F.; Del Pinto, R.; Ferri, C. An Overview of Chronic Kidney Disease Pathophysiology: The Impact of Gut Dysbiosis and Oral Disease. Biomedicines 2023, 11, 3033. [Google Scholar] [CrossRef] [PubMed]
- Altamura, S.; Del Pinto, R.; Pietropaoli, D.; Ferri, C. Oral health as a modifiable risk factor for cardiovascular diseases. Trends Cardiovasc. Med. 2024, 34, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Pietropaoli, D.; Del Pinto, R.; Ferri, C.; Wright, J.T.; Giannoni, M.; Ortu, E.; Monaco, A. Poor Oral Health and Blood Pressure Control Among US Hypertensive Adults: Results From the National Health and Nutrition Examination Survey 2009 to 2014. Hypertension 2018, 72, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Paul, O.; Arora, P.; Mayer, M.; Chatterjee, S. Inflammation in Periodontal Disease: Possible Link to Vascular Disease. Front. Physiol. 2021, 11, 609614. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Barranca-Enríquez, A.; Romo-González, T. Your health is in your mouth: A comprehensive view to promote general wellness. Front. Oral Health. 2022, 3, 971223. [Google Scholar] [CrossRef]
- Botelho, J.; Mascarenhas, P.; Viana, J.; Proença, L.; Orlandi, M.; Leira, Y.; Chambrone, L.; Mendes, J.J.; Machado, V. An umbrella review of the evidence linking oral health and systemic noncommunicable diseases. Nat. Commun. 2022, 13, 7614. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Ashworth, A.; Bescos, R. Dietary nitrate and blood pressure: Evolution of a new nutrient? Nutr. Res. Rev. 2017, 30, 208–219. [Google Scholar] [CrossRef]
- Siervo, M.; Scialò, F.; Shannon, O.M.; Stephan, B.C.M.; Ashor, A.W. Does dietary nitrate say NO to cardiovascular ageing? Current evidence and implications for research. Proc. Nutr. Soc. 2018, 77, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Rosier, B.T.; Takahashi, N.; Zaura, E.; Krom, B.P.; MartInez-Espinosa, R.M.; van Breda, S.G.J.; Marsh, P.D.; Mira, A. The Importance of Nitrate Reduction for Oral Health. J. Dent. Res. 2022, 101, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Morou-Bermúdez, E.; Torres-Colón, J.E.; Bermúdez, N.S.; Patel, R.P.; Joshipura, K.J. Pathways Linking Oral Bacteria, Nitric Oxide Metabolism, and Health. J. Dent. Res. 2022, 101, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, M.F.; Sundqvist, M.L.; Larsen, F.J.; Zhuge, Z.; Carlstrom, M.; Weitzberg, E.; Lundberg, J.O. Blood Pressure-Lowering Effect of Orally Ingested Nitrite Is Abolished by a Proton Pump Inhibitor. Hypertension 2017, 69, 23–31. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- DeMartino, A.W.; Kim-Shapiro, D.B.; Patel, R.P.; Gladwin, M.T. Nitrite and nitrate chemical biology and signalling. Br. J. Pharmacol. 2019, 176, 228–245. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; An, W.; Li, D.; Qin, L. Regulatory effect of dietary nitrate on blood pressure: A meta-analysis of randomized controlled trials. Food Funct. 2023, 14, 1839–1850. [Google Scholar] [CrossRef]
- Apte, M.; Nadavade, N.; Sheikh, S.S. A review on nitrates’ health benefits and disease prevention. Nitric Oxide 2024, 142, 1–15. [Google Scholar] [CrossRef]
- Bak, K.H.; Bauer, S.; Eisenreich, C.; Paulsen, P. Residual Nitrite, Nitrate, and Volatile N-Nitrosamines in Organic and Conventional Ham and Salami Products. Foods 2025, 14, 112. [Google Scholar] [CrossRef]
- Allaker, R.P.; Silva Mendez, L.S.; Hardie, J.M.; Benjamin, N. Antimicrobial effect of acidified nitrite on periodontal bacteria. Oral Microbiol. Immunol. 2001, 16, 253–256. [Google Scholar] [CrossRef]
- Backlund, C.J.; Sergesketter, A.R.; Offenbacher, S.; Schoenfisch, M.H. Antibacterial efficacy of exogenous nitric oxide on periodontal pathogens. J. Dent. Res. 2014, 93, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, J.J.; Krishnamurthy, H.K.; Bosco, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K. Oral Microbiome: A Review of Its Impact on Oral and Systemic Health. Microorganisms 2024, 12, 1797. [Google Scholar] [CrossRef]
- Chai, X.; Liu, L.; Chen, F. Oral nitrate-reducing bacteria as potential probiotics for blood pressure homeostasis. Front. Cardiovasc. Med. 2024, 11, 1337281. [Google Scholar] [CrossRef]
- Todorov, S.D.; Baretto Penna, A.L.; Venema, K.; Holzapfel, W.H.; Chikindas, M.L. Recommendations for the use of standardised abbreviations for the former Lactobacillus genera, reclassified in the year 2020. Benef. Microbes 2023, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.J.; Liu, X.F.; Wang, G.Q.; Zhang, H.; Xiong, Z.Q.; Sun, Y.; Ai, L.Z. Characterization and selection of Lactobacillus brevis starter for nitrite degradation of Chinese pickle. Food Control 2017, 78, 126–131. [Google Scholar] [CrossRef]
- Cakir, E.; Arici, M.; Durak, M.Z. Effect of starter culture sourdough prepared with Lactobacilli and Saccharomyces cerevisiae on the quality of hull-less barley-wheat bread. LWT-Food Sci. Technol. 2021, 152, 112230. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Zhao, Y.; Guan, H.; Jin, C.; Gong, H.; Sun, X.; Wang, P.; Li, H.; Liu, W. Effect of Levilactobacillus brevis as a starter on the flavor quality of radish paocai. Food Res. Int. 2023, 168, 112780. [Google Scholar] [CrossRef]
- Woo, S.H.; Park, J.; Sung, J.M.; Choi, E.J.; Choi, Y.S.; Park, J.D. Characterization of Lactic Acid Bacteria and Yeast from Grains as Starter Cultures for Gluten-Free Sourdough. Foods 2023, 12, 4367. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Xu, J.; Li, Q.; Xia, X.; Du, G. Characterization of a Lactobacillus brevis strain with potential oral probiotic properties. BMC Microbiol. 2018, 18, 221. [Google Scholar] [CrossRef]
- Tasli, L.; Mat, C.; De Simone, C.; Yazici, H. Lactobacilli lozenges in the management of oral ulcers of Behcet’s syndrome. Clin. Exp. Rheumatol. 2006, 24, S83–S86. [Google Scholar]
- Riccia, D.N.; Bizzini, F.; Perilli, M.G.; Polimeni, A.; Trinchieri, V.; Amicosante, G.; Cifone, M.G. Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral Dis. 2007, 13, 376–385. [Google Scholar] [CrossRef]
- Sharma, A.; Rath, G.K.; Chaudhary, S.P.; Thakar, A.; Mohanti, B.K.; Bahadur, S. Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: A randomized double-blind placebo-controlled study. Eur. J. Cancer 2012, 48, 875–881. [Google Scholar] [CrossRef]
- Niscola, P.; Tendas, A.; Scaramucci, L.; Giovannini, M.; Trinchieri, V.; De Fabritiis, P. Aphthous oral ulceration and its successful management by Lactobacillus brevis CD2 extract in an adult haemophilic patient. Haemophilia 2012, 18, e78–e79. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, T.; Hajishengallis, G. Topical treatment with probiotic Lactobacillus brevis CD2 inhibits experimental periodontal inflammation and bone loss. J. Periodontal Res. 2014, 49, 785–791. [Google Scholar] [CrossRef]
- Campus, G.; Cocco, F.; Carta, G.; Cagetti, M.G.; Simark-Mattson, C.; Strohmenger, L.; Lingstrom, P. Effect of a daily dose of Lactobacillus brevis CD2 lozenges in high caries risk schoolchildren. Clin. Oral Investig. 2014, 18, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kim, S.J.; Ko, S.H.; Ouwehand, A.C.; Ma, D.S. Modulation of the host response by probiotic Lactobacillus brevis CD2 in experimental gingivitis. Oral Dis. 2015, 21, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.P.; Gujjari, S.K.; Chandrasekhar, V.S. Long-term effect of Lactobacillus brevis CD2 (Inersan((R))) and/or doxycycline in aggressive periodontitis. J. Indian Soc. Periodontol. 2017, 21, 341–343. [Google Scholar] [CrossRef]
- Lai, S.; Lingstrom, P.; Cagetti, M.G.; Cocco, F.; Meloni, G.; Arrica, M.A.; Campus, G. Effect of Lactobacillus brevis CD2 containing lozenges and plaque pH and cariogenic bacteria in diabetic children: A randomised clinical trial. Clin. Oral Investig. 2021, 25, 115–123. [Google Scholar] [CrossRef]
- Altamura, S.; Augello, F.R.; Ortu, E.; Pietropaoli, D.; Cinque, B.; Giannoni, M.; Lombardi, F. Efficacy of the Probiotic L. brevis in Counteracting the Demineralizing Process of the Tooth Enamel Surface: Results from an In Vitro Study. Biomolecules 2024, 14, 605. [Google Scholar] [CrossRef]
- Altamura, S.; Lombardi, F.; Augello, F.R.; Barone, A.; Giannoni, M.; Cinque, B.; Pietropaoli, D. Levilactobacillus brevis CD2 as a multifaceted probiotic to preserve oral health: Results of a double-blind, randomized, placebo-controlled trial in healthy adults. J. Transl. Med. 2025, 23, 128. [Google Scholar] [CrossRef]
- Arias-Negrete, S.; Jiménez-Romero, L.A.; Solís-Martínez, M.O.; Ramírez-Emiliano, J.; Avila, E.E.; Cuéllar-Mata, P. Indirect determination of nitric oxide production by reduction of nitrate with a freeze-thawing-resistant nitrate reductase from Escherichia coli MC1061. Anal. Biochem. 2004, 328, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Rosier, B.T.; Johnston, W.; Carda-Diéguez, M.; Simpson, A.; Cabello-Yeves, E.; Piela, K.; Reilly, R.; Artacho, A.; Easton, C.; Burleigh, M.; et al. Nitrate reduction capacity of the oral microbiota is impaired in periodontitis: Potential implications for systemic nitric oxide availability. Int. J. Oral Sci. 2024, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, Y. Clinical Investigations of the Salivary Buffering Action. Acta Odontol. Scand. 1959, 17, 131–165. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Nurs. Ethics 2002, 9, 105–109. [Google Scholar] [CrossRef]
- Ashworth, A.; Cutler, C.; Farnham, G.; Liddle, L.; Burleigh, M.; Rodiles, A.; Sillitti, C.; Kiernan, M.; Moore, M.; Hickson, M.; et al. Dietary intake of inorganic nitrate in vegetarians and omnivores and its impact on blood pressure, resting metabolic rate and the oral microbiome. Free. Radic. Biol. Med. 2019, 138, 63–72. [Google Scholar] [CrossRef]
- Shetty, C.; Hedge, M.N.; Devadiga, D. Correlation between dental caries with salivary flow, pH, and buffering capacity in adult south Indian population: An in -vivo study. Int. J. Res. Ayurveda Pharm. 2013, 4, 219–223. [Google Scholar] [CrossRef]
- Andreadis, G.; Topitsoglou, V.; Kalfas, S. Acidogenicity and acidurance of dental plaque and saliva sediment from adults in relation to caries activity and chlorhexidine exposure. J. Oral Microbiol. 2015, 7, 26197. [Google Scholar] [CrossRef]
- Doel, J.J.; Hector, M.P.; Amirtham, C.V.; Al-Anzan, L.A.; Benjamin, N.; Allaker, R.P. Protective effect of salivary nitrate and microbial nitrate reductase activity against caries. Eur. J. Oral Sci. 2004, 112, 424–428. [Google Scholar] [CrossRef]
- Abruzzo, A.; Vitali, B.; Lombardi, F.; Guerrini, L.; Cinque, B.; Parolin, C.; Bigucci, F.; Cerchiara, T.; Arbizzani, C.; Gallucci, M.C.; et al. Mucoadhesive Buccal Films for Local Delivery of Lactobacillus brevis. Pharmaceutics 2020, 12, 241. [Google Scholar] [CrossRef]
- Marquis, R.E.; Bender, G.R.; Murray, D.R.; Wong, A. Arginine deiminase system and bacterial adaptation to acid environments. Appl. Environ. Microbiol. 1987, 53, 198–200. [Google Scholar] [CrossRef]
- Nascimento, M.M.; Gordan, V.V.; Garvan, C.W.; Browngardt, C.M.; Burne, R.A. Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol. Immunol. 2009, 24, 89–95. [Google Scholar] [CrossRef]
- Dipalma, G.; Inchingolo, F.; Patano, A.; Guglielmo, M.; Palumbo, I.; Campanelli, M.; Inchingolo, A.D.; Malcangi, G.; Palermo, A.; Tartaglia, F.C.; et al. Dental erosion and the role of saliva: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 10651–10660. [Google Scholar] [CrossRef]
- Rosier, B.T.; Marsh, P.D.; Mira, A. Resilience of the Oral Microbiota in Health: Mechanisms That Prevent Dysbiosis. J. Dent. Res. 2018, 97, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. In Sickness and in Health-What Does the Oral Microbiome Mean to Us? An Ecological Perspective. Adv. Dent. Res. 2018, 29, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Burne, R.A.; Marquis, R.E. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol. Lett. 2000, 193, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.M.; Alvarez, A.J.; Huang, X.; Hanway, S.; Perry, S.; Luce, A.; Richards, V.P.; Burne, R.A. Arginine Metabolism in Supragingival Oral Biofilms as a Potential Predictor of Caries Risk. JDR Clin. Trans. Res. 2019, 4, 262–270. [Google Scholar] [CrossRef]
- Remund, B.; Yilmaz, B.; Sokollik, C. D-Lactate: Implications for Gastrointestinal Diseases. Children 2023, 10, 945. [Google Scholar] [CrossRef]
- Hove, H.; Nørgaard, H.; Mortensen, P.B. Lactic acid bacteria and the human gastrointestinal tract. Eur. J. Clin. Nutr. 1999, 53, 339–350. [Google Scholar] [CrossRef]
- Levitt, M.D.; Levitt, D.G. Quantitative Evaluation of D-Lactate Pathophysiology: New Insights into the Mechanisms Involved and the Many Areas in Need of Further Investigation. Clin. Exp. Gastroenterol. 2020, 13, 321–337. [Google Scholar] [CrossRef]
- Rosier, B.T.; Moya-Gonzalvez, E.M.; Corell-Escuin, P.; Mira, A. Isolation and Characterization of Nitrate-Reducing Bacteria as Potential Probiotics for Oral and Systemic Health. Front. Microbiol. 2020, 11, 555465. [Google Scholar] [CrossRef]
- Wicaksono, D.P.; Washio, J.; Abiko, Y.; Domon, H.; Takahashi, N. Nitrite Production from Nitrate and Its Link with Lactate Metabolism in Oral Veillonella spp. Appl. Environ. Microbiol. 2020, 86, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Carlström, M.; Weitzberg, E. Metabolic Effects of Dietary Nitrate in Health and Disease. Cell Metab. 2018, 28, 9–22. [Google Scholar] [CrossRef]
- Kapil, V.; Haydar, S.M.; Pearl, V.; Lundberg, J.O.; Weitzberg, E.; Ahluwalia, A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free. Radic. Biol. Med. 2013, 55, 93–100. [Google Scholar] [CrossRef]
- Li, Y.; Qian, F.; Cheng, X.; Wang, D.; Wang, Y.; Pan, Y.; Chen, L.; Wang, W.; Tian, Y. Dysbiosis of Oral Microbiota and Metabolite Profiles Associated with Type 2 Diabetes Mellitus. Microbiol. Spectr. 2023, 11, e0379622. [Google Scholar] [CrossRef] [PubMed]
- Berends, J.E.; van den Berg, L.M.M.; Guggeis, M.A.; Henckens, N.F.T.; Hossein, I.J.; de Joode, M.; Zamani, H.; van Pelt, K.; Beelen, N.A.; Kuhnle, G.G.; et al. Consumption of Nitrate-Rich Beetroot Juice with or without Vitamin C Supplementation Increases the Excretion of Urinary Nitrate, Nitrite, and N-nitroso Compounds in Humans. Int. J. Mol. Sci. 2019, 20, 2277. [Google Scholar] [CrossRef] [PubMed]
- Rosier, B.T.; Buetas, E.; Moya-Gonzalvez, E.M.; Artacho, A.; Mira, A. Nitrate as a potential prebiotic for the oral microbiome. Sci. Rep. 2020, 10, 12895. [Google Scholar] [CrossRef]
- Milton-Laskibar, I.; Martínez, J.A.; Portillo, M.P. Current Knowledge on Beetroot Bioactive Compounds: Role of Nitrate and Betalains in Health and Disease. Foods 2021, 10, 1314. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altamura, S.; Augello, F.R.; Lombardi, F.; Palumbo, P.; Cinque, B.; Pietropaoli, D.; De Simone, C. In Vitro, Ex Vivo, and In Vivo Evidence of Nitrate-Reducing Activity in Levilactobacillus brevis CD2: A Potential Tool for Oral and Systemic Health Applications. Foods 2025, 14, 1512. https://doi.org/10.3390/foods14091512
Altamura S, Augello FR, Lombardi F, Palumbo P, Cinque B, Pietropaoli D, De Simone C. In Vitro, Ex Vivo, and In Vivo Evidence of Nitrate-Reducing Activity in Levilactobacillus brevis CD2: A Potential Tool for Oral and Systemic Health Applications. Foods. 2025; 14(9):1512. https://doi.org/10.3390/foods14091512
Chicago/Turabian StyleAltamura, Serena, Francesca Rosaria Augello, Francesca Lombardi, Paola Palumbo, Benedetta Cinque, Davide Pietropaoli, and Claudio De Simone. 2025. "In Vitro, Ex Vivo, and In Vivo Evidence of Nitrate-Reducing Activity in Levilactobacillus brevis CD2: A Potential Tool for Oral and Systemic Health Applications" Foods 14, no. 9: 1512. https://doi.org/10.3390/foods14091512
APA StyleAltamura, S., Augello, F. R., Lombardi, F., Palumbo, P., Cinque, B., Pietropaoli, D., & De Simone, C. (2025). In Vitro, Ex Vivo, and In Vivo Evidence of Nitrate-Reducing Activity in Levilactobacillus brevis CD2: A Potential Tool for Oral and Systemic Health Applications. Foods, 14(9), 1512. https://doi.org/10.3390/foods14091512









