The Role of Whey in Functional Microorganism Growth and Metabolite Generation: A Biotechnological Perspective
Abstract
1. Introduction
2. Chemical Composition of Whey
2.1. Protein Fraction
2.2. Lactose Content
2.3. Minerals and Vitamins
3. Functional Microorganisms Cultivated in Whey

4. Techniques for Whey Fermentation, Metabolite Production, and Their Industrial Applications
5. Limitations and Environmental–Social Impact of Using Whey as a Substrate for Functional Microorganism Cultivation
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostertag, F.; Krolitzki, E.; Berensmeier, S.; Hinrichs, J. Protein valorisation from acid whey–Screening of various micro-and ultrafiltration membranes concerning the filtration performance. Int. Dairy J. 2023, 146, 105745. [Google Scholar] [CrossRef]
- Milk and Milk Product Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Milk_and_milk_product_statistics (accessed on 18 January 2025).
- Mukherjee, P.; Raj, N.; Sivaprakasam, S. Harnessing valorization potential of whey permeate for D-lactic acid production using lactic acid bacteria. Biomass Conv. Bioref. 2023, 13, 15639–15658. [Google Scholar] [CrossRef]
- Zandona, E.; Blažić, M.; Režek Jambrak, A. Whey utilization: Sustainable uses and environmental approach. Food Technol. Biotechnol. 2021, 59, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, D.; Martindale, W.; Romeih, E.; Hebishy, E. Recent advances in whey processing and valorisation: Technological and environmental perspectives. Int. J. Dairy Technol. 2023, 76, 291–312. [Google Scholar] [CrossRef]
- Chourasia, R.; Phukon, L.C.; Abedin, M.M.; Padhi, S.; Singh, S.P.; Rai, A.K. Whey valorization by microbial and enzymatic bioprocesses for the production of nutraceuticals and value-added products. Bioresour. Technol. Rep. 2022, 19, 101144. [Google Scholar] [CrossRef]
- Abish, Z.A.; Alibekov, R.S.; Tarapoulouzi, M.; Bakhtybekova, A.R.; Kobzhasarova, Z.I. Review in deep processing of whey. Cogent Food Agric. 2024, 10, 2415380. [Google Scholar] [CrossRef]
- Kaya, B.; Wijayarathna, E.K.B.; Yüceer, Y.K.; Agnihotri, S.; Taherzadeh, M.J.; Sar, T. The use of cheese whey powder in the cultivation of protein-rich filamentous fungal biomass for sustainable food production. Front. Sustain. Food Syst. 2024, 8, 1386519. [Google Scholar] [CrossRef]
- Chizhayeva, A.; Oleinikova, Y.; Saubenova, M.; Sadanov, A.; Amangeldi, A.; Aitzhanova, A.; Yelubaeva, M. Impact of probiotics and their metabolites in enhancement of the functional properties of whey-based beverages. AIMS Agric. Food 2020, 5, 521–542. [Google Scholar] [CrossRef]
- Antone, U.; Ciprovica, I.; Zolovs, M.; Scerbaka, R.; Liepins, J. Propionic acid fermentation—Study of substrates, strains, and antimicrobial properties. Fermentation 2022, 9, 26. [Google Scholar] [CrossRef]
- Prete, R.; Alam, M.K.; Perpetuini, G.; Perla, C.; Pittia, P.; Corsetti, A. Lactic acid bacteria exopolysaccharides producers: A sustainable tool for functional foods. Foods 2021, 10, 1653. [Google Scholar] [CrossRef]
- Allen, M.M.; Pike, O.A.; Kenealey, J.D.; Dunn, M.L. Metabolomics of acid whey derived from Greek yogurt. J. Dairy Sci. 2021, 104, 11401–11412. [Google Scholar] [CrossRef]
- Soumati, B.; Atmani, M.; Benabderrahmane, A.; Benjelloun, M. Whey Valorization–Innovative Strategies for Sustainable Development and Value-Added Product Creation. J. Ecol. Eng. 2023, 24, 10. [Google Scholar] [CrossRef]
- Arshad, U.E.T.; Hassan, A.; Ahmad, T.; Naeem, M.; Chaudhary, M.T.; Abbas, S.Q.; Aadil, R.M. A recent glance on the valorisation of cheese whey for industrial prerogative: High-value-added products development and integrated reutilising strategies. Int. J. Food Sci. Technol. 2023, 58, 2001–2013. [Google Scholar] [CrossRef]
- Gallardo-Escamilla, F.J.; Kelly, A.L.; Delahunty, C.M. Sensory characteristics and related volatile flavor compound profiles of different types of whey. J. Dairy Sci. 2005, 88, 2689–2699. [Google Scholar] [CrossRef] [PubMed]
- Chandrapala, J.; Duke, M.C.; Gray, S.R.; Zisu, B.; Weeks, M.; Palmer, M.; Vasiljevic, T. Properties of acid whey as a function of pH and temperature. J. Dairy Sci. 2015, 98, 4352–4363. [Google Scholar] [CrossRef] [PubMed]
- Abdelhakam, K.E.; Sid Ahmed, S.M.F.; Farahat, F.H.; Bushara, A.M.; Ali, E.M.; Mohamed, A.A. Physicochemical properties, microbial load, and sensory attributes of sweet and sour whey of white cheese. J. Saudi Soc. Food Nutr. 2022, 15, 57–66. [Google Scholar]
- Poonia, A.; Rao, V.; Mann, B. Whey production status, types, characterization and functional properties. In Whey Valorization: Innovations, Technological Advancements, and Sustainable Exploitation; Trajkovska Petkoska, A., Ed.; Springer Nature: Singapore, 2023. [Google Scholar]
- Rodrigues, C.F.; Andrade, J.C. Milk proteins. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; Taylor & Francis: New York, NY, USA, 2015. [Google Scholar]
- Tapia-Hernández, J.A.; Madera-Santana, T.J.; Rodríguez-Félix, F.; Barreras-Urbina, C.G. Controlled and prolonged release systems of urea from micro-and nanomaterials as an alternative for developing a sustainable agriculture: A review. J. Nanomater. 2022, 2022, 5697803. [Google Scholar] [CrossRef]
- Figueroa-Enríquez, C.E.; Rodríguez-Félix, F.; Ruiz-Cruz, S.; Castro-Enriquez, D.D.; Gonzalez-Rios, H.; Perez-Alvarez, J.Á.; López-Peña, I.Y. Application of active packaging films for extending the shelf life of red meats: A review. Processes 2024, 12, 2115. [Google Scholar] [CrossRef]
- Ergasheva, Z.K.; Sultanov, S.A.; Saparov, J.E. Analysis of dairy whey food functional modules based on resource-saving technologies. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2023; Volume 1231, p. 012042. [Google Scholar]
- Pescuma, M.; Hébert, E.M.; De Valdez, G.F.; Mozzi, F. Functional fermented whey foods: Their role in human health. In Beneficial Microbes in Fermented and Functional Foods; CRC Press: Boca Raton, FL, USA, 2014; pp. 95–111. [Google Scholar]
- Bylund, G. Dairy Processing Handbook: Tetra Pak Processing Systems; Visit Lund AB: Lund, Sweden, 1995; Volume 5, pp. 13–36. [Google Scholar]
- Božanić, R.; Barulčić, I.; Lisak Jakopović, K.; Tratnik, L. Possibilities of whey utilisation. Godišnjak Akad. Teh. Znan. Hrvat. 2023, 1, 281–287. [Google Scholar]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy by-products: A review on the valorization of whey and second cheese whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- Papademas, P.; Kotsaki, P. Technological utilization of whey towards sustainable exploitation. J. Adv. Dairy Res. 2019, 7, 231. [Google Scholar]
- Quinn, E.M.; Slattery, H.; Thompson, A.P.; Kilcoyne, M.; Joshi, L.; Hickey, R.M. Mining Milk for Factors which Increase the Adherence of Bifidobacterium longum subsp. infantis to Intestinal Cells. Foods 2018, 7, 196. [Google Scholar] [PubMed]
- Mercier-Bonin, M.; Chapot-Chartier, M.P. Surface proteins of Lactococcus lactis: Bacterial resources for muco-adhesion in the gastrointestinal tract. Front. Microbiol. 2017, 8, 2247. [Google Scholar] [CrossRef]
- Simões, L.S.; Araújo, J.F.; Vicente, A.A.; Ramos, O.L. Design of β-lactoglobulin micro-and nanostructures by controlling gelation through physical variables. Food Hydrocoll. 2020, 100, 105357. [Google Scholar] [CrossRef]
- Chaneton, L.; Sáez, J.P.; Bussmann, L.E. Antimicrobial activity of bovine β-lactoglobulin against mastitis-causing bacteria. J. Dairy Sci. 2011, 94, 138–145. [Google Scholar] [CrossRef]
- Pescuma, M.; Hébert, E.M.; Bru, E.; de Valdez, G.F.; Mozzi, F. Diversity in growth and protein degradation by dairy relevant lactic acid bacteria species in reconstituted whey. J. Dairy Res. 2012, 79, 201–208. [Google Scholar] [CrossRef]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Barbana, C.; Sánchez, L.; Pérez, M.D. Bioactivity of α-lactalbumin related to its interaction with fatty acids: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 783–794. [Google Scholar] [CrossRef]
- Brown, L.; Pingitore, E.V.; Mozzi, F.; Saavedra, L.; Villegas, J.M.; Hebert, E.M. Lactic acid bacteria as cell factories for the generation of bioactive peptides. Protein Pept. Lett. 2017, 24, 146–155. [Google Scholar] [CrossRef]
- Bu, G.; Luo, Y.; Zhang, Y.; Chen, F. Effects of fermentation by lactic acid bacteria on the antigenicity of bovine whey proteins. J. Sci. Food Agric. 2010, 90, 2015–2020. [Google Scholar] [CrossRef]
- Boscaini, S.; Cabrera-Rubio, R.; Speakman, J.R.; Cotter, P.D.; Cryan, J.F.; Nilaweera, K.N. Dietary α-lactalbumin alters energy balance, gut microbiota composition and intestinal nutrient transporter expression in high-fat diet-fed mice. Nutrients 2019, 11, 2047. [Google Scholar] [CrossRef] [PubMed]
- Mukkur, T.K.S.; Froese, A. Isolation and characterization of IgM from bovine colostral whey. Immunochemistry 1971, 8, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.; Stiller, J.; Sowa, B. Immunoglobulin G1 Fc in colostral whey. Can. J. Comp. Med. 1984, 48, 410. [Google Scholar] [PubMed]
- Cakebread, J.; Hodgkinson, A.; Wallace, O.; Callaghan, M.; Hurford, D.; Wieliczko, R.; Haigh, B. Bovine milk derived skimmed milk powder and whey protein concentrate modulates Citrobacter rodentium shedding in the mouse intestinal tract. PeerJ 2018, 6, e5359. [Google Scholar] [CrossRef]
- Quinn, E.M.; Kilcoyne, M.; Walsh, D.; Joshi, L.; Hickey, R.M. A whey fraction rich in immunoglobulin G combined with Bifidobacterium longum subsp. infantis ATCC 15697 exhibits synergistic effects against Campylobacter jejuni. Int. J. Mol. Sci. 2020, 21, 4632. [Google Scholar]
- Qu, Y.; Kim, B.J.; Koh, J.; Dallas, D.C. Analysis of bovine kappa-casein glycomacropeptide by liquid chromatography-tandem mass spectrometry. Foods 2021, 10, 2028. [Google Scholar] [CrossRef]
- Feeney, S.; Joshi, L.; Hickey, R.M. Biological roles and production technologies associated with bovine glycomacropeptide. In Novel Proteins for Food, Pharmaceuticals and Agriculture: Sources, Applications and Advances; Wiley: Hoboken, NJ, USA, 2018; pp. 1–28. [Google Scholar]
- Hager-Mair, F.F.; Bloch, S.; Schäffer, C. Glycolanguage of the oral microbiota. Mol. Oral Microbiol. 2024, 39, 112–125. [Google Scholar] [CrossRef]
- Behren, S.; Yu, J.; Pett, C.; Schorlemer, M.; Heine, V.; Fischöder, T.; Westerlind, U. Fucose binding motifs on mucin core glycopeptides impact bacterial lectin recognition. Angew. Chem. 2023, 135, e202302437. [Google Scholar] [CrossRef]
- Taleb, V.; Liao, Q.; Narimatsu, Y.; García-García, A.; Compañón, I.; Borges, R.J.; Hurtado-Guerrero, R. Structural and mechanistic insights into the cleavage of clustered O-glycan patches-containing glycoproteins by mucinases of the human gut. Nat. Commun. 2022, 13, 4324. [Google Scholar] [CrossRef]
- Rackerby, B.; Le, H.N.M.; Haymowicz, A.; Dallas, D.C.; Park, S.H. Potential prebiotic properties of whey protein and glycomacropeptide in gut microbiome. Food Sci. Anim. Resour. 2024, 44, 299. [Google Scholar] [CrossRef]
- Brück, W.M.; Graverholt, G.; Gibson, G.R. A two-stage continuous culture system to study the effect of supplemental α-lactalbumin and glycomacropeptide on mixed cultures of human gut bacteria challenged with enteropathogenic Escherichia coli and Salmonella serotype Typhimurium. J. Appl. Microbiol. 2003, 95, 44–53. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, J.; Jia, Y.; Liu, X.; Yan, Y.; Pang, G. Modulation of mice fecal microbiota by administration of casein glycomacropeptide. Microbiol. Res. 2012, 3, e3. [Google Scholar] [CrossRef]
- Jiménez, M.; Cervantes-García, D.; Muñoz, Y.H.; García, A.; Haro, L.M., Jr.; Salinas, E. Novel mechanisms underlying the therapeutic effect of glycomacropeptide on allergy: Change in gut microbiota, upregulation of TGF-β, and inhibition of mast cells. Int. Arch. Allergy Immunol. 2017, 171, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Morozumi, M.; Wada, Y.; Tsuda, M.; Tabata, F.; Ehara, T.; Nakamura, H.; Miyaji, K. Cross-feeding among bifidobacteria on glycomacropeptide. J. Funct. Foods 2023, 103, 105463. [Google Scholar] [CrossRef]
- Maier, R.; Fries, M.R.; Buchholz, C.; Zhang, F.; Schreiber, F. Human versus bovine serum albumin: A subtle difference in hydrophobicity leads to large differences in bulk and interface behavior. Cryst. Growth Des. 2021, 21, 5451–5459. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Voronina, P.A.; Goncharov, N.V. Integrative role of albumin: Evolutionary, biochemical and pathophysiological aspects. J. Evol. Biochem. Physiol. 2021, 57, 1419–1448. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Wang, T.; Zhao, H.; Zhang, B. Bovine serum albumin plays an important role in the removal of acrylamide by Lactobacillus strains. LWT 2023, 174, 114413. [Google Scholar] [CrossRef]
- Marques, A.M.; Bourbon, A.I.; Rodrigues, R.M.; Teixeira, J.A.; Pastrana, L.M.; Cerqueira, M.A. Lactoferrin as a carrier of iron: Development and physicochemical characterization. Food Hydrocoll. 2023, 142, 108772. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, N.; Ashaolu, T.J. Prebiotic and modulatory evidence of lactoferrin on gut health and function. J. Funct. Foods 2023, 108, 105741. [Google Scholar] [CrossRef]
- Li, B.; Zhang, B.; Zhang, F.; Liu, X.; Zhang, Y.; Peng, W.; Wang, J. Interaction between Dietary Lactoferrin and Gut Microbiota in Host Health. J. Agric. Food Chem. 2024, 72, 7596–7606. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kennedy, J.F. Biotechnological approaches for the value addition of whey. Crit. Rev. Biotechnol. 2012, 32, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Bellés, A.; Abad, I.; Sánchez, L.; Grasa, L. Whey and buttermilk-based formulas modulate gut microbiota in mice with antibiotic-induced dysbiosis. Mol. Nutr. Food Res. 2023, 67, 2300248. [Google Scholar] [CrossRef] [PubMed]
- Lara Aguilar, S. Lactose Oxidase: A Preservative of Dairy Products. Master’s Thesis, Cornell University Graduate School, New York, NY, USA, 2018. [Google Scholar]
- Al-Shehri, S.S.; Duley, J.A.; Bansal, N. Xanthine oxidase-lactoperoxidase system and innate immunity: Biochemical actions and physiological roles. Redox Biol. 2020, 34, 101524. [Google Scholar] [CrossRef]
- Kozhakhmetova, M.H.; Akimbekov, N.S.; Tastambek, K.T. Characterization of functional and microbial profile of whey recovered from cottage cheese and cheese manufacturing. Exp. Biol. 2023, 94, 57–68. [Google Scholar] [CrossRef]
- Zhao, Y.; Saxena, J.; Truong, T.; Chandrapala, J. Lactose-Proteins-Minerals in Dairy Systems: A Review. In Milk Proteins-Technological Innovations, Nutrition, Sustainability, and Novel Applications; IntechOpen: London, UK, 2024; p. 75. [Google Scholar] [CrossRef]
- Neves, A.R.; Pool, W.A.; Solopova, A.; Kok, J.; Santos, H.; Kuipers, O.P. Towards enhanced galactose utilization by Lactococcus lactis. Appl. Environ. Microbiol. 2010, 76, 7048–7060. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Gaucher, F.; Cauty, C.; Jardin, J.; Le Loir, Y.; Jeantet, R.; Jan, G. Growth in hyper-concentrated sweet whey triggers multi-stress tolerance and spray drying survival in Lactobacillus casei BL23: From the molecular basis to new perspectives for sustainable probiotic production. Front. Microbiol. 2018, 9, 2548. [Google Scholar] [CrossRef]
- Kondybayev, A.; Konuspayeva, G.; Strub, C.; Loiseau, G.; Mestres, C.; Grabulos, J.; Achir, N. Growth and metabolism of Lacticaseibacillus casei and Lactobacillus kefiri isolated from qymyz, a traditional fermented central asian beverage. Fermentation 2022, 8, 367. [Google Scholar] [CrossRef]
- Zia, H.; Awais, M.; Masood, H.M.; Ali, N. Investigating the Impacts of Various Parameters on Lactic Acid Production; A Review. Korean Chem. Eng. Res. 2024, 62, 281–295. [Google Scholar]
- Alvarez, M.M.; Aguirre-Ezkauriatza, E.J.; Ramírez-Medrano, A.; Rodríguez-Sánchez, Á. Kinetic analysis and mathematical modeling of growth and lactic acid production of Lactobacillus casei var. rhamnosus in milk whey. J. Dairy Sci. 2010, 93, 5552–5560. [Google Scholar] [CrossRef]
- Parmar, R. Incorporation of Acid Whey Powders in Probiotic Yogurt. Master’s Thesis, South Dakota State University, Brookings, SD, USA, 2004. [Google Scholar]
- Wishon, L.M.; Song, D.F.; Ibrahim, S.A. Effect of Metals on Growth and Functionality of Lactobacillus and Bifidobacteria. Milchwissenschaft 2010, 65, 369–372. [Google Scholar]
- Amrane, A. Effect of inorganic phosphate on lactate production by Lactobacillus helveticus grown on supplemented whey permeate. J. Chem. Technol. Biotechnol. 2000, 75, 223–228. [Google Scholar] [CrossRef]
- Bremer, E.; Krämer, R. Responses of microorganisms to osmotic stress. Annu. Rev. Microbiol. 2019, 73, 313–334. [Google Scholar] [CrossRef]
- Scarpellini, E.; Balsiger, L.M.; Maurizi, V.; Rinninella, E.; Gasbarrini, A.; Giostra, N.; Rasetti, C. Zinc and gut microbiota in health and gastrointestinal disease under the COVID-19 suggestion. Biofactors 2022, 48, 294–306. [Google Scholar] [CrossRef]
- Meng, Y.; Liang, Z.; Yi, M.; Tan, Y.; Li, Z.; Du, P.; Liu, L. Enrichment of zinc in Lactobacillus plantarum DNZ-4: Impact on its characteristics, metabolites and antioxidant activity. LWT 2022, 153, 112462. [Google Scholar] [CrossRef]
- Cifric Mujezinovic, S. Effect of Mg, Zn, Ca, and Fe Supplements on Growth, Probiotic Potential, and Biofilm-Forming Capacity of Lactobacillus Bacteria. Bact. Emp. 2022, 5, e501. [Google Scholar] [CrossRef]
- Kong, L.; Xiong, Z.; Song, X.; Xia, Y.; Zhang, H.; Yang, Y.; Ai, L. Enhanced antioxidant activity in Streptococcus thermophilus by high-level expression of superoxide dismutase. Front. Microbiol. 2020, 11, 579804. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.C.; Robinson, A.K.; Rodríguez-Quiñones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [Google Scholar] [CrossRef]
- Kroll, J.; Klinter, S.; Schneider, C.; Voß, I.; Steinbüchel, A. Plasmid addiction systems: Perspectives and applications in biotechnology. Microb. Biotechnol. 2010, 3, 634–657. [Google Scholar] [CrossRef]
- Jauregui-Rincón, J.; Salinas-Miralles, E.; Chávez-Vela, N.; Jiménez-Vargas, M. Glycomacropeptide: Biological Activities and Uses. In Whey-Biological Properties and Alternative Use; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Bleoussi, R.T.; Konfo, C.T.; Tchekessi, C.C.; Sachi, P.A.; Banon, J.S.; Djogbe, A.A.; Innocent, B.Y. Nutritional quality and use of whey in human food for its valorization. World J. Adv. Res. Rev. 2020, 8, 284–293. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. From Metabolism to Vitality: Uncovering Riboflavin’s Importance in Poultry Nutrition. Animals 2023, 13, 3554. [Google Scholar] [CrossRef]
- Pan, X.; Nan, X.; Yang, L.; Jiang, L.; Xiong, B. Thiamine status, metabolism and application in dairy cows: A review. Br. J. Nutr. 2018, 120, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Sathe, R.R.; Paerl, R.W.; Hazra, A.B. Exchange of vitamin B1 and its biosynthesis intermediates shapes the composition of synthetic microbial cocultures and reveals complexities of nutrient sharing. J. Bacteriol. 2022, 204, e00503-21. [Google Scholar] [CrossRef] [PubMed]
- Saulnier, D.M.; Santos, F.; Roos, S.; Mistretta, T.A.; Spinler, J.K.; Molenaar, D.; Versalovic, J. Exploring metabolic pathway reconstruction and genome-wide expression profiling in Lactobacillus reuteri to define functional probiotic features. PLoS ONE 2011, 6, e18783. [Google Scholar] [CrossRef] [PubMed]
- Deptula, P.; Chamlagain, B.; Edelmann, M.; Sangsuwan, P.; Nyman, T.A.; Savijoki, K.; Varmanen, P. Food-like growth conditions support production of active vitamin B12 by Propionibacterium freudenreichii 2067 without DMBI, the lower ligand base, or cobalt supplementation. Front. Microbiol. 2017, 8, 368. [Google Scholar] [CrossRef]
- Kolodkin-Gal, I.; Parsek, M.R.; Patrauchan, M.A. The roles of calcium signaling and calcium deposition in microbial multicellularity. Trends Microbiol. 2023, 31, 1225–1237. [Google Scholar] [CrossRef]
- Walker, G.M. The roles of magnesium in biotechnology. Crit. Rev. Biotechnol. 1994, 14, 311–354. [Google Scholar] [CrossRef]
- Kamau, S.M.; Cheison, S.C.; Chen, W.; Liu, X.M.; Lu, R.R. Alpha-Lactalbumin: Its Production Technologies and Bioactive Peptides. Compr. Rev. Food Sci. Food Saf. 2010, 9, 197–212. [Google Scholar] [CrossRef]
- Locatelli, F.M.; Goo, K.S.; Ulanova, D. Effects of trace metal ions on secondary metabolism and the morphological development of streptomycetes. Metallomics 2016, 8, 469–480. [Google Scholar] [CrossRef]
- Dubey, M.K.; Meena, M.; Aamir, M.; Zehra, A.; Upadhyay, R.S. Regulation and role of metal ions in secondary metabolite production by microorganisms. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 259–277. [Google Scholar]
- Miriyala, S.; Spasojevic, I.; Tovmasyan, A.; Salvemini, D.; Vujaskovic, Z.; Clair, D.S.; Batinic-Haberle, I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 794–814. [Google Scholar] [CrossRef]
- Pescuma, M.; de Valdez, G.F.; Mozzi, F. Whey-derived valuable products obtained by microbial fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 6183–6196. [Google Scholar] [CrossRef]
- Vera-Santander, V.E.; Hernández-Figueroa, R.H.; Arrioja-Bretón, D.; Jiménez-Munguía, M.T.; Mani-López, E.; López-Malo, A. Utilization of Whey for Eco-Friendly Bio-Preservation of Mexican-Style Fresh Cheeses: Antimicrobial Activity of Lactobacillus casei 21/1 Cell-Free Supernatants (CFS). Int. J. Environ. Res. Public Health 2024, 21, 560. [Google Scholar] [CrossRef]
- Neviani, E.; Levante, A.; Gatti, M. The Microbial Community of Natural Whey Starter: Why Is It a Driver for the Production of the Most Famous Italian Long-Ripened Cheeses? Fermentation 2024, 10, 186. [Google Scholar] [CrossRef]
- González-González, F.; Delgado, S.; Ruiz, L.; Margolles, A.; Ruas-Madiedo, P. Functional bacterial cultures for dairy applications: Towards improving safety, quality, nutritional and health benefit aspects. J. Appl. Microbiol. 2022, 133, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Molero, M.S.; Briñez, W.J. Probiotics consumption increment through the use of whey-based fermented beverages. In Probiotics-Current Knowledge and Future Prospects; IntechOpen: London, UK, 2018. [Google Scholar]
- Utama, G.L.; Utba, F.; Cahyana, Y.; Balia, R.L. Mozzarella whey indigenous yeasts and their potential in amino acid and peptide production through fermentation. Syst. Rev. Pharm. 2021, 12, 9. [Google Scholar]
- Mora, R.H.; Macbeth, T.W.; MacHarg, T.; Gundarlahalli, J.; Holbrook, H.; Schiff, P. Enhanced bioremediation using whey powder for a trichloroethene plume in a high-sulfate, fractured granitic aquifer. Remediat. J. 2008, 18, 7–30. [Google Scholar] [CrossRef]
- Estrella, M.J.; Pieckenstain, F.L.; Marina, M.; Diaz, L.E.; Ruiz, O.A. Cheese whey: An alternative growth and protective medium for Rhizobium loti cells. J. Ind. Microbiol. Biotechnol. 2004, 31, 122–126. [Google Scholar] [CrossRef]
- Marasco, R.; Gazzillo, M.; Campolattano, N.; Sacco, M.; Muscariello, L. Isolation and identification of lactic acid bacteria from natural whey cultures of buffalo and cow milk. Foods 2022, 11, 233. [Google Scholar] [CrossRef]
- Pescuma, M.; Hébert, E.M.; Mozzi, F.; de Valdez, G.F. Whey fermentation by thermophilic lactic acid bacteria: Evolution of carbohydrates and protein content. Food Microbiol. 2008, 25, 442–451. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Robles-Porchas, G.R.; Martínez-Porchas, M.; Ramírez-Suárez, J.C.; García-Sifuentes, C.O.; Torres-Llanez, M.J.; Vallejo-Cordoba, B. Bacterial diversity and dynamics during spontaneous cheese whey fermentation at different temperatures. Fermentation 2022, 8, 342. [Google Scholar] [CrossRef]
- Mandal, B. Continuous pediocin production by Pediococcus acidilactici using dairy waste. Asian J. Pharm. Clin. Res. 2023, 16, 80–85. [Google Scholar] [CrossRef]
- Aragón-Rojas, S.; Ruiz-Pardo, R.Y.; Hernández-Sánchez, H.; Quintanilla-Carvajal, M.X. Optimization of the production and stress resistance of the probiotic Lactobacillus fermentum K73 in a submerged bioreactor using a whey-based culture medium. CyTA-J. Food 2018, 16, 1064–1070. [Google Scholar] [CrossRef]
- Jantzen, M.; Göpel, A.; Beermann, C. Direct spray drying and microencapsulation of probiotic Lactobacillus reuteri from slurry fermentation with whey. J. Appl. Microbiol. 2013, 115, 1029–1036. [Google Scholar] [CrossRef]
- Balciunas, E.M.; Al Arni, S.; Converti, A.; Leblanc, J.G.; Oliveira, R.P.S. Production of bacteriocin-like inhibitory substances (BLIS) by Bifidobacterium lactis using whey as a substrate. Int. J. Dairy Technol. 2016, 69, 236–242. [Google Scholar] [CrossRef]
- Orlova, T.N.; Ott, E.F.; Musina, O.N. Perspective of using propionic acid bacteria to produce functional foods based on milk whey. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2021; Volume 2419. [Google Scholar]
- Dumitru, M.; Ciurescu, G. Optimization of the fermentation conditions and survival of Bacillus licheniformis as freeze-dried powder for animal probiotic applications. Sci. Papers Ser. D Anim. Sci. 2023, LXVI, 109–115. [Google Scholar]
- Machado, T.S.; Decesaro, A.; Cappellaro, Â.C.; Machado, B.S.; van Schaik Reginato, K.; Reinehr, C.O.; Colla, L.M. Effects of homemade biosurfactant from Bacillus methylotrophicus on bioremediation efficiency of a clay soil contaminated with diesel oil. Ecotoxicol. Environ. Saf. 2020, 201, 110798. [Google Scholar] [CrossRef] [PubMed]
- Jahanshah, G.; Nahvi, I.; Zarkesh-Esfahani, S.H.; Ghanavati, H.; Khodaverdi, H.; Barani, M. Enhancing compost quality by using whey-grown biosurfactant-producing bacteria as inocula. Ann. Microbiol. 2013, 63, 91–100. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Kitamura, G.; Tian, X.; Suzuki, I.; Kobayashi, T.; Shimizu, T.; Ike, M. Temperature dependence of sequential chlorinated ethenes dechlorination and the dynamics of dechlorinating microorganisms. Chemosphere 2022, 287, 131989. [Google Scholar] [CrossRef]
- Bissonnette, N.; Lalande, R.; Bordeleau, L.M. Large-scale production of Rhizobium meliloti on whey. Appl. Environ. Microbiol. 1986, 52, 838–841. [Google Scholar] [CrossRef]
- Barrett, E.; Stanton, C.; Zelder, O.; Fitzgerald, G.; Ross, R.P. Heterologous expression of lactose-and galactose-utilizing pathways from lactic acid bacteria in Corynebacterium glutamicum for production of lysine in whey. Appl. Environ. Microbiol. 2004, 70, 2861–2866. [Google Scholar] [CrossRef]
- Shen, J.; Chen, J.; Jensen, P.R.; Solem, C. Development of a novel, robust and cost-efficient process for valorizing dairy waste exemplified by ethanol production. Microb. Cell Fact. 2019, 18, 51. [Google Scholar] [CrossRef]
- Herrero, O.M.; Alvarez, H.M. Whey as a renewable source for lipid production by Rhodococcus strains: Physiology and genomics of lactose and galactose utilization. Eur. J. Lipid Sci. Technol. 2016, 118, 262–272. [Google Scholar] [CrossRef]
- Noby, N.; Khattab, S.N.; Soliman, N.A. Sustainable production of bacterioruberin carotenoid and its derivatives from Arthrobacter agilis NP20 on whey-based medium: Optimization and product characterization. Bioresour. Bioprocess. 2023, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhao, G.; Gu, L.; Solem, C. A novel approach for accelerating smear development on bacterial smear-ripened cheeses reduces ripening time and inhibits the growth of Listeria and other unwanted microorganisms on the rind. LWT 2022, 170, 114109. [Google Scholar] [CrossRef]
- Gottardi, D.; Siroli, L.; Braschi, G.; Rossi, S.; Bains, N.; Vannini, L.; Lanciotti, R. Selection of Yarrowia lipolytica strains as possible solution to valorize untreated cheese whey. Fermentation 2023, 9, 51. [Google Scholar] [CrossRef]
- Ohstrom, A.M.; Buck, A.E.; Du, X.; Wee, J. Evaluation of Kluyveromyces spp. for conversion of lactose in different types of whey from dairy processing waste into ethanol. Front. Microbiol. 2023, 14, 1208284. [Google Scholar] [CrossRef]
- Löser, C.; Urit, T.; Stukert, A.; Bley, T. Formation of ethyl acetate from whey by Kluyveromyces marxianus on a pilot scale. J. Biotechnol. 2013, 163, 17–23. [Google Scholar] [CrossRef]
- Dinika, I.; Nurhadi, B.; Masruchin, N.; Balia, R.L.; Utama, G.L. The roles of candida tropicalis toward peptide and amino acid changes in cheese whey fermentation. Int. J. Technol. 2019, 10, 1533. [Google Scholar] [CrossRef]
- Estrada, M.; Navarrete, C.; Møller, S.; Procentese, A.; Martínez, J.L. Utilization of salt-rich by-products from the dairy industry as feedstock for recombinant protein production by Debaryomyces hansenii. Microb. Biotechnol. 2023, 16, 404–417. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J.; Méndez-Zavala, A.; Morales-Oyervides, L.; Montañez, J. Microbial Carotenoid Synthesis Optimization in Goat Cheese Whey Using the Robust Taguchi Method: A Sustainable Approach to Help Tackle Vitamin A Deficiency. Foods 2023, 12, 658. [Google Scholar] [CrossRef]
- Cristiani-Urbina, E.; Ruiz-Ordaz, N.; Galindez-Mayer, J. Differences in the growth kinetic behavior of Torulopsis cremoris in batch and continuous cultures. Biotechnol. Appl. Biochem. 1997, 26, 189–194. [Google Scholar] [CrossRef]
- Bansfield, D.; Spilling, K.; Mikola, A.; Piiparinen, J. Growth of fungi and yeasts in food production waste streams: A feasibility study. BMC Microbiol. 2023, 23, 328. [Google Scholar] [CrossRef]
- Santiesteban-Lopez, N.A.; Ceron-Carrillo, T.G.; Carmona-Silva, J.L.; Chavez-Medina, J. Cultivation of Aspergillus oryzae and Saccharomyces cerevisiae in whey for the production of single-celled protein intended for feeding cattle. Int. J. Food Sci. Biotechnol. 2020, 5, 12–21. [Google Scholar] [CrossRef]
- Crament, T.C.; Arendsen, K.; Rose, S.H.; Jansen, T. Cultivation of recombinant Aspergillus niger strains on dairy whey as a carbohydrate source. J. Ind. Microbiol. Biotechnol. 2024, 51, kuae007. [Google Scholar] [CrossRef]
- Kumura, H.; Ishido, T.; Shimazaki, K. Production and partial purification of proteases from Aspergillus oryzae grown in a medium based on whey protein as an exclusive nitrogen source. J. Dairy Sci. 2011, 94, 657–667. [Google Scholar] [CrossRef]
- Lopes, F.C.; Tichota, D.M.; Sauter, I.P.; Meira, S.M.; Segalin, J.; Rott, M.B.; Brandelli, A. Active metabolites produced by Penicillium chrysogenum IFL1 growing on agro-industrial residues. Ann. Microbiol. 2013, 63, 771–778. [Google Scholar] [CrossRef]
- Kumura, H.; Satoh, M.; Machiya, T.; Hosono, M.; Hayakawa, T.; Wakamatsu, J.I. Lipase and protease production of dairy Penicillium sp. on milk-protein-based solid substrates. Int. J. Dairy Technol. 2019, 72, 403–408. [Google Scholar] [CrossRef]
- Basto, B.; da Silva, N.R.; Teixeira, J.A.; Silvério, S.C. Production of natural pigments by Penicillium brevicompactum using agro-industrial byproducts. Fermentation 2022, 8, 536. [Google Scholar] [CrossRef]
- Subash, N.; Viji, J.; Sasikumar, C.; Meenakshisundaram, M. Isolation, Media Optimization and Formulation of Trichoderma harizanum in Agricultural Soil. J. Microbiol. Biotechnol. Res. 2013, 3, 61–64. [Google Scholar]
- Fan, X.; Rivera Flores, V.K.; DeMarsh, T.A.; Deriancho, D.L.; Alcaine, S.D. Aerobic cultivation of mucor species enables the deacidification of yogurt acid whey and the production of fungal oil. Foods 2023, 12, 1784. [Google Scholar] [CrossRef] [PubMed]
- Akpinar-Bayizit, A.; Ozcan, T.; Yilmaz-Ersan, L.; Basoglu, F. Single cell oil (SCO) production by Fusarium species using cheese whey as a substrate. Mljekarstvo 2014, 64, 111–118. [Google Scholar]
- Asunis, F.; De Gioannis, G.; Dessì, P.; Isipato, M.; Lens, P.N.; Muntoni, A.; Spiga, D. The dairy biorefinery: Integrating treatment processes for cheese whey valorisation. J. Environ. Manag. 2020, 276, 111240. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, J.; Liu, S. Advanced fermentation techniques for lactic acid production from agricultural waste. Fermentation 2023, 9, 765. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Shang, N.; Li, P. Microbial fermentation processes of lactic acid: Challenges, solutions, and future prospects. Foods 2023, 12, 2311. [Google Scholar] [CrossRef]
- Miller, A.L.; Renye Jr, J.A.; Oest, A.M.; Liang, C.; Garcia, R.A.; Plumier, B.M.; Tomasula, P.M. Bacteriocin production by lactic acid bacteria using ice cream co-product as the fermentation substrate. J. Dairy Sci. 2024, 107, 3468–3477. [Google Scholar] [CrossRef]
- Saubenova, M.; Oleinikova, Y.; Rapoport, A.; Maksimovich, S.; Yermekbay, Z.; Khamedova, E. Bioactive peptides derived from whey proteins for health and functional beverages. Fermentation 2024, 10, 359. [Google Scholar] [CrossRef]
- Wojtyniak, B.; Kołodziejczyk, J.; Szaniawska, D. Production of lactic acid by ultrafiltration of fermented whey obtained in bioreactor equipped with ZOSS membrane. Chem. Eng. J. 2016, 305, 28–36. [Google Scholar] [CrossRef]
- Malvido, M.C.; González, E.A.; Bazán Tantaleán, D.L.; Bendaña Jácome, R.J.; Guerra, N.P. Batch and fed-batch production of probiotic biomass and nisin in nutrient-supplemented whey media. Braz. J. Microbiol. 2019, 50, 915–925. [Google Scholar] [CrossRef]
- Chen, M.J.; Liu, J.R.; Sheu, J.F.; Lin, C.W.; Chuang, C.L. Study on skin care properties of milk kefir whey. Asian-Australas. J. Anim. Sci. 2006, 19, 905–908. [Google Scholar] [CrossRef]
- Hamdy, A.A.; Elattal, N.A.; Amin, M.A.; Ali, A.E.; Mansour, N.M.; Awad, G.E.; Farrag, A.R.H.; Esawy, M.A. In Vivo Assessment of possible probiotic properties of Bacillus subtilis and prebiotic properties of levan. Biocatal. Agric. Biotechnol. 2018, 13, 190–197. [Google Scholar] [CrossRef]
- Pan, L.; Han, Y.; Zhou, Z. In vitro prebiotic activities of exopolysaccharide from Leuconostoc pseudomesenteroides XG5 and its effect on the gut microbiota of mice. J. Funct. Foods 2020, 67, 103853. [Google Scholar] [CrossRef]
- Martínez-Burgos, W.J.; Ocán-Torres, D.Y.; Manzoki, M.C.; Scapini, T.; de Mello, A.F.M.; Pozzan, R.; Soccol, C.R. New trends in microbial gums production, patented technologies and applications in food industry. Discov. Food 2024, 4, 49. [Google Scholar] [CrossRef]
- Adesulu-Dahunsi, A.T.; Sanni, A.I.; Jeyaram, K. Production, characterization and in vitro antioxidant activities of exopolysaccharide from Weissella cibaria GA44. LWT 2018, 87, 432–442. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, L.; Jia, K.; Zhan, H.; Zhang, Z.; Shah, N.P.; Tao, X.; Wei, H. Sulfonation of Lactobacillus plantarum WLPL04 exopolysaccharide amplifies its antioxidant activities in vitro and in a Caco-2 cell model. J. Dairy Sci. 2019, 102, 5922–5932. [Google Scholar] [CrossRef]
- Merghni, A.; Dallel, I.; Noumi, E.; Kadmi, Y.; Hentati, H.; Tobji, S.; Amor, A.B.; Mastouri, M. Antioxidant and antiproliferative potential of biosurfactants isolated from Lactobacillus casei and their anti-biofilm effect in oral Staphylococcus aureus strains. Microb. Pathog. 2017, 104, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014, 63, 133–139. [Google Scholar] [CrossRef]
- Benmechernene, Z.; Fernandez-No, I.; Kihal, M.; Bohme, K.; Calo-Mata, P.; Barros-Velazquez, J. Recent patents on bacteriocins: Food and biomedical applications. Recent Pat. DNA Gene Seq. 2013, 7, 66–73. [Google Scholar] [CrossRef]
- Jeevaratnam, K.; Jamuna, M.; Bawa, A.S. Biological Preservation of Foods–Bacteriocins of Lactic Acid Bacteria. Indian J. Biotechnol. 2005, 4. [Google Scholar]
- Makhal, S.; Kanawjia, S.K.; Giri, A. Effect of microGARD on keeping quality of direct acidified Cottage cheese. J. Food Sci. Technol. 2015, 52, 936–943. [Google Scholar] [CrossRef]
- Saucier, L.; Champagne, C.P. Immobilised-cell technology and meat processing. In Applications of Cell Immobilisation Biotechnology; Springer: Dordrecht, The Netherlands, 2005; pp. 337–353. [Google Scholar]
- Chikindas, M.L. Safety, formulation, and in vitro antiviral activity of the antimicrobial peptide subtilosin against herpes simplex virus type 1. Probiotics Antimicrob. Proteins 2013, 5, 26–35. [Google Scholar]
- Olvera-Rosales, L.B.; Cruz-Guerrero, A.E.; García-Garibay, J.M.; Gómez-Ruíz, L.C.; Contreras-López, E.; Guzmán-Rodríguez, F.; González-Olivares, L.G. Bioactive peptides of whey: Obtaining, activity, mechanism of action, and further applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 10351–10381. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; del Mar Contreras, M.; Recio, I. Antihypertensive peptides: Production, bioavailability, and incorporation into foods. Adv. Colloid Interface Sci. 2011, 165, 23–35. [Google Scholar] [CrossRef]
- Chopada, K.; Basaiawmoit, B.; Sakure, A.A.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Hati, S. Purification and characterization of novel antihypertensive and antioxidative peptides from whey protein fermentate: In vitro, in silico, and molecular interactions studies. J. Am. Nutr. Assoc. 2023, 42, 598–617. [Google Scholar] [CrossRef] [PubMed]
- Prieto, A.; Guadix, M.; Guadix, A. Recent patents on whey protein hydrolysates manufactured by proteolysis coupled to membrane ultrafiltration. Recent Pat. Chem. Eng. 2010, 3, 115–128. [Google Scholar] [CrossRef]
- Peslerbes, M.; Fellenberg, A.; Jardin, J.; Deglaire, A.; Ibáñez, R.A. Manufacture of whey protein hydrolysates using plant enzymes: Effect of processing conditions and simulated gastrointestinal digestion on angiotensin-I-converting enzyme (ACE) inhibitory activity. Foods 2022, 11, 2429. [Google Scholar] [CrossRef]
- Millan, G.C.L.; Veras, F.F.; Stincone, P.; Pailliè-Jiménez, M.E.; Brandelli, A. Biological activities of whey protein hydrolysate produced by protease from the Antarctic bacterium Lysobacter sp. A03. Biocatal. Agric. Biotechnol. 2022, 43, 102415. [Google Scholar] [CrossRef]
- Fashogbon, R.O.; Samson, O.J.; Awotundun, T.A.; Olanbiwoninu, A.A.; Adebayo-Tayo, B.C. Microbial gamma-aminobutyric acid synthesis: A promising approach for functional food and pharmaceutical applications. Lett. Appl. Microbiol. 2024, 77, ovae122. [Google Scholar] [CrossRef]
- Icer, M.A.; Sarikaya, B.; Kocyigit, E.; Atabilen, B.; Çelik, M.N.; Capasso, R.; Budán, F. Contributions of gamma-aminobutyric acid (GABA) produced by lactic acid bacteria on food quality and human health: Current applications and future prospects. Foods 2024, 13, 2437. [Google Scholar] [CrossRef]
- Esin, R.G.; Esin, O.R.; Khakimova, A.R. Effective neuroprotection and organ protection: Activation of the endogenous mechanisms of sanogenesis. Neurol. Neuropsychiatry Psychosom. 2020, 12, 123–127. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, L.; Kang, Y.; Yang, G.; Zhao, Z.; Zhao, Y.; Li, S. Non-targeted metabolomics analysis reveals metabolite profiles change during whey fermentation with Kluyveromyces marxianus. Metabolites 2024, 14, 694. [Google Scholar] [CrossRef]
- Gómez, G.A.; Cuffia, F.; Nagel, O.G.; Althaus, R.L.; Ceruti, R.J. Fermentation of whey-derived matrices by Kluyveromyces marxianus: Alcoholic beverage development from whey and fruit juice mixes. J. Dairy Res. 2024, 91, 108–115. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Psarianos, C.; Koutinas, A.A.; Kanellaki, M.; Banat, I.M.; Marchant, R. Continuous whey fermentation using kefir yeast immobilized on delignified cellulosic material. J. Agric. Food Chem. 2002, 50, 2543–2547. [Google Scholar] [CrossRef]
- Risner, D.; Tomasino, E.; Hughes, P.; Meunier-Goddik, L. Volatile aroma composition of distillates produced from fermented sweet and acid whey. J. Dairy Sci. 2019, 102, 202–210. [Google Scholar] [CrossRef]
- Marcus, J.F.; DeMarsh, T.A.; Alcaine, S.D. Upcycling of whey permeate through yeast-and mold-driven fermentations under anoxic and oxic conditions. Fermentation 2021, 7, 16. [Google Scholar] [CrossRef]
- Gerós, H.; Cássio, F.; Leão, C. Utilization and transport of acetic acid in Dekkera anomala and their implications on the survival of the yeast in acidic environments. J. Food Prot. 2000, 63, 101–106. [Google Scholar] [CrossRef]
- Carpenter, C.E.; Broadbent, J.R. External concentration of organic acid anions and pH: Key independent variables for studying how organic acids inhibit growth of bacteria in mildly acidic foods. J. Food Sci. 2009, 74, R12–R15. [Google Scholar] [CrossRef]
- Mani-López, E.; García, H.S.; López-Malo, A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- Park, S.H.; Choi, M.R.; Park, J.W.; Park, K.H.; Chung, M.S.; Ryu, S.; Kang, D.H. Use of organic acids to inactivate Escherichia coli O157: H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh apples and lettuce. J. Food Sci. 2011, 76, M293–M298. [Google Scholar] [CrossRef]
- Abdelhamid, A.G.; Campbell, E.P.; Hawkins, Z.; Yousef, A.E. Efficient production of broad-spectrum antimicrobials by Paenibacillus polymyxa OSY–EC using acid whey-based medium and novel antimicrobial concentration approach. Front. Bioeng. Biotechnol. 2022, 10, 869778. [Google Scholar] [CrossRef]
- Urit, T.; Stukert, A.; Bley, T.; Löser, C. Formation of ethyl acetate by Kluyveromyces marxianus on whey during aerobic batch cultivation at specific trace element limitation. Appl. Microbiol. Biotechnol. 2012, 96, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Poblete-Castro, I.; Wittmann, C.; Nikel, P.I. Biochemistry, genetics and biotechnology of glycerol utilization in Pseudomonas species. Microb. Biotechnol. 2020, 13, 32–53. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Tan, G.Y.A.; Ge, L.; Chen, C.L.; Wang, J.Y. Two-stage microbial conversion of crude glycerol to 1, 3-propanediol and polyhydroxyalkanoates after pretreatment. J. Environ. Manag. 2019, 232, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Biadała, A.; Szablewski, T.; Lasik-Kurdyś, M.; Cegielska-Radziejewska, R. Antimicrobial activity of goat’s milk fermented by single strain of kefir grain microflora. Eur. Food Res. Technol. 2020, 246, 1231–1239. [Google Scholar] [CrossRef]
- Wee, Y.J.; Kim, J.N.; Ryu, H.W. Biotechnological production of lactic acid and its recent applications. Food Technol. Biotechnol. 2006, 44, 163–172. [Google Scholar]
- Bezirci, E.; Taşpınar-Demir, H.; Turanlı-Yıldız, B.; Erdem, A.; Alemdar, F.; Türker, M. Propionic acid production via two-step sequential repeated batch fermentations on whey and flour. Biochem. Eng. J. 2023, 192, 108816. [Google Scholar] [CrossRef]
- Vidra, A.; Németh, Á. Whey utilization in a two-stage fermentation process. Waste Treat. Recovery 2017, 2, 17–20. [Google Scholar] [CrossRef]
- Staniszewski, M.; Kujawski, M. The Dynamics of Vitamin B12 Synthesis by Some Strains of Propionic Fermentation Bacteria. Milchwiss.-Milk Sci. Int. 2007, 62, 428–430. [Google Scholar]
- Lind, H.; Jonsson, H.; Schnürer, J. Antifungal effect of dairy propionibacteria—Contribution of organic acids. Int. J. Food Microbiol. 2005, 98, 157–165. [Google Scholar] [CrossRef]
- Chimirri, F.; Bosco, F.; Ceccarelli, R.; Venturello, A.; Geobaldo, F. Succinic acid and its derivatives: Fermentative production using sustainable industrial agro-food by-products and its applications in the food industry. Ital. J. Food Sci. 2010, 22, 2. [Google Scholar]
- Pirozzi, D.; Fagnano, M.; Fiorentino, N.; Toscano, G.; Rugari, F.; Sannino, F.; Florio, C. Biotechnological synthesis of succinic acid by Actinobacillus succinogenes by exploitation of lignocellulosic biomass. Chem. Eng. Trans. 2017, 57, 1741–1746. [Google Scholar]
- Tripathi, A.; Pandey, V.K.; Panesar, P.S.; Taufeeq, A.; Mishra, H.; Rustagi, S.; Shaikh, A.M. Fermentative production of vitamin B12 by Propionibacterium shermanii and Pseudomonas denitrificans and its promising health benefits: A review. Food Sci. Nutr. 2024, 12, 8675–8691. [Google Scholar] [CrossRef]
- Massoud, R.; Khosravi-Darani, K.; Golshahi, M.; Sohrabvandi, S.; Mortazavian, A.M. Assessment of process variables on vitamin B12 production in fermented dairy product including propionic acid. Curr. Nutr. Food Sci. 2020, 16, 155–161. [Google Scholar] [CrossRef]
- Calvillo, Á.; Pellicer, T.; Carnicer, M.; Planas, A. Bioprocess strategies for vitamin B12 production by microbial fermentation and its market applications. Bioengineering 2022, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Zigova, J.; Šturdík, E. Advances in biotechnological production of butyric acid. J. Ind. Microbiol. Biotechnol. 2000, 24, 153–160. [Google Scholar] [CrossRef]
- Girotto, F.; Lavagnolo, M.C.; Pivato, A.; Cossu, R. Acidogenic fermentation of the organic fraction of municipal solid waste and cheese whey for bio-plastic precursors recovery–Effects of process conditions during batch tests. Waste Manag. 2017, 70, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Asunis, F.; De Gioannis, G.; Isipato, M.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. Control of fermentation duration and pH to orient biochemicals and biofuels production from cheese whey. Bioresour. Technol. 2019, 289, 121722. [Google Scholar] [CrossRef]
- Raganati, F.; Olivieri, G.; Procentese, A.; Russo, M.E.; Salatino, P.; Marzocchella, A. Butanol production by bioconversion of cheese whey in a continuous packed bed reactor. Bioresour. Technol. 2013, 138, 259–265. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Umar, M.; Hassan, F.U.; Alagawany, M.; Arif, M.; Taha, A.E.; Abd El-Hack, M.E. Applications of butyric acid in poultry production: The dynamics of gut health, performance, nutrient utilization, egg quality, and osteoporosis. Anim. Health Res. Rev. 2022, 23, 136–146. [Google Scholar] [CrossRef]
- Jiang, L.; Fu, H.; Yang, H.K.; Xu, W.; Wang, J.; Yang, S.T. Butyric acid: Applications and recent advances in its bioproduction. Biotechnol. Adv. 2018, 36, 2101–2117. [Google Scholar] [CrossRef]
- Ventura, I.; Chomon-García, M.; Tomás-Aguirre, F.; Palau-Ferré, A.; Legidos-García, M.E.; Murillo-Llorente, M.T.; Pérez-Bermejo, M. Therapeutic and immunologic effects of short-chain fatty acids in inflammatory bowel disease: A systematic review. Int. J. Mol. Sci. 2024, 25, 10879. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Hermansen, K. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- Li, H.B.; Xu, M.L.; Xu, X.D.; Tang, Y.Y.; Jiang, H.L.; Li, L.; Yang, T. Faecalibacterium prausnitzii attenuates CKD via butyrate-renal GPR43 axis. Circ. Res. 2022, 131, e120–e134. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Agate, S.; Velev, O.D.; Lucia, L.; Pal, L. A critical review of the performance and soil biodegradability profiles of biobased natural and chemically synthesized polymers in industrial applications. Environ. Sci. Technol. 2022, 56, 2071–2095. [Google Scholar] [CrossRef] [PubMed]
- Litti, Y.V.; Potekhina, M.A.; Zhuravleva, E.A.; Vishnyakova, A.V.; Gruzdev, D.S.; Kovalev, A.A.; Parshina, S.N. Dark fermentative hydrogen production from simple sugars and various wastewaters by a newly isolated Thermoanaerobacterium thermosaccharolyticum SP-H2. Int. J. Hydrogen Energy 2022, 47, 24310–24327. [Google Scholar] [CrossRef]
- Saygin, D.; Blanco, H.; Boshell, F.; Cordonnier, J.; Rouwenhorst, K.; Lathwal, P.; Gielen, D. Ammonia production from clean hydrogen and the implications for global natural gas demand. Sustainability 2023, 15, 1623. [Google Scholar] [CrossRef]
- Shah, N.; Wei, M.; Letschert, V.; Phadke, A. Benefits of Energy Efficient and Low-Global Warming Potential Refrigerant Cooling Equipment; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2019.
- Singh, H.; Poudel, M.R.; Dunn, B.L.; Fontanier, C.; Kakani, G. Effect of greenhouse CO2 supplementation on yield and mineral element concentrations of leafy greens grown using nutrient film technique. Agronomy 2020, 10, 323. [Google Scholar] [CrossRef]
- Rinne, F.; Minor, B.; Salem, K. Experimental study of R-134a alternative in a supermarket refrigeration system. ASHRAE Trans. 2011, 117, 124–132. [Google Scholar]
- Adebayo, V.; Abid, M.; Adedeji, M.; Dagbasi, M.; Bamisile, O. Comparative thermodynamic performance analysis of a cascade refrigeration system with new refrigerants paired with CO2. Appl. Therm. Eng. 2021, 184, 116286. [Google Scholar] [CrossRef]
- Stratford, M.; Nebe-von-Caron, G.; Steels, H.; Novodvorska, M.; Ueckert, J.; Archer, D.B. Weak-acid preservatives: pH and proton movements in the yeast Saccharomyces cerevisiae. Int. J. Food Microbiol. 2013, 161, 164–171. [Google Scholar] [CrossRef]
- Fotouh, Y.O. Controlling grey and blue mould diseases of apple fruits using acetic acid vapours. Arch. Phytopathol. Plant Protect. 2009, 42, 777–782. [Google Scholar] [CrossRef]
- Hassenberg, K.; Geyer, M.; Herppich, W.B. Effect of acetic acid vapour on the natural microflora and Botrytis cinerea of strawberries. Eur. J. Hortic. Sci. 2010, 75, 141. [Google Scholar] [CrossRef]
- Nayak, J.; Chakrabortty, S. Production of acetic acid and whey protein from cheese whey in a hybrid reactor under response surface optimized conditions. Fine Chem. Eng. 2023, 4, 58–73. [Google Scholar] [CrossRef]
- Lustrato, G.; Salimei, E.; Alfano, G.; Belli, C.; Fantuz, F.; Grazia, L.; Ranalli, G. Cheese whey recycling in traditional dairy food chain: Effects of vinegar from whey in dairy cow nutrition. Acetic Acid Bact. 2013, 2, e8. [Google Scholar] [CrossRef]
- Madhusudhan, V.L. Efficacy of 1% acetic acid in the treatment of chronic wounds infected with Pseudomonas aeruginosa: Prospective randomised controlled clinical trial. Int. Wound J. 2016, 13, 1129–1136. [Google Scholar] [CrossRef]
- Egan, J.; Salmon, S. Strategies and progress in synthetic textile fiber biodegradability. SN Appl. Sci. 2022, 4, 22. [Google Scholar] [CrossRef]
- Derwin, R.; Patton, D.; Avsar, P.; Strapp, H.; Moore, Z. The impact of topical agents and dressing on pH and temperature on wound healing: A systematic, narrative review. Int. Wound J. 2022, 19, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Savarino, E.V. Short-chain fatty acids and human health: From metabolic pathways to current therapeutic implications. Life 2024, 14, 559. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Verbeke, K. Short-chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Adhikari, D.; Mukai, M.; Kubota, K.; Kai, T.; Kaneko, N.; Araki, K.S.; Kubo, M. Degradation of bioplastics in soil and their degradation effects on environmental microorganisms. J. Agric. Chem. Environ. 2016, 5, 23–34. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. Zootechnol. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Mélo, E.D.D.; Almeida, R.D.; Pessoa, J.M.; França, K.B.; de Gusmão, T.A.S.; de Gusmão, R.P.; Nascimento, A.P.S. Effect of Commercial Bioprotective Lactic Cultures on Physicochemical, Microbiological, and Textural Properties of Yogurt. Fermentation 2024, 10, 585. [Google Scholar] [CrossRef]
- Gecse, G.; Vente, A.; Kilstrup, M.; Becker, P.; Johanson, T. Impact of elevated levels of dissolved CO2 on performance and proteome response of an industrial 2′-fucosyllactose producing Escherichia coli strain. Microorganisms 2022, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- Herselman, J.; Bradfield, M.F.; Vijayan, U.; Nicol, W. The effect of carbon dioxide availability on succinic acid production with biofilms of Actinobacillus succinogenes. Biochem. Eng. J. 2017, 117, 218–225. [Google Scholar] [CrossRef]
- Guadalupe-Daqui, M.; Goodrich-Schneider, R.M.; Sarnoski, P.J.; Carriglio, J.C.; Sims, C.A.; Pearson, B.J.; MacIntosh, A.J. The effect of CO2 concentration on yeast fermentation: Rates, metabolic products, and yeast stress indicators. J. Ind. Microbiol. Biotechnol. 2023, 50, kuad001. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kumar, A.; Choudhary, A.; Sharma, S.; Choudhary, D.R.; Mehta, S. Effect of elevated CO2 on plant growth, active constituents, and production. In Plants and Their Interaction to Environmental Pollution; Elsevier: Amsterdam, The Netherlands, 2023; pp. 61–77. [Google Scholar]
- Zikmanis, P.; Kolesovs, S.; Semjonovs, P. Production of biodegradable microbial polymers from whey. Bioresour. Bioprocess. 2020, 7, 36. [Google Scholar] [CrossRef]
- Lagoa-Costa, B.; Kennes, C.; Veiga, M.C. Exploiting cheese whey for efficient selection of polyhydroxyalkanoates-storing bacteria. Fermentation 2023, 9, 574. [Google Scholar] [CrossRef]
- Duque, A.F.; Oliveira, C.S.S.; Carmo, I.T.D.; Gouveia, A.R.; Pardelha, F.; Ramos, A.M.; Reis, M.A.M. Response of a three-stage process for PHA production by mixed microbial cultures to feedstock shift: Impact on polymer composition. New Biotechnol. 2014, 31, 276–288. [Google Scholar] [CrossRef]
- Xia, J.; He, J.; Xu, J.; Liu, X.; Qiu, Z.; Xu, N.; Su, L. Direct conversion of cheese whey to polymalic acid by mixed culture of Aureobasidium pullulans and permeabilized Kluyveromyces marxianus. Bioresour. Technol. 2021, 337, 125443. [Google Scholar] [CrossRef]
- Miu, D.M.; Eremia, M.C.; Moscovici, M. Polyhydroxyalkanoates (PHAs) as biomaterials in tissue engineering: Production, isolation, characterization. Materials 2022, 15, 1410. [Google Scholar] [CrossRef]
- Lim, J.; You, M.; Li, J.; Li, Z. Emerging bone tissue engineering via Polyhydroxyalkanoate (PHA)-based scaffolds. Mater. Sci. Eng. C 2017, 79, 917–929. [Google Scholar] [CrossRef]
- Rathbone, S.; Furrer, P.; Lübben, J.; Zinn, M.; Cartmell, S. Biocompatibility of polyhydroxyalkanoate as a potential material for ligament and tendon scaffold material. J. Biomed. Mater. Res. A 2010, 93, 1391–1403. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.; Liu, Y.; Zhang, X.; Guo, J.; Wang, J. Biohydrogen production from anaerobic digestion and its potential as renewable energy. Renew. Energy 2018, 129, 754–768. [Google Scholar] [CrossRef]
- Chatzipaschali, A.A.; Stamatis, A.G. Biotechnological utilization with a focus on anaerobic treatment of cheese whey: Current status and prospects. Energies 2012, 5, 3492–3525. [Google Scholar] [CrossRef]
- Yang, S.T.; Silva, E.M. Novel products and new technologies for use of a familiar carbohydrate, milk lactose. J. Dairy Sci. 1995, 78, 2541–2562. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mendoza, A.P.; Hernández-García, H.; Cocotle-Ronzón, Y.; Hernandez-Martinez, E. Methanogenesis of raw cheese whey: pH and substrate–inoculum ratio evaluation at mesophyll temperature range. J. Chem. Technol. Biotechnol. 2020, 95, 1946–1952. [Google Scholar] [CrossRef]
- Stamatelatou, K.; Antonopoulou, G.; Tremouli, A.; Lyberatos, G. Production of gaseous biofuels and electricity from cheese whey. Ind. Eng. Chem. Res. 2011, 50, 639–644. [Google Scholar] [CrossRef]
- Dannehl, D.; Kläring, H.P.; Schmidt, U. Light-mediated reduction in photosynthesis in closed greenhouses can be compensated for by CO2 enrichment in tomato production. Plants 2021, 10, 2808. [Google Scholar] [CrossRef]
- Szacherska, K.; Oleskowicz-Popiel, P.; Ciesielski, S.; Mozejko-Ciesielska, J. Volatile fatty acids as carbon sources for polyhydroxyalkanoates production. Polymers 2021, 13, 321. [Google Scholar] [CrossRef]
- Aremu, M.O.; Ishola, M.M.; Taherzadeh, M.J. Polyhydroxyalkanoates (PHAs) production from volatile fatty acids (VFAs) from organic wastes by Pseudomonas oleovorans. Fermentation 2021, 7, 287. [Google Scholar] [CrossRef]
- Vu, D.H.; Wainaina, S.; Taherzadeh, M.J.; Åkesson, D.; Ferreira, J.A. Production of polyhydroxyalkanoates (PHAs) by Bacillus megaterium using food waste acidogenic fermentation-derived volatile fatty acids. Bioengineered 2021, 12, 2480–2498. [Google Scholar] [CrossRef]
- Koukoumaki, D.I.; Papanikolaou, S.; Ioannou, Z.; Mourtzinos, I.; Sarris, D. Single-cell protein and ethanol production of a newly isolated Kluyveromyces marxianus strain through cheese whey valorization. Foods 2024, 13, 1892. [Google Scholar] [CrossRef]
- Somaye, F.; Marzieh, M.N.; Lale, N. Single Cell Protein (SCP) production from UF cheese whey by Kluyveromyces marxianus. In Proceedings of the 18th National Congress on Food Technology, Tehran, Iran, 15 October 2008. [Google Scholar]
- Yadav, J.S.S.; Bezawada, J.; Ajila, C.M.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Mixed culture of Kluyveromyces marxianus and Candida krusei for single-cell protein production and organic load removal from whey. Bioresour. Technol. 2014, 164, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Bratosin, B.C.; Darjan, S.; Vodnar, D.C. Single cell protein: A potential substitute in human and animal nutrition. Sustainability 2021, 13, 9284. [Google Scholar] [CrossRef]
- Molfetta, M.; Morais, E.G.; Barreira, L.; Bruno, G.L.; Porcelli, F.; Dugat-Bony, E.; Minervini, F. Protein sources alternative to meat: State of the art and involvement of fermentation. Foods 2022, 11, 2065. [Google Scholar] [CrossRef] [PubMed]
- Muniz, E.D.N.; Montenegro, R.T.D.Q.; da Silva, D.N.; D’Almeida, A.P.; Gonçalves, L.R.B.; de Albuquerque, T.L. Advances in biotechnological strategies for sustainable production of non-animal proteins: Challenges, innovations, and applications. Fermentation 2024, 10, 638. [Google Scholar] [CrossRef]
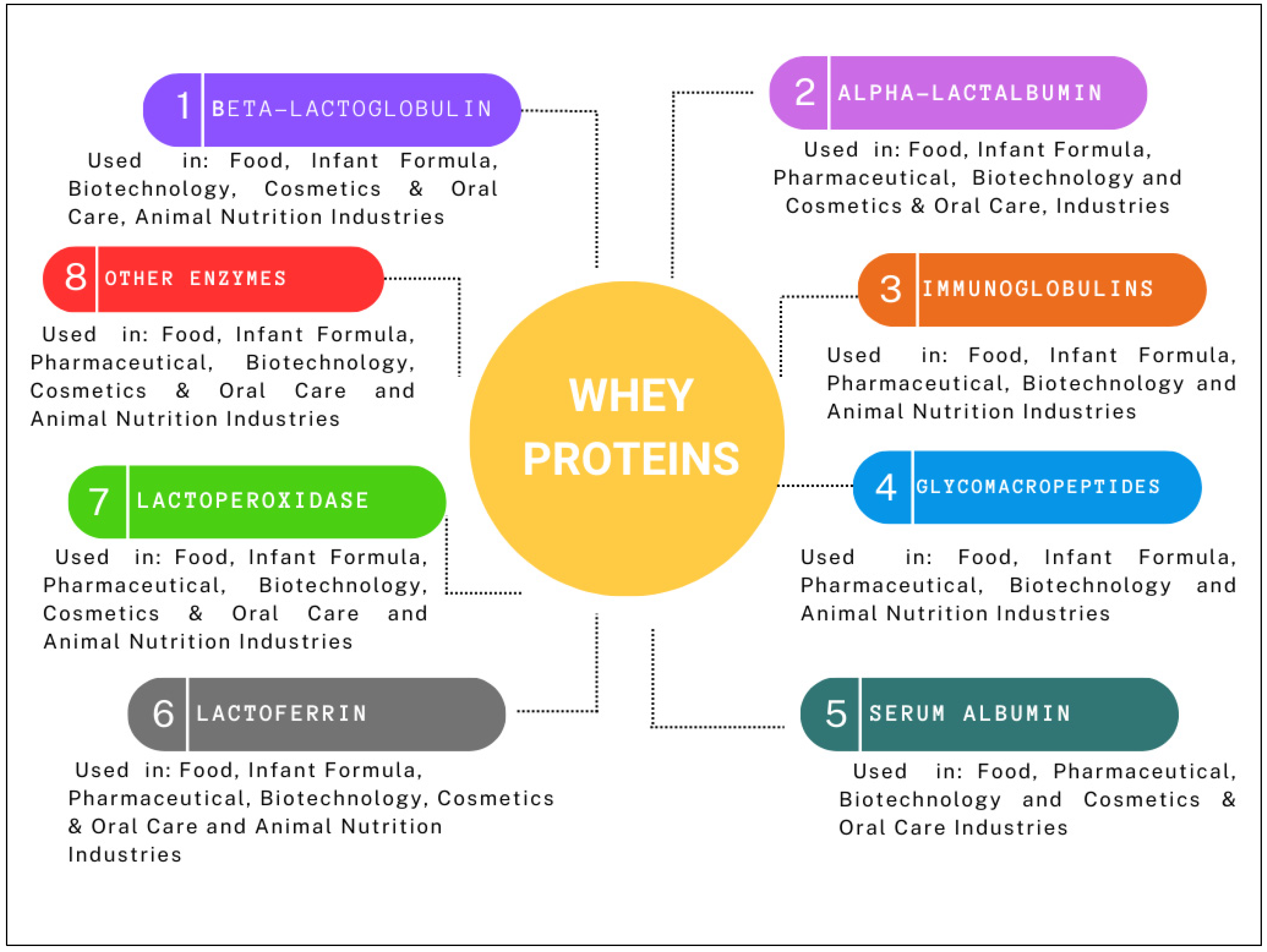
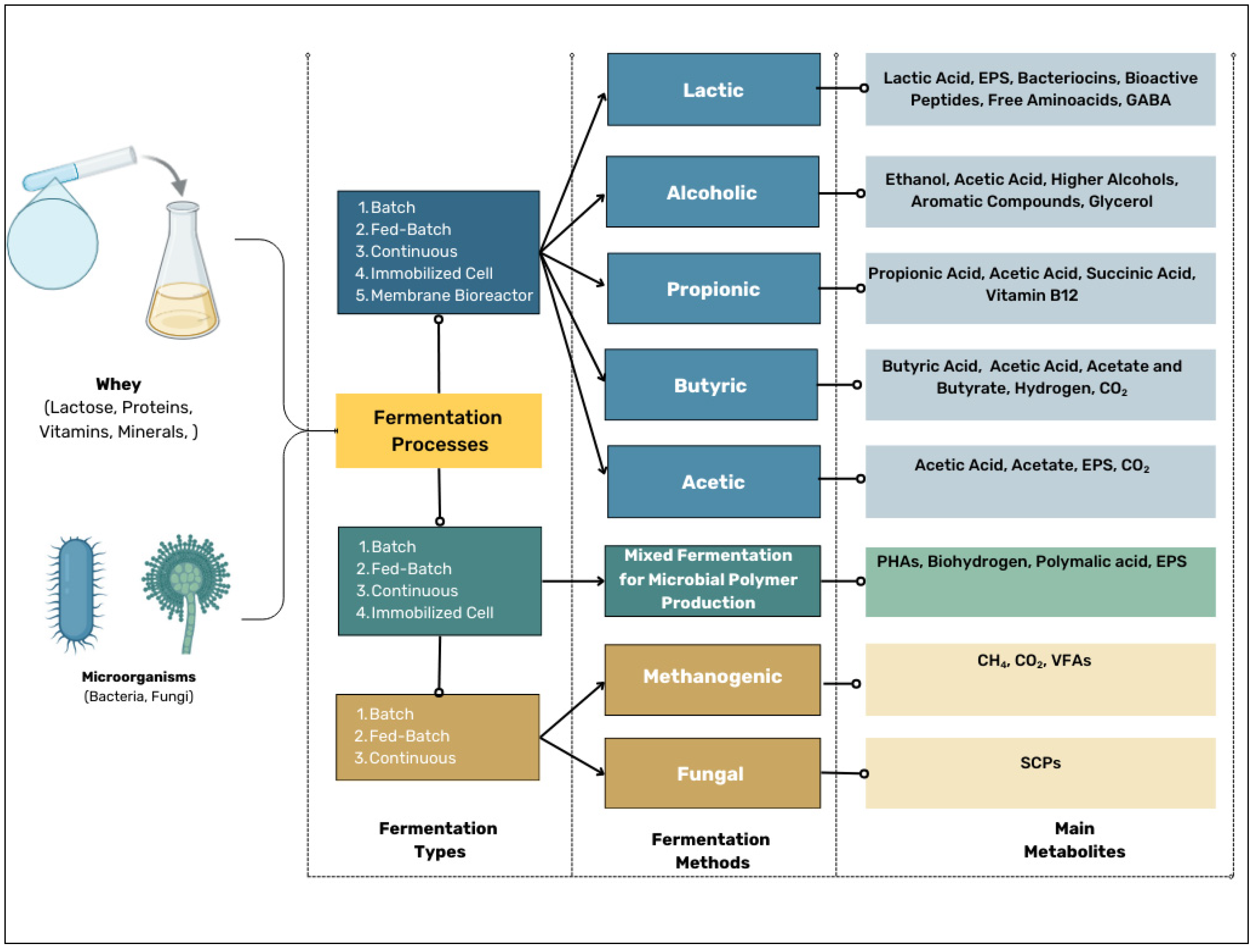
| Categories of Functional Microorganisms/Genus | Species Cultivated on Whey as Medium | Main Metabolic Activity | Optimal Temperature Range | Advantages | References |
|---|---|---|---|---|---|
| LABs Lactobacillus Streptococcus Lactiplantibacillus Limosilactobacillus Lacticaseibacillus Lactococcus Enterococcus Leuconostoc | Lactobacillus fermentum Lactobacillus delbrueckii Lactobacillus helveticus Streptococcus thermophilus Lactococcus lactis | Lactose fermentation, proteolysis, production of EPS and other bioactive metabolites | 37–42 °C | - Tolerant to acidic conditions; found in spontaneous whey cultures - Strong proteolytic activity; synergistic with S. thermophilus for bioactive peptide production - High proteolytic activity; produces antihypertensive and antioxidant peptides; isolated from natural whey cultures - Synergistic with L. delbrueckii in mixed fermentations - Used in food-grade fermentations; adapts well to whey | [13,35,100,101,102] |
| Probiotic bacteria Lactobacillus, Lactiplantibacillus, Limosilactobacillus, Lacticaseibacillus, Lactococcus, Enterococcus Bifidobacterium Propionibacterium Bacillus Pediococcus Weissella | Lactobacillus acidophilus Lactobacillus fermentum Lactobacillus helveticus Lactobacillus delbrueckii subsp. bulgaricus Lactiplantibacillus plantarum Limosilactobacillus reuteri Lacticaseibacillus casei Lacticaseibacillus rhamnosus Lactococcus lactis Enterococcus faecium Bifidobacterium animalis subsp. lactis Propionibacterium freudenreichii Bacillus subtilis Pediococcus acidilactici | Lactose fermentation, proteolysis, pH regulation and acidification of the gut environment, production of bioactive metabolites Fermentation of lactose and oligosaccharides, production of SCFAs, vitamin production Fermentation of lactose and lactate, production of propionic acid, production of vitamin B12 Production of extracellular enzymes, proteolysis Fermentation of carbohydrates, production of bacteriocins | 37–42 °C 37–45 °C around 30 °C 30–39 °C 30–37 °C | - Commonly used in mixed fermentations with other LABs to enhance the functional value of dairy products - Tolerant to acid and osmotic stress; enhanced growth and metabolic activity in whey-based optimized media - Strong proteolytic activity; produces antihypertensive and antioxidant peptides; frequently isolated from natural whey cultures - Thermophilic strain with synergistic action with Streptococcus thermophilus - Highly versatile in whey fermentation; prebiotic effect, antioxidant, and cholesterol-lowering properties - Demonstrates stability after spray-drying and microencapsulation; suitable for development of shelf-stable probiotic formulations - Highly adaptable to lactose-rich media - Strong lactose fermentation capacity; known for biofilm formation and high survival in functional foods - Widely used in food-grade fermentations; well adapted to different whey types - Potential probiotic activity - Widely used in probiotic dairy products - Suitable for functional food development due to antimicrobial and metabolic benefits - Ferments efficiently in dairy by-products; survives well in freeze-dried probiotic formulations - Used as natural food preservation | [13,103,104,105,106,107,108] |
| Bioremediation bacteria Bacillus Streptomyces Dehalococcoides | Bacillus methylotrophicus Streptomyces sp. Dehalococcoides sp. | Production of biosurfactants, degradation of organic compounds, production of enzymes, production of antimicrobial compounds, degradation of organochlorinated compounds | 30 °C 25–30 °C | - Suitable for eco-friendly soil bioremediation - Grown on whey to improve compost quality and microbial biomass; contributes to organic matter stabilization and nutrient cycling during composting - Activity enhanced in whey-fed bioremediation systems, particularly in sulfate-rich aquifers | [104,109,110,111] |
| Nitrogen-fixing microorganisms Rhizobium | Rhizobium meliloti | Nitrogen fixation, fermentation of carbohydrates | 30 °C | - Capable of large-scale biomass production using whey as a cost-effective substrate; suitable for bioinoculant formulations in agriculture | |
| Actinobacteria Streptomyces Corynebacterium Rhodococcus Arthrobacter Brevibacterium | Streptomyces sp.
Corynebacterium glutamicum Rhodococcus opacus Arthrobacter agilis Arthrobacter viscosus Brevibacterium linens | Production of biosurfactants, amino acids (lysine) production, lactose fermentation into ethanol, production of lipids Production of C50 carotenoids of the bacterioruberin type and its glycosylated derivatives, production of EPS, Metabolism of lactic acid and proteins, production of ammonia and carotenoids, inhibition of pathogenic microorganisms | 30 °C 30–37 °C 28–30 °C 20–30 °C 25–30 °C | - Enhances compost microbial structure and nutrient availability; supports organic waste stabilization - Engineered strains use lactose and galactose from whey to produce L-lysine - Exhibits high lipid accumulation rates - Sustainable for pigment production - Improves rind texture and suppresses Listeria and other spoilage microorganisms during cheese maturation - Improves flavor development and microbial stability in smear-maturation cheeses | [99,112,113,114,115,116,117] |
| Yeasts Kluyveromyces Yarrowia Candida Debaryomyces Rhodotorula Saccharomyces Torulopsis | Kluyveromyces lactis Kluyveromyces marxianus Candida tropicalis Debaryomyces hansenii Rhodotorula glutinis | Fermentation of carbohydrates, production of enzymes, production of aromatic compounds, production of lipids, production of carotenoids | 30–45 °C 30–37 °C 25–30 °C 28–30 °C | - Suitable for ethanol and lactic acid production from sweet and acid whey; grows well under mild industrial conditions - Thermotolerant and fast growing; highly scalable for industrial bioprocesses - Enhances proteolysis; useful for flavor enhancement - Suitable for recombinant protein production using salt-rich dairy by-products - Capable of synthesizing carotenoids (e.g., β-carotene, torulene) when grown on goat cheese whey | [118,119,120,121,122,123,124,125] |
| Filamentous fungi Aspergillus Penicillium Trichoderma Mucor Fusarium Rhizopus Geotrichum | Aspergillus oryzae Aspergillus niger Aspergillus flavus, Aspergillus awamori, Aspergillus tubingensis Aspergillus tamarii Penicillium chrysogenum Penicillium roqueforti Penicillium camemberti Penicillium brevicompactum Trichoderma harizanum Mucor genevensis Mucor circinelloides Mucor azygosporus Mucor miehei Fusarium semitectum Fusarium solani Fusarium culmorum Fusarium oxysporum kolhapuriensis Rhizopus oryzae Rhizopus arrhizus Geotrichum candidum | Fermentation of lactose, proteolysis, lipolysis, production of enzymes, production of secondary metabolites, degradation of organic compounds Production of lipids, production of organic acids | 25–37 °C 20–28 °C 25–30 °C 30–37 °C 25–30 °C | - Suitable for animal feed and enzymatic applications Recombinant strains used for enzyme production in industrial processes - Used for enzyme production - Used for protease production in dairy media - Used for the production of active metabolites and secondary compounds on agro-industrial residues including whey - Used in cheese ripening and flavor development involved in rind formation and aroma development in soft cheeses - Applicable in food colorants and functional ingredient production - Used as a biocontrol agent; applicable in biofertilizer production - Capable of acid-whey deacidification and lipid accumulation - Involved in biomass valorization for lipase production - Applicable in biofuel and nutritional lipid production - Involved in studies on oil accumulation for industrial uses - Used for Single-Cell Proteins (SCPs) production and deacidification of acid whey - Involved in cheese surface microbiota; contributes to rind development and deacidification | [10,126,127,128,129,130,131,132,133,134] |
| Fermentation Methods | Main Metabolites Produced | Functional Properties Highlighted in Various Studies | Industrial Applications | References |
|---|---|---|---|---|
| Lactic fermentation | Lactic acid | Lactic acid inhibits Propionibacterium acnes at concentrations above 60 mg/mL, which supports its role in the treatment of acne and skin whitening. Its efficacy may vary depending on the stability and concentration of the formulation. | Food preservation, dairy products, bioplastics, cosmetics, pharmaceuticals | [13,38,99,136,137,138,139] |
| EPS | EPSs exhibit a wide range of strain-specific bioactivities: EPS from Leuconostoc pseudomesenteroides increased Lactobacillus counts by 20% and reduced E. coli by 15% in rats; EPS from Bifidobacterium longum reduced lung eosinophils by 40% in an asthma model; sulfonated EPS from Lactiplantibacillus plantarum increased antioxidant activity by 35%, while EPS from L. lactis subsp. cremoris lowered cholesterol by 25% in rats. HePS inhibited biofilms by 70%, and Lactobacillus gasseri EPS suppressed E. coli, L. monocytogenes, and S. aureus by 60%. EPS-loaded nanoparticles reduced tumor volume by 70%, indicating a promising but context-dependent therapeutic potential. | Functional foods, dairy products, prebiotics, biopolymers | [140,141,142,143,144] | |
| Bacteriocins | Bacteriocins exhibit strong antimicrobial activity, positioning them as effective natural food preservatives. In situ production using starter cultures is promising for fermented foods; however, their application still depends on strain compatibility and product matrix. Commercial examples include Lactococcus lactis subsp. lactis BS-10 (BioSafe™) for cheese and Leuconostoc carnosum (Bactoferm™ B-SF-43) or Lactobacillus sakei (Bactoferm™ B-2) for vacuum-sealed meat. Lacticin 3147 has been shown to improve the quality of cheddar cheese by controlling non-starter LABs. Bacteriocin-producing LABs are also used to preserve plant-based foods, seafood, and fermented vegetables, although their wider industrial use may be limited by production stability, regulatory approval, and interaction with complex food systems. | Food preservation, pharmaceuticals, antimicrobial coatings | [145,146,147,148,149,150,151,152,153] | |
| Bioactive peptides and free amino acids | The antihypertensive effect of peptides from whey is primarily associated with angiotensin-converting enzyme (ACE) inhibition, with whey protein hydrolysate lowering systolic blood pressure by 30% in hypertensive rats. Co-fermentation of Lactobacillus paracasei and Saccharomyces cerevisiae has shown that novel ACE inhibitory and antioxidant peptides can be produced from whey protein concentrate, increasing serum antioxidant capacity by 40%. These peptides also showed antimicrobial activity by inhibiting the growth of Listeria and E. coli by 80% at 0.2 mg/mL and increasing the proliferation of immune cells by 50% in vitro. While these multifunctional properties suggest considerable therapeutic potential, their efficacy and stability in various biological systems and routes of administration need to be further validated for practical applications. | Functional foods (infant formula, sports nutrition, medical nutrition), nutraceuticals, pharmaceuticals | [145,154,155,156,157,158] | |
| γ-aminobutyric acid (GABA) | Fermented rice flour that contains 750.55 ± 26.03 mg GABA/100 g has been shown to reduce oxidative stress and improve neuroprotection. GABA also contributes to blood pressure regulation, as shown by a reduction in systolic pressure of 5.5 ± 3.9 mmHg over 12 weeks after daily consumption of 50 g cheddar cheese containing 16 mg GABA. A single dose of chocolate enriched with 28 g GABA from Lactobacillus hilgardii K-3 reduced stress levels. These results underline the multifunctional health potential of GABA. The physiological effects of GABA may vary depending on delivery matrix, dosage, and individual response, so further studies on standardized use in functional foods are needed. | Functional beverages, food supplements, pharmaceuticals | [159,160,161,162,163] | |
| Alcoholic fermentation | Ethanol | Fermentation studies have reported ethanol concentrations reaching 4.52% after 20 days, though such yields are strongly influenced by fermentation conditions and substrate composition. Kluyveromyces marxianus produces ethyl acetate under aerobic conditions, which can be economically recovered from bioreactors for use in flavors and solvents. Recent studies indicate that metabolite production by K. marxianus is significantly dependent on oxygen availability, medium composition, and fermentation mode, which may affect process consistency and scalability in industrial applications. | Bioethanol production, pharmaceutical solvents, beverages | [164,165,166,167,168,169,170] |
| Acetic acid | Dekkera anomala thrives under aerobic conditions and produces acetic acid with remarkable antimicrobial and preservative activity. Fermentation with D. anomala yields 9.18 g/L acetic acid after 34 days, supporting its potential use in food preservation at concentrations of 0.1–0.5%. Ethanol and acetic acid produced by these yeasts serve as effective antimicrobial agents; ethanol from whey-based disinfectants significantly reduced bacterial contamination, while acetic acid inhibited Listeria monocytogenes and E. coli in food matrices. L. monocytogenes was reduced by 90% at 0.5% and 99.9% at 2% acetic acid, while E. coli was reduced by 99% at 1.5% and 99.9% at 3%. A 2.5% acetic acid solution reduced L. monocytogenes in meat products by 3.7 log CFU/g after 24 h at 4 °C. Despite these promising results, the relatively slow fermentation rate and the concentration-dependent efficacy underline the need for process optimization and product-specific validation in industrial applications. | Food industry, pharmaceuticals, polymers, textiles, agrochemicals, cosmetics, chemical industry | [171,172,173,174,175,176,177,178] | |
| Higher alcohols and aromatic compounds | Higher alcohols from whey fermentation improve sensory properties and show antimicrobial activity. Isoamyl and phenylethyl alcohol produced during alcoholic fermentation inhibit spoilage organisms—phenylethyl alcohol at a concentration of 0.3% suppresses ~95% of Zygosaccharomyces bailii, while isoamyl alcohol at a concentration of 0.5% inhibits 90% of Penicillium species. Although these effects are promising, their application may be limited by concentration thresholds and product compatibility. | Biofuels, alcoholic beverages, chemical synthesis; dairy industry, flavoring agents, bakery products | ||
| Glycerol | Glycerol is frequently used in pharmaceutical formulations and cosmetics due to its moisture-retaining and stabilizing effect in concentrations of 5–30%. In biotechnology, it acts as a metabolic regulator at 0.5–10% (w/v) and can be converted by microbial fermentation into high-value products such as 1,3-propanediol and polyhydroxyalkanoates (PHAs). Although its multifunctionality is well documented, optimization of conversion efficiency remains essential for broader industrial integration. | Cosmetics, pharmaceutical stabilizers, food processing | ||
| Propionic fermentation | Propionic acid | Propionic acid serves as a natural preservative for food and effectively inhibits mold growth in baked goods and dairy products. Studies indicate strong antifungal activity, particularly against Aspergillus fumigatus, Penicillium roqueforti, Pichia anomala, and Kluyveromyces marxianus, with minimum inhibitory concentration (MIC) values between 20 and 120 mM at pH 5 and below 10 mM at pH 3. P. roqueforti was the most sensitive, showing inhibition at 50 mM even at pH 7. While the pH-dependent efficacy highlights its potential, optimization of formulation conditions is key to ensuring consistent antifungal performance in different food systems. | Food preservatives, bioplastics, functional foods, nutraceuticals, pharmaceuticals, feed supplements, cosmetics agrochemicals | [12,179,180,181,182,183,184,185,186,187] |
| Acetic acid | Acetic acid shows remarkable antimicrobial activity, with MIC values of 30–120 mM at pH 5 and effective suppression of fungal growth. The enhanced antifungal activity observed when combined with other organic acids, such as propionic acid, highlights its potential for natural food preservation, although the synergy of the formulation and pH sensitivity require careful optimization for consistent efficacy. | Food industry, pharmaceuticals, polymers, textiles; agrochemicals, cosmetics, chemical industry | ||
| Succinic acid | Succinic acid from whey fermentation can achieve yields of 25–30 g/L with over 98% purity after processing. It is used as a flavor enhancer, acidity regulator, and gelling agent in foods, while succinic acid and its derivatives, such as sodium succinate, are used in packaging and as slow-raising agents. As an important precursor for biodegradable plastics, it supports the production of co-polyesters for sustainable food packaging. It also acts as an ion chelator and surfactant in pharmaceuticals and as an acidifier and stabilizer in cosmetics. Despite its versatility, wider industrial application may depend on cost-efficient production and integration into existing manufacturing processes. | Polymers, pharmaceuticals, food industry, cosmetics, chemical industry, agriculture | ||
| Vitamin B12 | Propionibacterium freudenreichii subsp. shermanii efficiently synthesizes vitamin B12 under optimal conditions: pH 6–7, lactate as carbon source, and fed-batch fermentation. Whey is a cost-effective medium when using a 24 h inoculum, 5 mg/L iron, 4% lactose, and 0.5% (NH4)2HPO4, with fermentation typically involving anaerobic and aerobic phases at 30 °C. While yields are promising, maintaining these specific parameters is critical for consistent B12 production on a large scale. | Pharmaceuticals, nutraceuticals, food industry, animal nutrition, biotechnology, cosmetics | ||
| Butyric fermentation | Butyric acid | Butyric acid has been shown to be effective against pathogens such as Salmonella and E. coli while supporting beneficial gut microbiota. Supplementation with 0.63 g/kg reduced Salmonella colonization in chickens, and 0.4% in broilers improved weight gain and feed conversion, and reduced E. coli levels, comparable to antibiotics. This dose also lowered pH in the upper part of the digestive tract, increased villus length and crypt depth, improved carcass yield, and reduced abdominal fat. While these results highlight its potential as an alternative to antibiotics in poultry feed, consistent performance under variable rearing conditions is worth further validation. | Polymers, chemical industry, food and nutraceutical industry, feed industry, pharmaceuticals, bioenergy | [188,189,190,191] |
| Acetic acid | Acetic acid strongly inhibits Saccharomyces cerevisiae and outperforms sorbic acid at higher concentrations. Its antifungal effect is pH dependent: 100–115 mM completely inhibited Fusarium oxysporum at a pH of 4.8 (tomato), and 45–60 mM reduced spore germination of Penicillium expansum by 78–86% at a pH of 4.2 (apple juice). Although the effect is effective in acidic foods, the results may vary depending on the matrix and strain. | Food industry, pharmaceuticals, polymers, textiles, agrochemicals, cosmetics, chemical industry | [192,193,194,195] | |
| Acetate and butyrate | Clinical studies report that 2–5 g/day of butyrate increases Faecalibacterium prausnitzii by 28–47% and reduces inflammatory markers by 23–35%. Acetate at a concentration of 10–30 mM in the colon decreases E. coli adhesion by 42%. In bioplastics, acetate-based PHAs (15–25% acetate) are 76% biodegradable within 180 days. In livestock, 0.3–0.5% sodium butyrate improves poultry feed conversion by 8.7–12.3% and reduces intestinal pathogens by 18.5%. Acetate at 1–3 g/kg increases soil microbial biomass by 27% and organic carbon by 9–15%. Both acids are used as industrial solvents, with global production of acetate and butyrate exceeding 3.2 million and 80,000 tons, respectively. Despite their broad functionality, application efficiency varies by sector and depends on formulation, dosage, and environmental conditions. | Food and nutraceutical industry, pharmaceuticals, bioplastics, chemical industry, agriculture, feed industry, biofuels | [193,196,197,198,199] | |
| Hydrogen | Fermentative hydrogen production yields 1.5–2.8 mol H2/mol glucose with 15–24% conversion efficiency in optimized bioreactors. It plays a key role in biofuels and industrial cooling, where hydrogen systems operate at –253 °C with a coefficient of performance of 0.3–0.5. Blending hydrogen with natural gas (5–20%) reduces carbon emissions from heating by 7–13%. In the chemical industry, green hydrogen reduces the carbon footprint of ammonia synthesis by 65–90%, with modern Haber–Bosch plants consuming 28–37 GJ/ton of ammonia. Despite its potential, scalability and energy consumption remain critical factors for sustainable use. | Renewable energy, chemical industry, fertilizers | [200,201,202] | |
| Carbon dioxide (CO2) | CO2, a by-product of fermentation, is often used in the food industry to carbonate beverages at 3.5–7.0 g/L. In refrigeration, it is considered an environmentally friendly alternative with a GWP of 1, far lower than conventional refrigerants (GWP 1430–4000), and it achieves energy efficiency values of 2.5–3.8 in transcritical cycles. In greenhouse agriculture, CO2 enrichment at 800–1200 ppm increases tomato yields by 27–42% and lettuce biomass by 25–35% compared with ambient levels (~410 ppm). While the benefits are well documented, the efficiency of implementation depends on system design, cost, and sector-specific integration. | Food industry, industrial refrigeration, greenhouse agriculture | [203,204,205,206] | |
| Acetic fermentation | Acetic acid | Acetic acid is widely known for its antimicrobial properties. It reduces E. coli O157:H7 by 99.7% at 0.1–0.5% in 24 h and lowers Listeria monocytogenes by 3–5 log CFU/g at 2–3% in meat. In pharmaceuticals, 0.25–1.0% solutions inhibit Pseudomonas aeruginosa at MICs of 0.16–0.31%. It is also used in acetate fibers (25–30 MPa tensile strength; >1.5 Mt/year production) and cosmetics (0.1–0.5%) to regulate pH (3.5–5.0) and improve the skin barrier by 18–27%. With a global production of over 13.8 Mt/year, acetic acid is an important chemical intermediate. Despite its versatility, its efficacy and safety depend on the exact formulation and context of use. | Food industry, pharmaceuticals, polymers, textiles, agrochemicals, cosmetics, chemical industry | [207,208,209,210,211,212,213] |
| Acetate | An acetate content of 60–80 mM in the gut increases butyrate-producing bacteria by 35–40% and reduces inflammatory markers by 25%. In pharma, sodium acetate buffers enhance protein stability by 28–42%. Acetate-based PHAs (15–25%) show 76–90% in soil within 180 days. In animal feed and agriculture, 0.2–0.5% acetate improves digestion, increases feed conversion in poultry by 7–12%, and reduces CH4 emissions in ruminants by 15–22%. The chemical industry uses acetate in solvents, adhesives, and coatings, with a global production of over 3.5 Mt/year. Although it is versatile, its effectiveness depends on the formulation and sector-specific conditions. | Food and nutraceutical industry, pharmaceuticals, bioplastics, chemical industry, agriculture, feed industry | [214,215,216] | |
| EPS | EPS increases the viscosity of yogurt by 150–300% at 0.05–0.2% and reduces syneresis by 45–60%. In pharmaceuticals, 1.5–3 g/day increases SCFA production by 24–36% and bifidobacteria by 18–27%. In biotechnology, 2–5 g/L EPS increases the binding of heavy metals by 65–80% through biofilm formation. Biomedical applications include wound healing and drug delivery, where EPS hydrogels improve wound closure by 40–55% and prolong drug release by 30–45%. Despite their broad functionality, their efficacy is context- and formulation-dependent. | Food industry, pharmaceuticals | [217,218,219,220] | |
| CO2 | Controlled CO2 levels can enhance the productivity of industrial fermentation by 15–30% by optimizing dissolved CO2 and improving kinetics and metabolite synthesis. Specific CO2 concentrations also reduce unwanted by-products by 25–40% through effects on metabolic flux and substrate uptake. In penicillin production, precise CO2 control increases antibiotic titers by 18–24% and improves microbial performance. In addition, CO2 optimization reduces energy consumption by 12–19% through improved glucose–oxygen efficiency. While these results are promising, they depend on tightly controlled process conditions. | Food industry, biotechnology | ||
| Mixed fermentation for microbial polymer production | PHAs | PHAs containing 10–30% hydroxyvalerate exhibit greater flexibility, with elongation at break increasing from 5–10% to 30–450% compared with pure PHB, making them ideal for bioplastics. In tissue engineering, PHA-based scaffolds improve cell adhesion and proliferation. They exhibit 75–90% better biocompatibility and 65–85% higher cell viability than conventional synthetic polymers. PHAs with 10–30% hydroxyvalerate show increased flexibility, with elongation at break rising from 5–10% to 30–450% versus pure PHB, supporting their use in bioplastics. In tissue engineering, PHA scaffolds enhance cell adhesion and proliferation, with 75–90% better biocompatibility and 65–85% higher viability than synthetic polymers. Despite these advantages, performance can vary with composition and application context. | Polymers | [141,221,222,223,224] |
| Biohydrogen | Biohydrogen is gaining relevance as a renewable energy source, with fermentative yields of 1.8–2.5 mol H2/mol glucose under optimized conditions. Hydrogen-enriched fuels (15–20% H2) reduce CO2 emissions by 10–15% and NOx by 20–50%. Microbial reactors achieve up to 40% energy efficiency and produce 0.5–3.5 L H2/day in continuous operation. Practical scalability depends on whether these performance levels can be maintained in real systems. | [225,226,227,228] | ||
| Polymalic acid (PMA) | PMA, a biodegradable polyester, is widely studied for biomedical and pharmaceutical purposes. PMA nanocarriers enable 50–70% extended drug release over 7–21 days with 15–30% loading efficiency. In bioplastics, PMA films offer a tensile strength of 20–35 MPa and an oxygen permeability of 2.5–8.7 cm3-mm/m2-day-atm, making them suitable for sustainable packaging. During composting, PMA biodegrades 85–95% within 90 days. Despite its promising properties, performance can vary depending on the formulation and application environment. | |||
| EPS | EPS-based hydrogels improve water retention by 60–80%, retain 20–35 g water/g dry matter, and are suitable for biomedical dressings and agriculture. In biopolymers, EPS improves the flexibility of films by reducing the modulus of elasticity by 30–45%. Plastics with 5–15% EPS degrade 85% faster and achieve 90–95% biodegradation in 60–180 days compared with more than 365 days for conventional plastics. While the effect is effective, performance can vary depending on matrix compatibility and environmental conditions. | |||
| Methanogenic fermentation | CH4 | CH4 is an important renewable fuel in biogas production, with optimized anaerobic digestion of whey yielding 0.3–0.5 m3 CH4/kg COD. CH4-fueled engines reduce CO2 emissions by 15–25% compared with diesel, while bio-CNG in public transportation reduces NOx by 40–60% and particulates by 85–95%. Biogas plants using cheese whey achieve 0.34–0.48 m3 CH4/kg COD, with 55–68% energy recovery and 3.5–7 years payback. Despite strong potential, the results depend on the plant scale and process optimization. | Renewable energy, fuel for heating, transportation | [16,229,230,231,232,233,234,235,236] |
| CO2 | Optimized CO2 levels in sparkling water (5.8–6.2 g/L) increase consumer preference by 32–41%, while sparkling wines require more than 1.2 g/L for perceptible mouthfeel. At retail, transcritical CO2 systems reduce energy consumption by 18–26%, with advanced designs (e.g., two-phase ejector, heat exchanger) improving COP by 12% and energy efficiency by 16% and reducing electricity consumption by 10%. Cascade CO2 systems achieve an efficiency of 3.2–4.1 for medium-temperature cooling. In biotechnology, controlled CO2 increases the expression of recombinant proteins by 25–33%. Although the benefits are significant, system design and application context remain critical for consistent performance. | Food industry, industrial refrigeration, greenhouse agriculture | ||
| Volatile fatty acids (VFAs) | VFAs can be converted to PHAs with yields of 0.2–0.6 g PHA/g VFA. Industrial processes using mixed VFAs from wastes achieve 50–150 g/L PHA and 1.0–3.5 g/L/h productivity. The resulting PHAs have a tensile strength of 20–40 MPa and an elongation at break of 5–500%, with an oxygen permeability of 3–55 cm3-mm/m2-day-atm and a water vapor permeability of 1–5 g-mm/m2-day, suitable for sustainable packaging. VFAs also support the production of solvents and coatings, with acetate to ethanol conversion at 85–92% and propionate-based adhesives achieving bond strengths of 2.5–4.8 MPa. While performance and efficiency are promising, they are highly dependent on feedstock variability and process control. | Polymers | ||
| Fungal fermentation for SCPs production | SCPs | SCPs-enriched bread formulations with 5–10% fungal protein increase the protein content by 18–25%, with sensory acceptability at 85% of control products. SCP (mycoprotein) derived from Fusarium venenatum has been shown to reduce postprandial blood glucose levels by 20–35% and insulin response by 15–27% compared with reference proteins, thus supporting metabolic health. In the animal feed industry, SCPs are used as a high-protein supplement for livestock and aquaculture. Replacing 20–40% of fishmeal with SCPs from Aspergillus oryzae in aqua feed improves fish growth rates by 10–18% and increases feed conversion in tilapia and rainbow trout by 12–20. In poultry, the inclusion of 5–15% fungal SCPs in feed increases weight gain by 8–14% while improving gut health by reducing Salmonella by 22–30% and increasing Lactobacillus populations in the cecum by 15–22%. The production of SCPs for waste utilization and enzyme production is being researched. Trichoderma reesei efficiently utilizes whey as a carbon source and achieves biomass yields of 0.45–0.65 g/g lactose, with protein contents of 45–55% and cultivation productivity of 0.8–1.2 g/L/h in optimized bioreactors. In addition, SCP-producing fungi secrete valuable enzymes such as proteases and cellulases, which can be used for biofuels and increase enzyme activity by 50–75% under optimized fermentation conditions. T. reesei, which grows on lignocellulosic substrates, reaches cellulase activities of 1.2–2.8 FPU/mL. Fungal SCPs are analyzed for their bioactive properties. SCP-derived peptides show antimicrobial effects, with Saccharomyces cerevisiae protein hydrolysates inhibiting E. coli and Staphylococcus aureus by 65–80% at 0.5–1.5 mg/mL, with MIC of 0.18–0.42 mg/mL against common foodborne pathogens. In addition, SCP-derived β-glucans improve immune function by increasing macrophage activity by 40–60% and natural killer (NK) cell cytotoxicity by 25–38% at a dosage of 100–250 mg/day, making them promising functional ingredients for immunological supplements. SCP bread enriched with 5–10% mushroom protein increases the protein content by 18–25% while retaining 85% of the sensory acceptability. Trichoderma reesei also produces enzymes such as cellulases (1.2–2.8 FPU/mL), with 50–75% higher activity under optimized conditions. SCP-derived peptides show antimicrobial activity and inhibit E. coli and S. aureus by 65–80% at 0.5–1.5 mg/mL (MIC 0.18–0.42 mg/mL), while β-glucans improve immune function by increasing macrophage activity by 40–60% and NK cell cytotoxicity by 25–38% at 100–250 mg/day. Although SCPs show multifunctional potential in various areas, consistent bioactivity and integration into existing systems require further standardization and validation. | Food industry, feed industry, biotechnology, pharmaceuticals | [237,238,239,240,241,242] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malos, I.G.; Ghizdareanu, A.-I.; Vidu, L.; Matei, C.B.; Pasarin, D. The Role of Whey in Functional Microorganism Growth and Metabolite Generation: A Biotechnological Perspective. Foods 2025, 14, 1488. https://doi.org/10.3390/foods14091488
Malos IG, Ghizdareanu A-I, Vidu L, Matei CB, Pasarin D. The Role of Whey in Functional Microorganism Growth and Metabolite Generation: A Biotechnological Perspective. Foods. 2025; 14(9):1488. https://doi.org/10.3390/foods14091488
Chicago/Turabian StyleMalos, Iuliu Gabriel, Andra-Ionela Ghizdareanu, Livia Vidu, Catalin Bogdan Matei, and Diana Pasarin. 2025. "The Role of Whey in Functional Microorganism Growth and Metabolite Generation: A Biotechnological Perspective" Foods 14, no. 9: 1488. https://doi.org/10.3390/foods14091488
APA StyleMalos, I. G., Ghizdareanu, A.-I., Vidu, L., Matei, C. B., & Pasarin, D. (2025). The Role of Whey in Functional Microorganism Growth and Metabolite Generation: A Biotechnological Perspective. Foods, 14(9), 1488. https://doi.org/10.3390/foods14091488









