Metschnikowia pulcherrima and Lachancea thermotolerans Killer Toxins: Contribution to Must Bioprotection
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Growth Media
2.3. Killer Assay of Bioprotective Yeast Species
2.4. Assessing Killer Activity in Co-Cultures of Bioprotectant Against Saccharomyces cerevisiae
2.5. Monitoring Bioprotectant Killing Activity in Synthetic Must
2.6. Partial Production and Purification of Killer Toxins
2.7. Quantification of Protein Concentration
2.8. Evaluation of Toxin’s Killing Activity
2.9. Assessment of Killer Toxin’s Inhibitory Potentiality in Synthetic Must
2.10. Evaluation of Metabolite Production as Mode of Action
2.10.1. Lactic Acid and Acetic Acid Analysis
2.10.2. Ethanol, Malic Acid, Acetic Acid, and Total Acidity Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Screening for Potential Yeast Strains with Killer Activity Against Brettanomyces bruxellensis and Hanseniaspora uvarum
3.2. Co-Culture of Bioprotectant Yeast Species with Saccharomyces cerevisiae
3.3. Bioprotective Potentiality in Synthetic Must
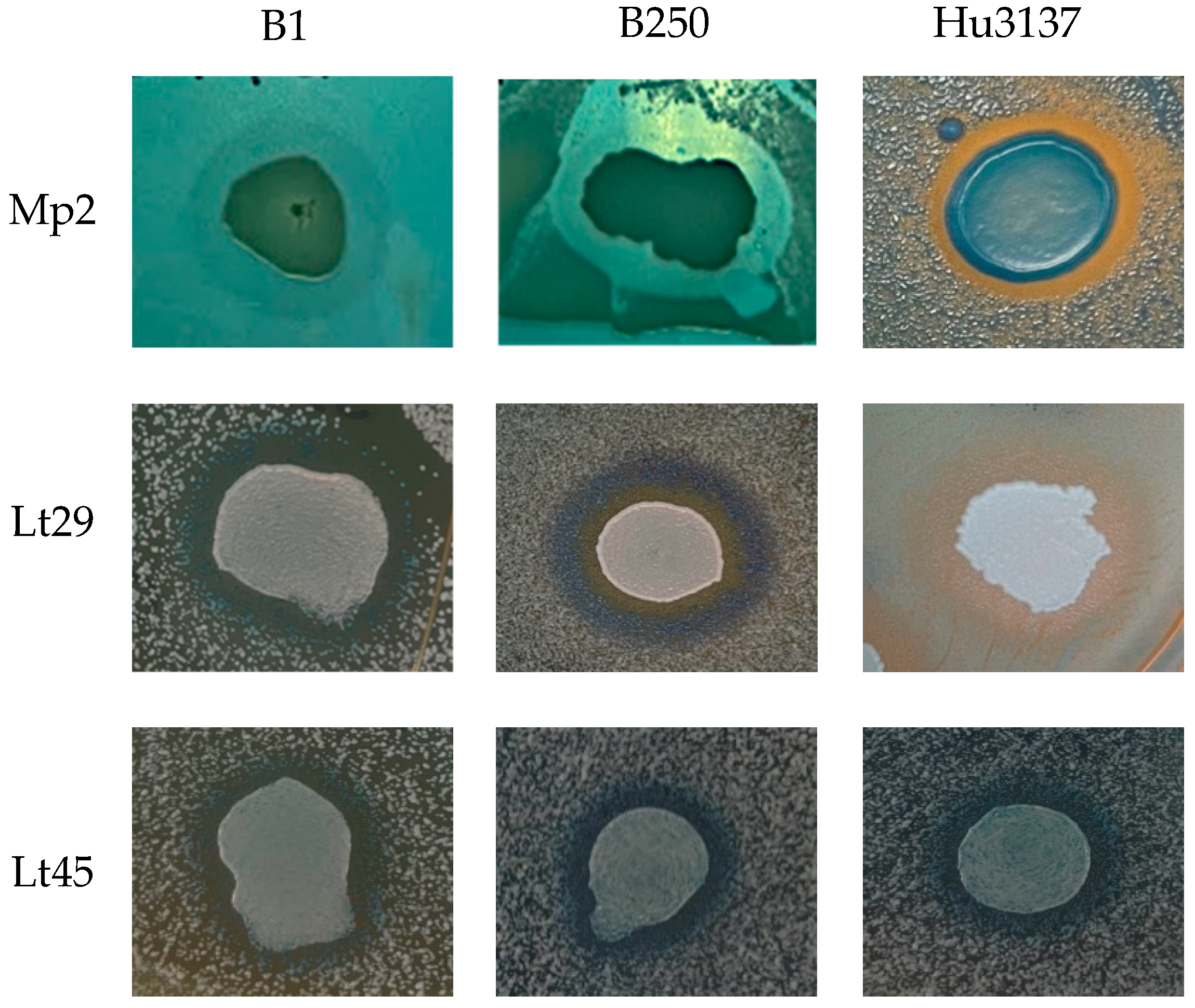
3.4. Assessment of Killer Toxin Activity on Agar
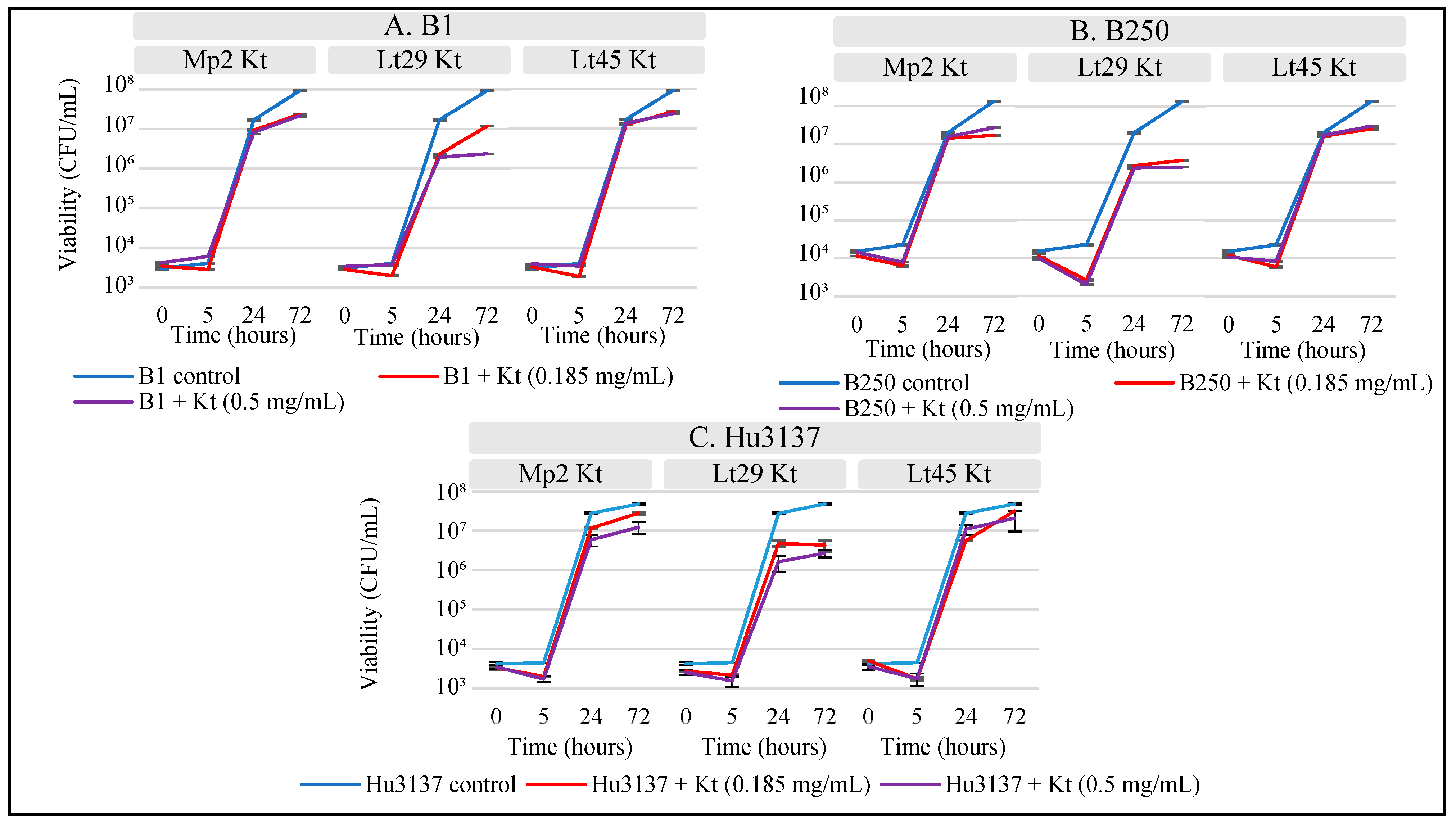
3.5. Inhibitory Effect of Killer Toxins on Spoilage Yeasts in Synthetic Must
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, B.D.; Divol, B. Brettanomyces bruxellensis, a Survivalist Prepared for the Wine Apocalypse and Other Beverages. Food Microbiol. 2016, 59, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Grbin, P.R.; Henschke, P.A. Mousy Off-Flavour Production in Grape Juice and Wine by Dekkera and Brettanomyces yeasts. Aust. J. Grape Wine Res. 2000, 6, 255–262. [Google Scholar] [CrossRef]
- Roullier-Gall, C.; Hemmler, D.; Gonsior, M.; Li, Y.; Nikolantonaki, M.; Aron, A.; Coelho, C.; Gougeon, R.D.; Schmitt-Kopplin, P. Sulfites and the Wine Metabolome. Food Chem. 2017, 237, 106–113. [Google Scholar] [CrossRef]
- Giacosa, S.; Río Segade, S.; Cagnasso, E.; Caudana, A.; Rolle, L.; Gerbi, V. SO2 in Wines. In Red Wine Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 309–321. ISBN 978-0-12-814399-5. [Google Scholar]
- Windholtz, S.; Vinsonneau, E.; Farris, L.; Thibon, C.; Masneuf-Pomarède, I. Yeast and Filamentous Fungi Microbial Communities in Organic Red Grape Juice: Effect of Vintage, Maturity Stage, SO2, and Bioprotection. Front. Microbiol. 2021, 12, 748416. [Google Scholar] [CrossRef]
- Gianvito, P.D.; Englezos, V.; Rantsiou, K.; Cocolin, L. Bioprotection Strategies in Winemaking. Int. J. Food Microbiol. 2022, 364, 109532. [Google Scholar] [CrossRef]
- Alexandre, H.; Puyo, M.; Tourdot-Maréchal, R. Bioprotection in Winemaking. In New Advances in Saccharomyces; IntechOpen: London, UK, 2023; pp. 135–158. [Google Scholar]
- Windholtz, S.; Nioi, C.; Thibon, C.; Bécquet, S.; Vinsonneau, E.; Coulon, J.; Masneuf-Pomarède, I. Bioprotection as an Alternative to SO2 in the Pre-Fermentation Phase: Original Language of the Article: English. IVES Technol. Rev. Vine Wine 2023. [Google Scholar] [CrossRef]
- Windholtz, S.; Redon, P.; Lacampagne, S.; Farris, L.; Lytra, G.; Cameleyre, M.; Barbe, J.-C.; Coulon, J.; Thibon, C.; Masneuf-Pomarède, I. Non-Saccharomyces Yeasts as Bioprotection in the Composition of Red Wine and in the Reduction of Sulfur Dioxide. LWT-Food Sci. Technol. 2021, 149, 111781. [Google Scholar] [CrossRef]
- Lücke, F.-K. Utilization of Microbes to Process and Preserve Meat. Meat Sci. 2000, 56, 105–115. [Google Scholar] [CrossRef]
- Yao, M.; Wang, F.; Arpentin, G. Bioprotection as a Tool to Produce Natural Wine: Impact on Physicochemical and Sensory Analysis. BIO Web Conf. 2023, 56, 02019. [Google Scholar] [CrossRef]
- OIV. Use of Bioprotection Strains in Winemaking; Expertise Document; OIV: Dijon, France, 2024. [Google Scholar]
- Bordet, F.; Joran, A.; Klein, G.; Roullier-Gall, C.; Alexandre, H. Yeast–Yeast Interactions: Mechanisms, Methodologies and Impact on Composition. Microorganisms 2020, 8, 600. [Google Scholar] [CrossRef]
- Zilelidou, E.A.; Nisiotou, A. Understanding Wine through Yeast Interactions. Microorganisms 2021, 9, 1620. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Canonico, L.; Agarbati, A.; Ciani, M. Biocontrol and Probiotic Function of Non-Saccharomyces Yeasts: New Insights in Agri-Food Industry. Microorganisms 2023, 11, 1450. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Degraeve, P.; Oulahal, N. Bioprotective Yeasts: Potential to Limit Postharvest Spoilage and to Extend Shelf Life or Improve Microbial Safety of Processed Foods. Heliyon 2024, 10, e24929. [Google Scholar] [CrossRef] [PubMed]
- Siedler, S.; Rau, M.H.; Bidstrup, S.; Vento, J.M.; Aunsbjerg, S.D.; Bosma, E.F.; McNair, L.M.; Beisel, C.L.; Neves, A.R. Competitive Exclusion Is a Major Bioprotective Mechanism of Lactobacilli against Fungal Spoilage in Fermented Milk Products. Appl. Environ. Microbiol. 2020, 86, e02312-19. [Google Scholar] [CrossRef]
- Rollero, S.; Bloem, A.; Ortiz-Julien, A.; Camarasa, C.; Divol, B. Altered Fermentation Performances, Growth, and Metabolic Footprints Reveal Competition for Nutrients between Yeast Species Inoculated in Synthetic Grape Juice-like Medium. Front. Microbiol. 2018, 9, 196. [Google Scholar] [CrossRef]
- Seguinot, P.; Ortiz-Julien, A.; Camarasa, C. Impact of Nutrient Availability on the Fermentation and Production of Aroma Compounds Under Sequential Inoculation with M. pulcherrima and S. cerevisiae. Front. Microbiol. 2020, 11, 305. [Google Scholar] [CrossRef]
- Zhang, H.; Du, H.; Xu, Y. Volatile Organic Compound-Mediated Antifungal Activity of Pichia spp. and Its Effect on the Metabolic Profiles of Fermentation Communities. Appl. Environ. Microbiol. 2021, 87, e02992-20. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, P.; Zhou, X.; Zheng, J.; Ma, Y.; Liu, C.; Wu, T.; Li, H.; Wang, X.; Wang, H.; et al. Isolation, Identification, and Characterization of an Acid-Tolerant Pichia kudriavzevii and Exploration of Its Acetic Acid Tolerance Mechanism. Fermentation 2023, 9, 540. [Google Scholar] [CrossRef]
- Melvydas, V.; Svediene, J.; Skridlaite, G.; Vaiciuniene, J.; Garjonyte, R. In Vitro Inhibition of Saccharomyces cerevisiae Growth by Metschnikowia spp. Triggered by Fast Removal of Iron via Two Ways. Braz. J. Microbiol. 2020, 51, 1953–1964. [Google Scholar] [CrossRef]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. Interaction between Hanseniaspora uvarum and Saccharomyces cerevisiae during Alcoholic Fermentation. Int. J. Food Microbiol. 2015, 206, 67–74. [Google Scholar] [CrossRef]
- Puyo, M.; Simonin, S.; Bach, B.; Klein, G.; Alexandre, H.; Tourdot-Maréchal, R. Bio-Protection in Oenology by Metschnikowia pulcherrima: From Field Results to Scientific Inquiry. Front. Microbiol. 2023, 14, 1252973. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, Natural Antimicrobials for Food Preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Ingeniis De, J.; Pepe, L.; Mannazzu, I.; Ciani, M. Pichia anomala and Kluyveromyces wickerhamii Killer Toxins as New Tools against Dekkera/Brettanomyces Spoilage Yeasts. FEMS Microbiol. Lett. 2004, 238, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; San Mauro, M.; Bravo, E.; Marquina, D. PMKT2, a New Killer Toxin from Pichia membranifaciens, and Its Promising Biotechnological Properties for Control of the Spoilage Yeast Brettanomyces bruxellensis. Microbiology 2009, 155, 624–634. [Google Scholar] [CrossRef]
- Mannazzu, I.; Domizio, P.; Carboni, G.; Zara, S.; Comitini, F.; Budroni, M.; Ciani, M. Yeast Killer Toxins: From Ecological Significance to Application. Crit. Rev. Biotechnol. 2019, 39, 603–617. [Google Scholar] [CrossRef]
- Comitini, F.; Ciani, M. The Zymocidial Activity of Tetrapisispora phaffii in the Control of Hanseniaspora uvarum during the Early Stages of Winemaking: Tetrapisispora phaffii Killer Toxin in Winemaking. Lett. Appl. Microbiol. 2010, 50, 50–56. [Google Scholar] [CrossRef]
- Santos, A.; Navascués, E.; Bravo, E.; Marquina, D. Ustilago maydis Killer Toxin as a New Tool for the Biocontrol of the Wine Spoilage Yeast Brettanomyces bruxellensis. Int. J. Food Microbiol. 2011, 145, 147–154. [Google Scholar] [CrossRef]
- Agarbati, A.; Ciani, M.; Esin, S.; Agnolucci, M.; Marcheggiani, F.; Tiano, L.; Comitini, F. Comparative Zymocidial Effect of Three Different Killer Toxins against Brettanomyces bruxellensis Spoilage Yeasts. Int. J. Mol. Sci. 2023, 24, 1309. [Google Scholar] [CrossRef]
- Büyüksırıt Bedir, T.; Kuleaşan, H. A Natural Approach, the Use of Killer Toxin Produced by Metschnikowia pulcherrima in Fresh Ground Beef Patties for Shelf Life Extention. Int. J. Food Microbiol. 2021, 345, 109154. [Google Scholar] [CrossRef]
- Ciani, M.; Fatichenti, F. Killer Toxin of Kluyveromyces phaffii DBVPG 6076 as a Biopreservative Agent to Control Apiculate Wine Yeasts. Appl. Environ. Microbiol. 2001, 67, 3058–3063. [Google Scholar] [CrossRef]
- Comitini, F.; Pietro, N.D.; Zacchi, L.; Mannazzu, I.; Ciani, M. Kluyveromyces phaffii Killer Toxin Active against Wine Spoilage Yeasts: Purification and Characterization. Microbiology 2004, 150, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Mehlomakulu, N.N.; Setati, M.E.; Divol, B. Characterization of Novel Killer Toxins Secreted by Wine-Related Non-Saccharomyces Yeasts and Their Action on Brettanomyces spp. Int. J. Food Microbiol. 2014, 188, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Mehlomakulu, N.N.; Prior, K.J.; Setati, M.E.; Divol, B. Candida pyralidae Killer Toxin Disrupts the Cell Wall of Brettanomyces bruxellensis in Red Grape Juice. J. Appl. Microbiol. 2017, 122, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Carboni, G.; Fancello, F.; Zara, G.; Zara, S.; Ruiu, L.; Marova, I.; Pinna, G.; Budroni, M.; Mannazzu, I. Production of a Lyophilized Ready-to-Use Yeast Killer Toxin with Possible Applications in the Wine and Food Industries. Int. J. Food Microbiol. 2020, 335, 108883. [Google Scholar] [CrossRef]
- De Ullivarri, M.F.; Mendoza, L.M.; Raya, R.R. Killer Activity of Saccharomyces cerevisiae Strains: Partial Characterization and Strategies to Improve the Biocontrol Efficacy in Winemaking. Antonie Van Leeuwenhoek 2014, 106, 865–878. [Google Scholar] [CrossRef]
- Abu-Mejdad, N.M.J.A.; Al-Badran, A.I.; Al-Saadoon, A.H. Purification and Characterization of Two Killer Toxins Originated from Torulaspora delbrueckii (Lindner) and Wickerhamomyces anomalus (E.C.Hansen) Kurtzman, Robnett, and Basehoar-Powers. Bull. Natl. Res. Cent. 2020, 44, 48. [Google Scholar] [CrossRef]
- Villalba, M.L.; Susana Sáez, J.; Del Monaco, S.; Lopes, C.A.; Sangorrín, M.P. TdKT, a New Killer Toxin Produced by Torulaspora delbrueckii Effective against Wine Spoilage Yeasts. Int. J. Food Microbiol. 2016, 217, 94–100. [Google Scholar] [CrossRef]
- Comitini, F.; Agarbati, A.; Canonico, L.; Galli, E.; Ciani, M. Purification and Characterization of WA18, a New Mycocin Produced by Wickerhamomyces anomalus Active in Wine Against Brettanomyces bruxellensis Spoilage Yeasts. Microorganisms 2020, 9, 56. [Google Scholar] [CrossRef]
- Evers, M.S.; Roullier-Gall, C.; Morge, C.; Sparrow, C.; Gobert, A.; Vichi, S.; Alexandre, H. Thiamine and Biotin: Relevance in the Production of Volatile and Non-Volatile Compounds during Saccharomyces cerevisiae Alcoholic Fermentation in Synthetic Grape Must. Foods 2023, 12, 972. [Google Scholar] [CrossRef]
- El Dana, F.; Hayar, S.; Colosio, M.-C. Selection of Three Indigenous Lebanese Yeast Saccharomyces cerevisiae with Physiological Traits from Grape Varieties in Western Semi-Desert and Pedoclimatic Conditions in the Bekaa Valley. Fermentation 2021, 7, 280. [Google Scholar] [CrossRef]
- Villalba, M.L.; Mazzucco, M.B.; Lopes, C.A.; Ganga, M.A.; Sangorrín, M.P. Purification and Characterization of Saccharomyces eubayanus Killer Toxin: Biocontrol Effectiveness against Wine Spoilage Yeasts. Int. J. Food Microbiol. 2020, 331, 108714. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.R.; Bevan, E.A. Studies on the Nature of the Killer Factor Produced by Saccharomyces cerevisiae. J. Gen. Microbiol. 1968, 51, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Oro, L.; Ciani, M.; Comitini, F. Antimicrobial Activity of Metschnikowia pulcherrima on Wine Yeasts. J. Appl. Microbiol. 2014, 116, 1209–1217. [Google Scholar] [CrossRef]
- MacDonald, J. Biosynthesis of Pulcherriminic Acid. Biochem. J. 1965, 96, 533–538. [Google Scholar] [CrossRef]
- Büyüksırıt-Bedir, T.; Kuleaşan, H. Purification and Characterization of a Metschnikowia pulcherrima Killer Toxin with Antagonistic Activity against Pathogenic Microorganisms. Arch. Microbiol. 2022, 204, 337. [Google Scholar] [CrossRef]
- Vicente, J.; Navascués, E.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. An Integrative View of the Role of Lachancea thermotolerans in Wine Technology. Foods 2021, 10, 2878. [Google Scholar] [CrossRef]
- Petitgonnet, C.; Klein, G.L.; Roullier-Gall, C.; Schmitt-Kopplin, P.; Quintanilla-Casas, B.; Vichi, S.; Julien-David, D.; Alexandre, H. Influence of Cell-Cell Contact between L. thermotolerans and S. cerevisiae on Yeast Interactions and the Exo-Metabolome. Food Microbiol. 2019, 83, 122–133. [Google Scholar] [CrossRef]
- Vion, C.; Yeramian, N.; Hranilovic, A.; Masneuf-Pomarède, I.; Marullo, P. Influence of Yeasts on Wine Acidity: New Insights into Saccharomyces cerevisiae. OENO One 2024, 58, 4. [Google Scholar] [CrossRef]
- Anderson, T.R.; Slotkin, T.A. Maturation of the Adrenal Medulla—IV. Effects of Morphine. Biochem. Pharmacol. 1975, 24, 1469–1474. [Google Scholar] [CrossRef]
- Sadoudi, M.; Rousseaux, S.; David, V.; Alexandre, H.; Tourdot-Maréchal, R. Metschnikowia pulcherrima Influences the Expression of Genes Involved in PDH Bypass and Glyceropyruvic Fermentation in Saccharomyces cerevisiae. Front. Microbiol. 2017, 8, 1137. [Google Scholar] [CrossRef]
- Puyo, M.; Mas, P.; Roullier-Gall, C.; Romanet, R.; Lebleux, M.; Klein, G.; Alexandre, H.; Tourdot-Maréchal, R. Bioprotection Efficiency of Metschnikowia Strains in Synthetic Must: Comparative Study and Metabolomic Investigation of the Mechanisms Involved. Foods 2023, 12, 3927. [Google Scholar] [CrossRef] [PubMed]
- Hranilovic, A.; Gambetta, J.M.; Jeffery, D.W.; Grbin, P.R.; Jiranek, V. Lower-Alcohol Wines Produced by Metschnikowia pulcherrima and Saccharomyces cerevisiae Co-Fermentations: The Effect of Sequential Inoculation Timing. Int. J. Food Microbiol. 2020, 329, 108651. [Google Scholar] [CrossRef] [PubMed]
- Escott, C.; Vaquero, C.; Loira, I.; López, C.; González, C.; Morata, A. Synergetic Effect of Metschnikowia pulcherrima and Lachancea thermotolerans in Acidification and Aroma Compounds in Airén Wines. Foods 2022, 11, 3734. [Google Scholar] [CrossRef]
- Vaquero, C.; Escott, C.; Heras, J.M.; Carrau, F.; Morata, A. Co-Inoculations of Lachancea thermotolerans with Different Hanseniaspora spp.: Acidification, Aroma, Biocompatibility, and Effects of Nutrients in Wine. Food Res. Int. 2022, 161, 111891. [Google Scholar] [CrossRef]
- Tristezza, M.; Tufariello, M.; Capozzi, V.; Spano, G.; Mita, G.; Grieco, F. The Oenological Potential of Hanseniaspora uvarum in Simultaneous and Sequential Co-Fermentation with Saccharomyces cerevisiae for Industrial Wine Production. Front. Microbiol. 2016, 7, 670. [Google Scholar] [CrossRef]
- Cibrario, A.; Miot-Sertier, C.; Paulin, M.; Bullier, B.; Riquier, L.; Perello, M.-C.; De Revel, G.; Albertin, W.; Masneuf-Pomarède, I.; Ballestra, P.; et al. Brettanomyces bruxellensis Phenotypic Diversity, Tolerance to Wine Stress and Wine Spoilage Ability. Food Microbiol. 2020, 87, 103379. [Google Scholar] [CrossRef]
- Nisiotou, A.; Mallouchos, A.; Tassou, C.; Banilas, G. Indigenous Yeast Interactions in Dual-Starter Fermentations May Improve the Varietal Expression of Moschofilero Wine. Front. Microbiol. 2019, 10, 1712. [Google Scholar] [CrossRef]
- Pereznevado, F.; Albergaria, H.; Hogg, T.; Girio, F. Cellular Death of Two Non-Saccharomyces Wine-Related Yeasts during Mixed Fermentations with Saccharomyces cerevisiae. Int. J. Food Microbiol. 2006, 108, 336–345. [Google Scholar] [CrossRef]
- Albertin, W.; Miot-Sertier, C.; Bely, M.; Marullo, P.; Coulon, J.; Moine, V.; Colonna-Ceccaldi, B.; Masneuf-Pomarede, I. Oenological Prefermentation Practices Strongly Impact Yeast Population Dynamics and Alcoholic Fermentation Kinetics in Chardonnay Grape Must. Int. J. Food Microbiol. 2014, 178, 87–97. [Google Scholar] [CrossRef]
- Freer, S.N. Acetic Acid Production by Dekkera/Brettanomyces yeasts. World J. Microbiol. Biotechnol. 2002, 18, 271–275. [Google Scholar] [CrossRef]
- Capusoni, C.; Arioli, S.; Zambelli, P.; Moktaduzzaman, M.; Mora, D.; Compagno, C. Effects of Oxygen Availability on Acetic Acid Tolerance and Intracellular pH in Dekkera bruxellensis. Appl. Environ. Microbiol. 2016, 82, 4673–4681. [Google Scholar] [CrossRef] [PubMed]
- Fernández De Ullivarri, M.; Bulacios, G.A.; Navarro, S.A.; Lanza, L.; Mendoza, L.M.; Chalón, M.C. The Killer Yeast Wickerhamomyces anomalus Cf20 Exerts a Broad Anti-Candida Activity through the Production of Killer Toxins and Volatile Compounds. Med. Mycol. 2020, 58, 1102–1113. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.-J.; Mei, W.-C.; Li, T.; Tao, Y.-S. Increase of Medium-Chain Fatty Acid Ethyl Ester Content in Mixed H. uvarum/S. cerevisiae Fermentation Leads to Wine Fruity Aroma Enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Ciani, M. Kluyveromyces wickerhamii Killer Toxin: Purification and Activity towards Brettanomyces/Dekkera yeasts in Grape Must: Kwkt Killer Toxin Purification. FEMS Microbiol. Lett. 2011, 316, 77–82. [Google Scholar] [CrossRef] [PubMed]
 acetic acid (Ac);
acetic acid (Ac);  L-lactic acid (La);
L-lactic acid (La);  ethanol (EtOH).
ethanol (EtOH).
 acetic acid (Ac);
acetic acid (Ac);  L-lactic acid (La);
L-lactic acid (La);  ethanol (EtOH).
ethanol (EtOH).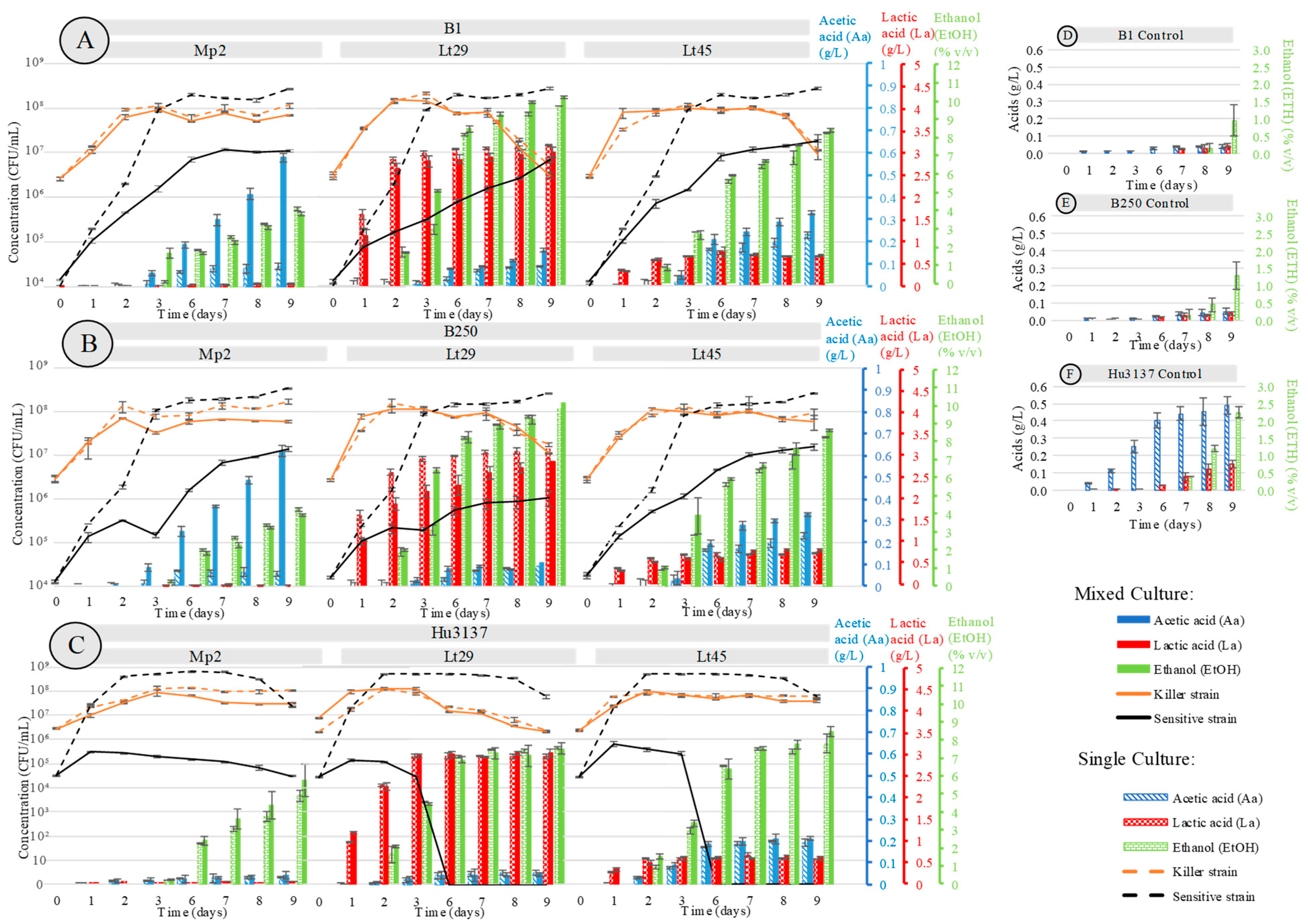



| Single Control Culture (CFU/mL) | Co-Culture Mix (CFU/mL) | ||
|---|---|---|---|
| Sensitive Strains | Bioprotectant Strains | ||
| Brettanomyces bruxellensis single culture (B1 or B250) | 1 × 104 | - | |
| Hanseniaspora uvarum single culture (Hu3137) | 1 × 104 | - | |
| Lachancea thermotolerans single culture (Lt29 or Lt45) | 1 × 106 | - | |
| Metschnikowia pulcherrima single culture (Mp2) | 1 × 106 | - | |
| Co-culture of bioprotectant × sensitive strains | - | 1 × 104 | 1 × 106 |
| Killer Yeast Codes | Killer Genera | Isolation Source | Killer Activity Agar Medium | ||||
|---|---|---|---|---|---|---|---|
| B1 | B250 | B3 | B7 | Hu3137 | |||
| Sb 36340 | Starmerella bacillaris | Carignan | ++ | + | + | +/− | Nd |
| Sb 36341 | Starmerella bacillaris | Tempranillo | +/− | + | + | − | Nd |
| Lt 28606 | Lachancea thermotolerans | Karech Noir Must | + | + | + | +/− | Nd |
| Lt 28607 | Lachancea thermotolerans | Karech Noir Must | + | + | ++ | +/− | Nd |
| Lt 28615 | Lachancea thermotolerans | Karech Noir Must | +/− | + | + | ++ | Nd |
| Lt 28645 | Lachancea thermotolerans | Karech Noir Must | + | ++ | + | ++ | + |
| Lt 28655 | Lachancea thermotolerans | Karech Noir Must | + | ++ | + | ++ | Nd |
| Lt 29126 | Lachancea thermotolerans | Grenache Must | + | +/− | + | + | Nd |
| Lt 29128 | Lachancea thermotolerans | Grenache Must | ++ | ++ | + | ++ | Nd |
| Lt 29129 | Lachancea thermotolerans | Grenache Must | ++ | ++ | ++ | ++ | ++ |
| Lt 29130 | Lachancea thermotolerans | Grenache Must | ++ | ++ | ++ | ++ | Nd |
| Lt 29134 | Lachancea thermotolerans | Grenache Must | ++ | ++ | +/− | + | Nd |
| Lt 29136 | Lachancea thermotolerans | Grenache Must | +/− | +/− | +/− | +/− | Nd |
| Lt 29139 | Lachancea thermotolerans | Grenache Must | +/− | +/− | ++ | ++ | Nd |
| Lt 29140 | Lachancea thermotolerans | Grenache Must | ++ | ++ | +/− | +/− | Nd |
| Lt 29143 | Lachancea thermotolerans | Grenache Must | ++ | ++ | ++ | ++ | Nd |
| Lt 29147 | Lachancea thermotolerans | Grenache Must | ++ | ++ | ++ | ++ | Nd |
| Lt 29694 | Pichia kudriavzevii | Mawardi Rouge Must | − | − | +/− | +/− | Nd |
| Lt 29698 | Pichia kudriavzevii | Mawardi Rouge Must | − | − | +/− | +/− | Nd |
| Lt 29702 | Pichia kudriavzevii | Mawardi Rouge Must | − | − | +/− | +/− | Nd |
| Mp2 | Metschnikowia pulcherrimin | Commercial | + | ++ | ++ | + | ++ |
| Selected Bioprotectant Strains | Target S. cerevisiae Strains | |||
|---|---|---|---|---|
| S342 | S340 | S334 | VL2 | |
| Mp2 | K− R+ | K− R+ | K− R+ | Nd |
| Lt29 | K− R+ | K− R+ | K− R+ | K− R+ |
| Lt45 | K− R+ | K− R+ | K+ R+ | K+ R+ |
| Sensitive Strain | Killer Strain 2 | μ max (h−1) | Final Population (CFU/mL) (9 Days) |
| B1 1 | 0.126 ± 0.001 a,3 | 2.72·108 ± 0.11·108 a | |
| Mp2 | 0.058 ± 0.003 c | 1.09·107 ± 0.08·107 c | |
| Lt29 | 0.044 ± 0.001 b | 8.93·106 ± 0.94·106 c | |
| Lt45 | 0.065 ± 0.001 c | 1.86·107 ± 0.14·107 b | |
| Sensitive Strain | Killer Strain 2 | μ max (h−1) | Final Population (CFU/mL) (9 Days) |
| B250 1 | 0.123 ± 0.002 a,3 | 2.7·108 ± 0.06·108 a | |
| Mp2 | 0.034 ± 0.002 c | 1.15·107 ± 0.16·107 c | |
| Lt29 | 0.045 ± 0.001 c | 2.07·106 ± 0.41·106 b | |
| Lt45 | 0.073 ± 0.004 b | 1.54·107 ± 0.24·107 | |
| Sensitive Strain | Killer Strain 2 | μ max (h−1) | Final Population (CFU/mL) (9 Days) |
| Hu3137 1 | 0.204 ± 0.001 a,3 | 5.8·107 ± 1.2·107 a | |
| Mp2 | 0.03 ± 0.002 b,d | 2.64·104 ± 1.94·104 b | |
| Lt29 | 0.03 ± 0.003 b | 0 b | |
| Lt45 | 0.053 ± 0.004 d | 0 b |
| Killer Toxins | Halo Diameter (mm) | |||||
|---|---|---|---|---|---|---|
| H. uvarum “Hu3137” | B. bruxellensis “B1” | B. bruxellensis “B250” | ||||
| 0.500 mg/mL | 0.185 mg/mL | 0.500 mg/mL | 0.185 mg/mL | 0.500 mg/mL | 0.185 mg/mL | |
| Lt29Kt | 10 | 7 | 9 | 5 | 8 | 5 |
| Lt45Kt | 0 | 0 | 4 | 1 | 4 | 2 |
| Mp2Kt | 6 | 0 | 5 | 3 | 5 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Dana, F.; David, V.; Hallal, M.A.; Tourdot-Maréchal, R.; Hayar, S.; Colosio, M.-C.; Alexandre, H. Metschnikowia pulcherrima and Lachancea thermotolerans Killer Toxins: Contribution to Must Bioprotection. Foods 2025, 14, 1462. https://doi.org/10.3390/foods14091462
El Dana F, David V, Hallal MA, Tourdot-Maréchal R, Hayar S, Colosio M-C, Alexandre H. Metschnikowia pulcherrima and Lachancea thermotolerans Killer Toxins: Contribution to Must Bioprotection. Foods. 2025; 14(9):1462. https://doi.org/10.3390/foods14091462
Chicago/Turabian StyleEl Dana, Fatima, Vanessa David, Mohammad Ali Hallal, Raphaëlle Tourdot-Maréchal, Salem Hayar, Marie-Charlotte Colosio, and Hervé Alexandre. 2025. "Metschnikowia pulcherrima and Lachancea thermotolerans Killer Toxins: Contribution to Must Bioprotection" Foods 14, no. 9: 1462. https://doi.org/10.3390/foods14091462
APA StyleEl Dana, F., David, V., Hallal, M. A., Tourdot-Maréchal, R., Hayar, S., Colosio, M.-C., & Alexandre, H. (2025). Metschnikowia pulcherrima and Lachancea thermotolerans Killer Toxins: Contribution to Must Bioprotection. Foods, 14(9), 1462. https://doi.org/10.3390/foods14091462









